Abstract
The mechanism of oxidative phosphorylation and its regulation remain one of the main problems of bioenergetics. Efficiency of the mitochondrial energization is determined by the relationship between the rate of generation of electrochemical potential of hydrogen ions and the rate of its expenditure on the synthesis of ATP and the use of ATP in endergonic reactions. Uncoupling (partial or complete), which occurs in the process of uncontrolled and controlled leakage of ions through the inner mitochondrial membrane, on the one hand leads to the decrease in the relative synthesis of ATP, and on the other, being consistent with the law of conservation of energy, leads to the formation of heat, generation of which is an essential function of the organism. In addition to increased thermogenesis, the increase of non-phosphorylating oxidation of various substrates is accompanied by the decrease in transmembrane potential, production of reactive oxygen species, and activation of oxygen consumption, water and carbon dioxide production, increase in the level of intracellular ADP and acidification of the cytosol. In this analysis, each of these factors will be considered separately for its role in regulating metabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Oxidative phosphorylation in the modern interpretation means coupling of the substrate oxidation, accompanied by generation of the electrochemical potential of hydrogen ions with its use by ATP synthase. This concept of coupling a priori assumes that the chemical energy stored during the substrate oxidation is initially transformed into electrochemical energy, and then again into chemical energy stored in the high-energy ATP bond. The degree of coupling between these processes determines efficiency of the integral process, that is, to what degree the oxidation energy is converted into the chemical energy of the macroergic bond in the ATP molecule. This means that at the high degree of coupling of oxidation with phosphorylation (that is, at the maximum possible conversion of the energy released by the electron transfer along the mitochondria respiratory chain into the energy stored in ATP), a large amount of ATP is formed (maximum three molecules per one molecule of oxidized NADH), and at the low degree of coupling less than three ATP molecules is formed. It is known from experiments that in any case, at least 1/3 of the oxidation energy is not associated with the synthesis of ATP, but rather is released as heat [1]. Literally speaking, the oxidative metabolism of substrates produces ATP, water, CO2, and releases heat. We will briefly discuss separately all these components and some ways to regulate them, taking into account the coupling elements.
THERMOGENESIS
It is improper to consider the heat released as a result of mitochondrial oxidation as an unnecessary and useless loss, since this released heat is the main way to maintain temperature, primarily in homoiothermic animals, to which humans belong. Moreover, the release of heat as a result of mitochondrial oxidation should not be considered as an imperative parasitic leak, but as a finely regulated physiologically necessary process that affects the coupling mechanism of oxidative phosphorylation.
This seemingly trivial idea was first suggested by V. P. Skulachev in 1960 on the basis of measurements of the P/O coefficient in the mitochondria of the skeletal muscles of a pigeon exposed to cold [2], and the data were presented at the 5th International Congress of Biochemistry in Moscow [3]. In particular, it was shown that during the oxidation of pyruvate and malate, the P/O coefficient in the mitochondria from the control birds was 2.07, and in the mitochondria from the birds exposed to cold, 1.0. It should be noted that these changes were accompanied by activation of respiration in the birds exposed to cold, meaning that the rate of oxidation of substrates increased. Immediately, the question about the mechanism of such regulation was raised. A little bit later, free fatty acids were suggested as the first candidates for the role of such regulator, which can indeed be called natural uncouplers of oxidative phosphorylation [4], and they became the subject of research by the Skulachev’s team for many years.
It must be taken into consideration that activation of the cell respiration within the organ can lead to a possible imbalance between the delivery and consumption of oxygen and substrates, which theoretically can lead to ischemia of the organ, which is undesirable, so the massive loss of coupling could cause many pathogenetic moments, including not only a decrease in pO2 in the tissue, but also changes in the levels of ATP and ADP and intracellular pH, which will be discussed later. Based on this, a moderate loss of coupling is physiologically acceptable, which is currently referred to as “mild uncoupling”. It is likely that the need to prevent ischemia is responsible for the high degree of vascular supply (vascularization) of the brown adipose tissue, which predominantly specializes in thermogenic function due to the presence in the tissue of the uncoupling protein UCP1 [5], and provides thermogenesis mediated by fatty acids. In general, the problem of matching the supply of energy to the tissue compartments and the energy demands of the tissue is one of the most important problems in physiological terms, the solution of which is very significant [6]. Perhaps, Nature has found a similar solution of this problem by very high vascularization of the thyroid gland, which produces thyroid hormones. These hormones, with certain limitations, are also recognized by some researchers as natural mild uncouplers, although the uncoupling effect of thyroid hormones is not always can be realized directly, which indicates that if they do uncouple, this process should have some mediators [7-9].
