Abstract
The development of express method for detection of endocrine-disrupting chemicals (EDC) such as alkylphenols is required for ecological monitoring. Several attempts have been made to produce antibodies against 4-nonylphenol (NP) in recent years. This work describes the production of new antibodies against NP and also summarizes the characterization of antibodies obtained earlier. Three approaches used to produce alkylphenol-specific antibodies are compared; these are based on:
-
1.
ω-(4-hydroxyphenyl)nonanoic or ω-(4-hydroxyphenyl)heptanoic acid NP derivatives designed to mimic the linear NP isomer;
-
2.
4-aminophenol, which potentially mimics various substituted phenolic compounds with different side-chain structures at position 4 of the benzene ring; and
-
3.
a mixture of branched NP isomers, conjugated to the carrier protein via a benzene ring by the Mannich reaction, and expected to be the closest mimic of NP structure by preserving its natural alkyl moiety.
Fluorescence polarization immunoassays based on different combinations of antibody and labeled antigen for screening detection of NP were developed and structural aspects of assay sensitivity and specificity were investigated. The assays based on the antisera raised against ω-(4-hydroxyphenyl)nonanoic acid and NP conjugate via Mannich reaction are capable of express detection of NP with detection limit of 7 μg mL−1 and assay dynamic range of 18–300 μg mL−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A variety of hazardous environmental pollutants has been identified in recent years, including a large group of endocrine-disrupting chemicals (EDC). These compounds mimic natural hormones and thus can adversely affect humans and wildlife by interaction with endocrine systems. Alkylphenols (AP) such as 4-nonylphenol (NP) and 4-octylphenol have been reported to have an estrogenic effect [1], interfering the natural hormone system and inducing the proliferation of estrogen-dependent MCF7 human breast tumor cells in vitro [2]. AP are stable degradation products of the widely used group of non-ionic surfactants, alkylphenolethoxylates (APEO), and are capable of accumulation in water reservoirs and aquatic organisms. For instance, surveys of AP in environmental samples performed in many countries revealed the presence of NP at concentrations of 10–220 μg L−1 in river water [3, 4] and 20–30 μg kg−1 in fish tissues [5, 6]. Toxicity, genotoxicity and estrogenic potency data for AP are scarce in the literature, and very much dependent on the type of organism used to evaluate the possible biological effects of these compounds. For instance, EC50 values (50% bioluminescence inhibition) determined from bioassays based on Vibrio fischeri and Escherichia coli were 0.36 and 500 μg mL−1, respectively [7].
AP and many other EDC are routinely measured by tandem gas chromatography–mass spectrometry (GC–MS) [8, 9], and liquid chromatography (LC) with various detection techniques [10, 11, 12]. Although these techniques are sensitive and specific, they do not enable rapid processing of large numbers of samples. Alternatively, immunochemical methods enable high-throughput sample screening and are now increasingly applied for EDC analysis. Recent examples include APEO [13], linear alkylbenzene sulfonates (LAS) [14, 15, 16] and bisphenol A (BPA) [17, 18]. AP are low-molecular-weight compounds with hydrophobic alkyl chains; the lack of specific antigenic epitopes makes it difficult to raise antibodies against these analytes. Another property of AP which hampers the production of high-affinity antibodies is their structural heterogeneity because of the large number of isomers with different alkyl chain-lengths and positions on the benzene ring. Although polyclonal [16, 19] and monoclonal [20] antibodies against NP have been described, it has not been possible to develop a sensitive and, at the same time, specific assay for heterogeneous NP mixtures.
We recently reported the development of a fluorescence polarization immunoassay (FPIA) for screening detection of NP [19]. FPIA is a rapid homogeneous immunoassay technique, especially suitable for the screening detection of target analytes, because the assay time per sample is about 1 min. In our previous work hapten design was based either on an NP derivative with an active terminal carboxylic group, ω-(4-hydroxyphenyl)nonanoic acid [16], or on 4-aminophenol cross-linked to the carrier protein via glutaraldehyde [19]. In this investigation we describe antibodies raised against new derivatives:
-
1.
ω-(4-hydroxyphenyl)heptanoic acid, and
-
2.
NP itself, conjugated to the carrier protein via Mannich reaction with formaldehyde.
Antibodies obtained as a result of various strategies for hapten design are compared in this work in terms of sensitivity and selectivity of FPIA for screening detection of NP in water samples.
Experimental
Reagents
Bovine serum albumin (BSA), N,N′-dicyclohexylcarbodiimide (DCC), N-hydroxysuccinimide (NHS), 4-aminophenol (4-AP), tyramine (TA), glutaraldehyde (GA) 25% v/v aqueous solution, formaldehyde (FA) 37% v/v aqueous solution, triethylamine, and fluorescein isothiocyanate (FITC) isomer I were purchased from Sigma (St Louis, MO, USA). Soybean trypsin inhibitor (STI) and chicken ovalbumin (OVA) were from Reanal (Budapest, Hungary). Freund’s complete and incomplete adjuvants were from ICN Biomedicals (Aurora, Ohio, USA). Nonylphenol (NP, technical mixture of branched isomers) was supplied by Aldrich (Steinheim, Germany). The compounds tested for cross-reactivity, such as 4-hydroxybenzoic acid, 4-chlorophenol, 2,4-dimethylphenol, 2,4-dinitrophenol, 2-amino-4-chlorophenol, and phenol were from Sigma. Alkylphenolpolyethoxylates (APEO), octylphenolethoxylate (OPEO), nonylphenolbiethoxycarboxylate (NP2EO), and 4-tert-octylphenol were kindly provided by Dr Jenny Emnéus (Department of Analytical Chemistry, Lund University, Lund, Sweden). Dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) were purchased from Merck (Darmstadt, Germany). All the other chemicals were of analytical grade. Pre-coated silica gel 60 F254 (0.25 mm) aluminium-backed sheets for thin-layer chromatography (TLC) were acquired from Merck.