Uncoupling of oxidative phosphorylation, especially in the mild form, which allows moderate synthesis of ATP by mitochondria, has been the subject of many studies, and was examined in detail by V. P. Skulachev [10]. The main idea expressed by him and supported by the world scientific community is that the mild uncoupling is a mechanism for reducing unnecessary and excessive endogenous production of reactive oxygen species (ROS). This conclusion was made based on the obtained exponential dependence of the generation of ROS, primarily hydrogen peroxide, on the levels of the transmembrane potential in mitochondria with the maximum generation of ROS observed in the hyperpolarized mitochondria [11]. This indirectly implies that generation of ROS by mitochondria could occur as a natural, finely regulated process, during which mitochondria perform a number of essential energetic and non-energetic functions, rather than being a result of some uncontrolled leakage of electrons from the components of the respiratory chain [12]. Due to this, they stabilize the level of intracellular ROS, thus turning into some sort of ROS-stat. In this regard, mitochondrial thermogenesis is a powerful regulator of ROS levels in mitochondria and in cells. In particular, an increase in thermogenesis will help to avoid unwanted avalanche generation of ROS [13-15], which is of remarkable pathogenetic significance [16].
Several years ago, it has been found that the cyclic transport process through bilayer membranes carried out by fatty acids and other weak and strong uncouplers in protonated and non-protonated forms [17] is not the only mechanism that provides the discharge of membrane potential on the inner mitochondrial membrane. In addition, the uncoupling of oxidative phosphorylation is achieved by the presence of uncoupling carrier proteins, including not only UCP1, but also an ADP/ATP antiporter [18] and a dicarboxylate carrier residing in the inner mitochondrial membrane [19, 20]. On the other hand, there are many unproven assumptions in the suggested uncoupling mechanism mediated by fatty acids, but in general form, we can talk about the possible natural protonophoric activity of fatty acids observed on bilayer models and mediated protonophoric activity caused by the interaction of fatty acids with the proteins of the inner membrane of mitochondria, with an important factor to consider, namely, that the value of the transmembrane potential is associated with the efficiency of uncoupling [21]. The confirmation of proton transport through the adenine nucleotide translocator mediated by fatty acids, which is very similar in nature to the mechanism of uncoupling action of fatty acids when interacting with the uncoupling protein UCP1, has been a breakthrough, as it also confirmed the possible thermogenic function of the ADP/ATP antiporter [22]. There is evidence that the mitochondria themselves are quite resistant to a significant increase in temperature [23], so that the calorigenic activity of mitochondrial carriers may be not detrimental to the functional activity of mitochondria. The process of inhibition of the action of even most powerful uncouplers by alpha-ketocholestanol remains a mystery [24] suggesting participation of lipids in the process of uncoupling.
WATER
The vast majority of researchers undoubtedly recognize the oxygen-consuming function of mitochondria, but the resulting formation of water in mitochondria is mainly ignored. It should not be forgotten that for one molecule of oxygen consumed, two water molecules are formed. On the one hand, while a huge number of publications are devoted to the problem of oxygen delivery to the tissue and to the mitochondria themselves, it seems that the problem of water outflow from the mitochondria and the whole cell does not exist. On the other hand, local or global (high-amplitude) swelling of mitochondria (i.e., at the micro level [25-28]) or edema of the entire cell or organ (i.e., at the macro level) can be observed in a pathological events [29, 30]. Note that the fatal brain edema is the ultimate cause of death in the stroke patients [31].
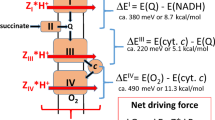
The main place of water formation in mitochondria is cytochrome oxidase, which releases water molecules into the intermembrane space (Fig. 1) [32]. There are two possible destinations of the formed water molecules. In one scenario, almost all formed water molecules go into the cytosol. Although it is recognized that the membranes are permeable for water, the easiest way for water to escape mitochondria is through the voltage-dependent anion channel (VDAC, or as it is also called, the porin channel [33]). As to the second option, in principle, water could enter mitochondrial matrix, where the osmotic + oncotic pressure is particularly high, and normally the orthodox configuration of the mitochondrial matrix is one of the indicators of mitochondrial homeostasis. In this regard, the presence of aquaporins in the inner mitochondrial membrane [34, 35] looks absurd, because their presence would accelerate the flow of water into the mitochondrial matrix. Although, to be fair, it should be noted that the rapid balancing of the mitochondrial volume after osmotic challenge [36] may indicate that passive water transport even without the presence of aquaporins may be sufficient to afford osmotic regulation, and the specific function of aquaporins is not limited to water transport at all, but something else, for example, ammonium ions, associated with water transport, which was assumed for aquaporin 8 [37].
We repeat that the swelling of the intermembrane space and high-amplitude swelling of the mitochondria in general, observed at the ultrastructural level, are signs of pathogenic stress. On the other hand, a slight swelling of the mitochondria is not only a pathological event, but on the contrary, a regulatory one leading to activation of mitochondrial metabolism as an adaptive response to moderate stress. In cardiomyocytes, an increase of the mitochondria volume caused by oxidative stress by only few percent leads to activation of respiration by one-third with the proportional increase in the synthesis of ATP, generation of ROS, which enables mobilization of the active protective signaling systems [38] and facilitates balance between the energy supply and demand [6]. This small increase in the volume of mitochondria was observed under the action of hormones, in particular glucagon, which also provides activation of metabolism [39]. In the early 1970s, we observed activation of the tissue respiration, accompanied by a slight increase in the in situ intermembrane space and enlightenment of the mitochondrial matrix of the diaphragm tissue of rats exposed to cold stress (Fig. 2). Thus, mitochondrial swelling also follows the general rule “multet nocem” (excess is harmful), which dominates biological world [15] and which should not be forgotten when pharmacological drugs are used. It is important to emphasize that a small swelling of mitochondria is not only physiologically acceptable, but also necessary for metabolism mobilization and its compliance with the increased needs, while more extensive swelling of mitochondria can lead to the cell death. We can only assume that this small change in the volume of mitochondria is not accompanied by the rupture of their outer membrane, while the larger swelling, up to the so-called high-amplitude, leads to the increased rupturing of the outer membrane, which is accompanied by the release of signal proteins from the intermembrane space that induce cell death. Note that the high-amplitude mitochondrial swelling observed with the opening of the mitochondrial megachannel (induction of nonspecific permeability) is accompanied by complete de-energization, on the one hand, and, on the other hand, with inability to provide normal (orthodox) conformation of the mitochondrial matrix, which definitely indicates interrelationship between these two processes/conditions.
Mitochondrial ultrastructure in the rat diaphragm. Electron microscopy after standard fixation and sample preparation. Viewing was performed with a Hitachi HU-12 microscope. a) Diaphragm of a control rat maintained at 28°C; b) diaphragm of a rat exposed to cold stress (2°C, 15 min); c) diaphragm of a control rat after addition of 40 µM 2,4-dinitrophenol in vitro at 28°C.
Considering the first option of the destination of water formed in mitochondria, namely its exit through VDAC, we assume that this huge flux of water would be able to generate a reactive impulse sufficient to provide motion of the individual mitochondria in the cells with poorly structured cytoplasm (such as fibroblasts or astroglial cells). Uneven distribution of VDAC over the surface of the outer mitochondrial membrane has been demonstrated earlier [40], and it can theoretically provide the reactive movement of the entire organelles or their parts, which has been observed. This reactive motion can be considered as another mechanism of mitochondrial mobility, complementing the already known mechanism of the mitochondrial movement, which involves adaptive motor proteins associated with the cytoskeleton [41, 42]. These types of movements, although different in mechanisms, imply that the movements of mitochondria mediated by the motor proteins are strictly energy-dependent, requiring ATP (possibly and/or membrane potential [43]), while the proposed water-jet mechanism under the non-phosphorylating conditions (with de-energization of mitochondria corresponding to the 3u state or close to it) due to activation of respiration and corresponding enhanced generation and expulsion of water from mitochondria would be more active than in the resting state (state 4 or close to it). We suggest that if such mechanism of the mitochondrial mobility exists, then, by definition, there would be a direct relationship between the decrease in the degree of mitochondrial coupling and increase in the rate of formation of H2O by cytochrome oxidase and, accordingly, creation of the reactive pulse capable of moving mitochondria.
ATP
We understand that extensive discussion of the role of ATP in the cell is simply impractical within the framework of such a review, so we will briefly focus only on those issues that are rarely discussed in the scientific literature.
The higher is contribution of the nonphosphorylating oxidation to the integral oxygen consumption of mitochondria, the greater is the effect on the levels of intracellular ATP, even while maintaining energy costs, not to mention physiological situations that involve activation of metabolism. But here, first of all, everybody needs to understand that changes in the levels of ATP will inevitably cause changes in the content of its partners according to the simple scheme of hydrolysis-synthesis, which very schematically looks like ATP = ADP + Pi + H+, that is, there is a complete coupling of the release and binding of proton in the system with the levels of ATP or ADP (here we omit the role of Mg ions, considering very similar chelating abilities of ATP and ADP). In short, in any cell compartment, an increase in the content of ATP accompanies some alkalization of the compartment, and its decrease is accompanied by acidification of the compartment. It is this property that determines occurrence of acidosis in the tissue that has problems with delivery of the energy substrates and oxygen (for example, in ischemia), which leads to the drop in the levels of ATP in the ischemic area. It is clear that intracellular buffers largely extinguish the acidic “fire”, but these buffers also have limited capacities, as a result of which acid gradients appear in the tissue, and degradation processes are activated in the areas with low values of intracellular pH [44-46]. We repeat that it is the ATPase activity necessary for performing intracellular functions that determines the degree of acidosis, rather than it is the result of switching to the glycolytic pathway, incorrectly called “lactate acidosis”, because the pathway of converting glucose to lactic acid is not associated with the total proton generation (read the detailed explanation in [47]).
In addition, it is important to know that the increase of the concentration of ADP associated with ATP hydrolysis can lead to the shift of the equilibrium of adenylate kinase reaction toward the increase of AMP, which is very important, in particular due to its role in regulation of AMP kinase activity [48].
Another point, although little to do with energetics, is that ATP located outside of the cell, can be considered as an inducer of the native inflammatory process, due to the fact that ATP belongs to the group of DAMPs (damage-associated molecular patterns) [49]; and its exit from the cell is possible in particular during massive or local tissue damage or as a result of necrotic death of individual cells. In addition to ATP, a powerful extracellular regulatory function belongs to adenosine, which is formed from AMP [50]. It should be noted that there is a possible relationship between the intra- and extracellular levels of adenine nucleotides, given the presence of pannexins in the cell membrane [51, 52].
CARBON DIOXIDE
One of the main pH buffers in the organism is the chemically balanced bicarbonate/carbon dioxide (HCO3–/CO2) system [53, 54], which emphasizes the important role of the stationary level of CO2 and its production via acid homeostasis in mitochondria, cytoplasm, extracellular space, and blood. By definition, increase in the level of CO2 leads to acidosis, while its decrease – to alkalosis in the biological compartment. In mitochondria, CO2 is formed in the tricarboxylic acid cycle in two consecutive reactions from isocitrate (with isocitrate dehydrogenase catalysis) and alpha-ketoglutarate (with alpha-ketoglutarate dehydrogenase catalysis), while the more active the Krebs cycle is, the more CO2 is formed and the greater is the probability of acidification of the mitochondrial matrix. Given that under activation of respiration, in particular, when the coupling of oxidative phosphorylation decreases, the flow through the Krebs cycle increases proportionally, generation of CO2 also increases, making an additional contribution to the overall acidification.
It seems that moderate acidification has protective properties, which was shown in ischemia as an example [55], in particular, due to activation of the protein inhibitor (IF1) binding to the ATP synthase complex [56], preventing under the conditions of energy crisis the undesirable consumption of ATP required to maintain the mitochondrial membrane potential observed in hypoxia/ischemia [43, 57]. However, it was found that the role of CO2 is not limited to a simple effect on pH changes. It has become known that increasing levels of carbon dioxide and bicarbonate associated with it, for example, in reperfusion after ischemia, directly affect the autophagy process by inhibiting it [58] and thereby preventing elimination of the non-functional mitochondria, which could cause further damage to the biological system [59]. However, in addition to their role in pH regulation, CO2 and bicarbonate have a significant signaling function, in particular, they participate in a number of important redox reactions [60-62], while the increasing level of bicarbonate provokes protein oxidation [63]. Very weak membrane permeability for bicarbonate is offset by the presence of specific carriers for it [64-66], the defect of which leads to pathologies, including systemic acidosis, brain and kidney dysfunction, and hypertension (summarized in [63]).
Within the physiological pH range, CO2 in its pure form is almost nonexistent, mainly being converted into bicarbonate, which regulates a variety of enzymes. These include adenylate cyclase [67], succinate dehydrogenase [68], mitochondrial ATPase [69, 70], and ATP synthase [71], which makes it possible to attribute the CO2/bicarbonate system to a fairly powerful factor regulating oxidative phosphorylation. The recent finding of the coupling effect of bicarbonate [72] confirms this suggestion, providing another control point in the process of uncoupling of oxidative phosphorylation.
References
Brand, M. D., Chien, L. F., Ainscow, E. K., Rolfe, D. F., and Porter, R. K. (1994) The causes and functions of mitochondrial proton leak, Biochim. Biophys. Acta, 1187, 132-139, https://doi.org/10.1016/0005-2728(94)90099-x.
Skulachev, V. P., and Maslov, S. P. (1960) The role of oxidation in thermopregulation, Biochemistry (Moscow), 25, 1058-1064.
Skulachev, V. P. (1961) Regulation of the coupling of oxidation and phosphorylation, 5th Internat. Cong. of Biochem. (Moscow), 5, 367-373.
Skulachev, V. P., Maslov, S. P., Sivkova, V. G., Kalinichenko, L. P., and Maslova, G. M. (1963) Cold-induced uncoupling of oxidation and phosphorylation in muscles of white mice, Biochemistry (Moscow), 28, 70-79.
Nedergaard, J., and Cannon, B. (2018) Brown adipose tissue as a heat-producing thermoeffector, Handb. Clin. Neurol., 156, 137-152, https://doi.org/10.1016/B978-0-444-63912-7.00009-6.
Yaniv, Y., Juhaszova, M., Nuss, H. B., Wang, S., Zorov, D. B., Lakatta, E. G., and Sollott, S. J. (2010) Matching ATP supply and demand in mammalian heart: in vivo, in vitro, and in silico perspectives, Ann. N. Y. Acad. Sci., 1188, 133-142, https://doi.org/10.1111/j.1749-6632.2009.05093.x.
Maley, G. F., and Lardi, H. A. (1953) Metabolic effects of thyroid hormones in vitro. II. Influence of thyroxine and triiodothyronine on oxidative phosphorylation, J. Biol. Chem., 204, 435-444, PMID: 13084614.
Hoch, F. L., and Lipmann, F. (1954) The uncoupling of respiration and phosphorylation by thyroid hormones, Proc. Natl. Acad. Sci. USA, 40, 909-921, https://doi.org/10.1073/pnas.40.10.909.
Dickens, F., and Salmony, D. (1956) Effects of thyroid hormones in vitro on tissue respiration, oxidative phosphorylation and the swelling of mitochondria, Biochem. J., 64, 645-651, https://doi.org/10.1042/bj0640645.
Skulachev, V. P. (1998) Uncoupling: new approaches to an old problem of bioenergetics, Biochim. Biophys. Acta, 1363, 100-124, https://doi.org/10.1016/s0005-2728(97)00091-1.
Korshunov, S. S., Skulachev, V. P., and Starkov, A. A. (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria, FEBS Lett., 416, 15-18, https://doi.org/10.1016/s0014-5793(97)01159-9.
Zorov, D. B., Krasnikov, B. F., Kuzminova, A. E., Vysokikh, M. Yu., and Zorova, L. D. (1997) Mitochondria revisited. Alternative functions of mitochondria, Bios. Rep., 17, 507-520, https://doi.org/10.1023/a:1027304122259.
Zorov, D. B., Filburn, C. R., Klotz, L. O., Zweier, J. L., and Sollott, S. J. (2000) Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes, J. Exp. Med., 192, 1001-1014, https://doi.org/10.1084/jem.192.7.1001.
Zorov, D. B., Juhaszova, M., and Sollott, S. J. (2006) Mitochondrial ROS-induced ROS release: an update and review, Biochim. Biophys. Acta, 1757, 509-517, https://doi.org/10.1016/j.bbabio.2006.04.029.
Zorov, D. B., Juhaszova, M., and Sollott, S. J. (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release, Physiol. Rev., 94, 909-950, https://doi.org/10.1152/physrev.00026.2013.
Zorov, D. B., Bannikova, S. Y., Belousov, V. V., Vyssokikh, M. Y., Zorova, L. D., Isaev, N. K., Krasnikov, B. F., and Plotnikov, E. Y. (2005) Reactive oxygen and nitrogen species: friends or foes, Biochemistry (Moscow), 70, 215-221.
Liberman, E. A., Topaly, V. P., Tsofina, L. M., Jasaitis, A. A., and Skulachev, V. P. (1969) Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria, Nature, 222, 1076-1078, https://doi.org/10.1038/2221076a0.
Andreyev, A. Yu., Bondareva, T. O., Dedukhova, V. I., Mokhova, E. N., Skulachev, V. P., Tsofina, L. M., Volkov, N. I., and Vygodina, T. V. (1989) The ATP/ADP-antiporter is involved in the uncoupling effect of fatty acids on mitochondria, Eur. J. Biochem., 182, 585-592, https://doi.org/10.1111/j.1432-1033.1989.tb14867.x.
Wieckowski, M. R., and Wojtczak, L. (1997) Involvement of the dicarboxylate carrier in the protonophoric action of long-chain fatty acids in mitochondria, Biochem. Biophys. Res. Commun., 232, 414-417, https://doi.org/10.1006/bbrc.1997.6298.
Samartsev, V. N., Smirnov, A. V., Zeldi, I. P., Markova, O. V., Mokhova, E. N., and Skulachev, V. P. (1997) Involvement of aspartate/glutamate antiporter in fatty acid-induced uncoupling of liver mitochondria, Biochim. Biophys. Acta, 1319, 251-257, https://doi.org/10.1016/s0005-2728(96)00166-1.
Rupprecht, A., Sokolenko, E. A., Beck, V., Ninnemann, O., Jaburek, M., Trimbuch, T., Klishin, S. S., Jezek, P., Skulachev, V. P., and Pohl, E. E. (2010) Role of the transmembrane potential in the membrane protein leak, Biophys. J., 98, 1503-1511, https://doi.org/10.1016/j.bpj.2009.12.4301.
Bertholet, A. M., Chouchani, E. T., Kazak, L., Angelin, A., Fedorenko, A., et al. (2019) H+ transport is an integral function of the mitochondrial ADP/ATP carrier, Nature, 571, 515-520, https://doi.org/10.1038/s41586-019-1400-3.
Chrétien, D., Bénit, P., Ha, H. H., Keipert, S., El-Khoury, R., et al. (2018) Mitochondria are physiologically maintained at close to 50°C, PLoS Biol., 16, e2003992, https://doi.org/10.1371/journal.pbio.2003992.
Starkov, A. A., Dedukhova, V. I., and Skulachev, V. P. (1994) 6-ketocholestanol abolishes the effect of the most potent uncouplers of oxidative phosphorylation in mitochondria, FEBS Lett., 355, 305-308, https://doi.org/10.1016/0014-5793(94)01211-3.
Lehninger, A. L. (1962) Water uptake and extrusion by mitochondria in relation to oxidative phosphorylation, Physiol. Rev., 42, 467-517, https://doi.org/10.1152/physrev.1962.42.3.467.
Solenski, N. J., diPierro, C. G., Trimmer, P. A., Kwan, A. L., and Helm, G. A. (2002) Ultrastructural changes of neuronal mitochondria after transient and permanent cerebral ischemia, Stroke, 33, 816-824, https://doi.org/10.1161/hs0302.104541.
Kaasik, A., Kuum, M., Joubert, F., Wilding, J., Ventura-Clapier, R., and Veksler, V. (2010) Mitochondria as a source of mechanical signals in cardiomyocytes, Cardiovasc. Res., 87, 83-91, https://doi.org/10.1093/cvr/cvq039.
Zorov, D. B., Vorobjev, I. A., Popkov, V. A., Babenko, V. A., Zorova, L. D., et al. (2019) Lessons from the discovery of mitochondrial fragmentation (fission): a review and update, Cells, 8, 175, https://doi.org/10.3390/cells8020175.
Ware, A. J., D’Agostino, A. N., and Combes, B. (1971) Cerebral edema: a major complication of massive hepatic necrosis, Gastroenterology, 61, 877-884, PMID: 125688.
Durward, Q. J., Del Maestro, R. F., Amacher, A. L., and Farrar, J. K. (1983) The influence of systemic arterial pressure and intracranial pressure on the development of cerebral vasogenic edema, J. Neurosurg., 59, 803-809, https://doi.org/10.3171/jns.1983.59.5.0803.
Preston, E., and Webster, J. (2004) A two-hour window for hypothermic modulation of early events that impact delayed opening of the rat blood-brain barrier after ischemia, Acta Neuropathol., 108, 406-412, https://doi.org/10.1007/s00401-004-0905-4.
Schmidt, B., McCracken, J., and Ferguson-Miller, S. (2003) A discrete water exit pathway in the membrane protein cytochrome c oxidase, Proc. Natl. Acad. Sci. USA, 100, 15539-15542, https://doi.org/10.1073/pnas.2633243100.
Zimmerberg, J., and Parsegian, V. A. (1987) Water movement during channel opening and closing, J. Bioenerg. Biomembr., 19, 351-358, https://doi.org/10.1007/BF00768538.
Gena, P., Fanelli, E., Brenner, C., Svelto, M., and Calamita, G. (2009) News and views on mitochondrial water transport, Front. Biosci. (Landmark Ed.), 14, 4189-4419, https://doi.org/10.2741/3522.
Lee, W. K., and Thévenod, F. (2006) A role for mitochondrial aquaporins in cellular life-and-death decisions? Am. J. Physiol. Cell Physiol., 291, 195-202, https://doi.org/10.1152/ajpcell.00641.2005.
Calamita, G., Gena, P., Meleleo, D., Ferri, D., and Svelto, M. (2006) Water permeability of rat liver mitochondria: a biophysical study, Biochim. Biophys. Acta, 1758, 1018-1024, https://doi.org/10.1016/j.bbamem.2006.07.008.
Saparov, S. M., Liu, K., Agre, P., and Pohl, P. (2007) In vitro analysis and modification of aquaporin pore selectivity, J. Biol. Chem., 282, 5296-5301, https://doi.org/10.1074/jbc.M609343200.
Juhaszova, M., Zorov, D. B., Kim, S. H., Pepe, S., Fu, Q., et al. (2004) Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore, J. Clin. Invest., 113, 1535-1549, https://doi.org/10.1172/JCI19906.
Halestrap, A. P. (1989) The regulation of the matrix volume of mammalian mitochondria in vivo and in vitro and its role in the control of mitochondrial metabolism, Biochim. Biophys. Acta, 973, 355-382, https://doi.org/10.1016/s0005-2728(89)80378-0.
Konstantinova, S. A., Mannella, C. A., Skulachev, V. P., and Zorov, D. B. (1995) Immunoelectron microscopic study of the distribution of porin on outer membranes of rat heart mitochondria, J. Bioenerg. Biomembr., 27, 93-99, https://doi.org/10.1007/BF02110336.
Maeder, C. I., Shen, K., and Hoogenraad, C. C., (2014) Axon and dendritic trafficking, Curr. Opin. Neurobiol., 27, 165-170, https://doi.org/10.1016/j.conb.2014.03.015.
Melkov, A., and Abdu, U. (2018) Regulation of long-distance transport of mitochondria along microtubules, Cell. Mol. Life Sci., 75, 163-176, https://doi.org/10.1007/s00018-017-2590-1.
Zorova, L. D., Popkov, V. A., Plotnikov, E. Y., Silachev, D. N., Pevzner, I. B., et al. (2018) Mitochondrial membrane potential, Anal. Biochem., 552, 50-59, https://doi.org/10.1016/j.ab.2017.07.009.
Rizack, M. A. (1964) An epinephrine-sensitive lipolytic activity in adipose tissue, J. Biol. Chem., 236, 657-662, PMID: 14169136.
Eastman, A. (1994) Deoxyribonuclease II in apoptosis and the significance of intracellular acidification, Cell Death Differ., 1, 7-9, PMID: 17180001.
Gottlieb, R. A., Giesing, H. A., Zhu, J. Y., Engler, R. L., and Babior, B. M. (1995) Cell acidification in apoptosis: granulocyte colony-stimulating factor delays programmed cell death in neutrophils by up-regulating the vacuolar H(+)-ATPase, Proc. Natl. Acad. Sci. USA, 92, 5965-5968, https://doi.org/10.1073/pnas.92.13.5965.
Silachev, D. N., Gulyaev, M. V., Zorova, L. D., Khailova, L. S., Gubsky, L. V., et al. (2015) Magnetic resonance spectroscopy of the ischemic brain under lithium treatment. Link to mitochondrial disorders under stroke, Chem. Biol. Interact., 237, 175-182, https://doi.org/10.1016/j.cbi.2015.06.012.
Steinberg, G. R., and Kemp, B. E. (2009) AMPK in health and disease, Physiol. Rev., 89, 1025-1078, https://doi.org/10.1152/physrev.00011.2008.
Zhang, Q., Raoof, M., Chen, Y., Sumi, Y., Sursal, T., Junger, W., Brohi, K., Itagaki, K., and Hauser, C. J. (2010) Circulating mitochondrial DAMPs cause inflammatory responses tom injury, Nature, 464, 104-107, https://doi.org/10.1038/nature08780.
Borea, P. A., Gessi, S., Merighi, S., Vincenzi, F., and Varani, K. (2015) Pharmacology of adenosine receptors: the state of the art, Physiol. Rev., 98, 1591-1625, https://doi.org/10.1152/physrev.00049.2017.
Kang, J., Kang, N., Lovatt, D., Torres, A., Zhao, Z., Lin, J., and Nedergaard, M. (2008) Connexin 43 hemichannels are permeable to ATP, J. Neurosci., 28, 4702-4711, https://doi.org/10.1523/JNEUROSCI.5048-07.2008.
Chekeni, F. B., Elliott, M. R., Sandilos, J. K., Walk, S. F., Kinchen, J. M., et al. (2010) Pannexin 1 channels mediate “find-me” signal release and membrane permeability during apoptosis, Nature, 467, 863-867, https://doi.org/10.1038/nature09413.
Roos, A., and Boron, W. F. (1981) Intracellular pH, Physiol. Rev., 61, 296-434, https://doi.org/10.1152/physrev.1981.61.2.296.
Aickin, C. C. (1986) Intracellular pH regulation by vertebrate muscle, Annu. Rev. Physiol., 48, 349-361, https://doi.org/10.1146/annurev.ph.48.030186.002025.
Inserte, J., Barba, I., Hernando, V., Abellán, A., Ruiz-Meana, M., Rodríguez-Sinovas, A., and Garcia-Dorado, D. (2008) Effect of acidic reperfusion on prolongation of intracellular acidosis and myocardial salvage, Cardiovasc. Res., 77, 782-790, https://doi.org/10.1093/cvr/cvm082.
Pullman, M. E., and Monroe, G. C. (1963) A naturally occurring inhibitor of mitochondrial adenosine triphosphatase, J. Biol. Chem., 238, 3762-3769, PMID: 14109217.
Di Lisa, F., Blank, P. S., Colonna, R., Gambassi, G., Silverman, H. S., Stern, M. D., and Hansford, R. G. (1995) Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition, J. Physiol., 486, 1-13, https://doi.org/10.1113/jphysiol.1995.sp020786.
Queliconi, B. B., Kowaltowski, A. J., and Gottlieb, R. A. (2016) Bicarbonate increases ischemia-reperfusion damage by inhibiting mitophagy, PLoS One, 11, e0167678, https://doi.org/10.1371/journal.pone.0167678.
Zorov, D. B., Popkov, V. A., Zorova, L. D., Vorobjev, I. A., Pevzner, I. B., et al. (2017) Mitochondrial aging: is there a mitochondrial clock? J. Gerontol. A Biol. Sci. Med. Sci., 72, 1171-1179, https://doi.org/10.1093/gerona/glw184.
Liochev, S. I., and Fridovich, I. (2002) Copper, zinc superoxide dismutase and H2O2. Effects of bicarbonate on inactivation and oxidations of NADPH and urate, and on consumption of H2O2, J. Biol. Chem., 277, 34674-34678, https://doi.org/10.1074/jbc.M204726200.
Medinas, D. B., Cerchiaro, G., Trindade, D. F., and Augusto, O. (2007) The carbonate radical and related oxidants derived from bicarbonate buffer, IUBMB Life, 59, 255-262, https://doi.org/10.1080/15216540701230511.
Queiroz, R. F., Paviani, V., Coelho, F. R., Marques, E. F., Di Mascio, P., and Augusto, O. (2013) The carbonylation and covalent dimerization of human superoxide dismutase 1 caused by its bicarbonate-dependent peroxidase activity is inhibited by the radical scavenger tempol, Biochem. J., 455, 37-46, https://doi.org/10.1042/BJ20130180.
Queliconi, B. B., Marazzi, T. B., Vaz, S. M., Brookes, P. S., Nehrke, K., Augusto, O., and Kowaltowski, A. J. (2013) Bicarbonate modulates oxidative and functional damage in ischemia-reperfusion, Free Radic. Biol. Med., 55, 46-53, https://doi.org/10.1016/j.freeradbiomed.2012.11.007.
Kumar, S., Flacke, J., Kostin, S., Appukuttan, A., Reusch, H. P., and Ladilov, Y. (2011) SLC4A7 sodium bicarbonate cotransporter controls mitochondrial apoptosis in ischaemic coronary endothelial cells, Cardiovasc. Res., 89, 392-400, https://doi.org/10.1093/cvr/cvq330.
Alka, K., and Casey, J. R. (2014) Bicarbonate transport in health and disease, IUBMB Life, 66, 596-615, https://doi.org/10.1002/iub.1315.
Nozik-Grayck, E., Huang, Y.-C. T., Carraway, M. S., and Piantadosi, C. (2003) Bicarbonate-dependent superoxide release and pulmonary artery tone, Am. J. Physiol. Heart Circ. Physiol., 285, 2327-2335, https://doi.org/10.1152/ajpheart.00507.2003.
Acin-Perez, R., Salazar, E., Kamenetsky, M., Buck, J., Levin, L. R., and Manfredi, G. (2009) Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation, Cell Metabol., 9, 265-276, https://doi.org/10.1016/j.cmet.2009.01.012.
Zeylemaker, W. P., Klaasse, A. D., Slater, E. C., and Veeger, C. (1970) Studies on succinate dehydrogenase. VI. Inhibition by monocarboxylic acids, Biochim. Biophys. Acta, 198, 415-422, https://doi.org/10.1016/0005-2744(70)90120-8.
Kasho, V. N., and Boyer, P. D. (1984) Relationships of inosine triphosphate and bicarbonate effects on F1-ATPase to the binding change mechanism, J. Bioenerg. Biomembr., 16, 407-419, https://doi.org/10.1007/BF00743235.
Roveri, O. A., and Calcaterra, N. B. (1985) Steady-state kinetic of F1-ATPase. Mechanism of anion activation, FEBS Lett., 192, 123-127, https://doi.org/10.1016/0014-5793(85)80056-9.
Lodeyro, A. F., Calcaterra, N. B., and Roveri, O. A. (2001) Inhibition of steady-state mitochondrial ATP synthesis by bicarbonate, an activating anion of ATP hydrolysis, Biochim. Biophys. Acta, 1506, 236-243, https://doi.org/10.1016/s0005-2728(01)00221-3.
Khailova, L. S., Vygodina, T. V., Lomakina, G. Y., Kotova, E. A., and Antonenko, Y. N. (2020) Bicarbonate suppresses mitochondrial membrane depolarization induced by conventional uncouplers, Biochem. Biophys. Res. Commun., 530, 29-34, https://doi.org/10.1016/j.bbrc.2020.06.131.
Funding
This work was financially supported by the Russian Science Foundation (project no. 19-14-00173).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest in financial or any other sphere. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Zorov, D.B., Andrianova, N.V., Babenko, V.A. et al. Nonphosphorylating Oxidation in Mitochondria and Related Processes. Biochemistry Moscow 85, 1570–1577 (2020). https://doi.org/10.1134/S0006297920120093
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297920120093