Apparatus
Fluorescence polarization data were recorded with a Beacon 2000 (PanVera, USA) fluorescence polarization system equipped with a temperature-control unit at temperature 22 °C. The inhibition curves were analyzed by use of a four-parameter sigmoidal curve in Origin 6.0 for Windows. The structure of the fluorescent conjugates was confirmed by recording atmospheric pressure chemical ionization (APCI) tandem mass spectrometry data using an API 3000 LC–MS–MS instrument (AME Bioscience, Toroed, Norway) in turbo ion spray mode.
Buffers and solutions
The PBS buffer was 50 mmol L−1 potassium-phosphate buffer, containing 0.8% w/v NaCl, pH 7.4. Borate buffer was 25 mmol L−1 sodium borate containing 1% w/v NaN3, pH 8.0. Bicarbonate buffer was 0.01 mol L−1 sodium hydrogen carbonate, pH 10.0. Stock solutions of NP and cross-reacting compounds were prepared in methanol (10 mg mL−1) and subsequently diluted with borate buffer to the concentrations 0.1, 1, 10, 30, 100, 300 and 1000 μg mL−1 before analysis.
Synthesis of haptens and protein conjugates
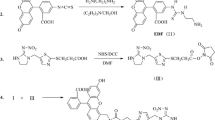
Structural NP analogs with a terminal carboxyl group, ω-(4-hydroxyphenyl)heptanoic acid (NP7) and ω-(4-hydroxyphenyl)nonanoic acid (NP9), were synthesized in six steps starting with α,ω-alkanedicarboxylic acid as described elsewhere [21]. NP7 and NP9 were conjugated to BSA and STI by NHS-ester method in a manner similar to that previously described for NP9 [16]. Protein conjugate 4AP-BSA was synthesized by cross-linking via GA according to Ref. [19]. Brief descriptions of the syntheses are given below.
Synthesis of the NP7-BSA and NP9-OVA conjugates
A mixture of 44 mg (0.2 mmol) NP7 or 50 mg NP9, 125 mg (1.2 mmol) NHS, and 250 mg (1.2 mmol) DCC in 1 mL DMF was stirred under room temperature for 3 h. The supernatant was added dropwise to a solution of 133 mg (2 μmol) BSA or 89 mg OVA in 10 mL bicarbonate buffer (pH 10.0) with vigorous stirring. The reaction was left to proceed overnight at 4 °C and the mixture was then dialyzed for 48 h in PBS at 4 °C and the conjugates were lyophilized.
Synthesis of the 4AP-BSA conjugate
A solution of 22 mg (0.2 mmol) 4AP in 1 mL DMF was added dropwise to a mixture of 0.9 mL distilled water and 0.1 mL (0.2 mmol) 25% w/v glutaraldehyde. The reaction mixture was gently shaken for 10 min, then slowly added to a solution of 136 mg (2 μmol) BSA in 10 mL carbonate buffer and stirred for 15 min at room temperature (RT). To reduce the Schiff bases, 10 mg (200 μmol) sodium borohydride was added and the reaction mixture was incubated for 3 h. The solution was then dialyzed against 3 L PBS (five changes of buffer) and lyophilized.
Synthesis of NPFA-STI and NPFA-BSA conjugates
Nonylphenol was conjugated to BSA and STI by Mannich reaction with formaldehyde (FA) in a manner similar to that described for preparation of the immunogen for 3-methoxy-4-hydroxyphenylglycol [22]. NP (20.6 mg) was dissolved in 0.62 mL DMSO then 0.116 mL NP solution in DMSO was mixed with 2.0 mL BSA solution in bicarbonate buffer (pH 10) in a dark glass vial and then 0.2 mL 35% FA was added dropwise to the mixture with stirring. Analogously, 0.248 mL NP in DMSO was mixed with 2.14 mL STI in bicarbonate buffer (pH 10). FA (0.214 mL, 35%) was added dropwise and the mixture was stirred. Reaction mixtures were incubated for 5 days at 37 °C and stirred periodically. The resulting conjugates (NPFA-BSA and NPFA-STI) were separated from low molecular weight components in the reaction mixture by dialysis against PBS (pH 7.4).
Production of antibodies
To obtain antibodies against NP7-BSA, NPFA-BSA, and NPFA-STI, eight Chinchilla rabbits weighing 3–4 kg were immunized with the corresponding conjugates. The immunogens (NPFA-STI, NPFA-BSA or NP7-BSA conjugate) were dissolved in PBS and then emulsified with an equal volume of Freund’s complete adjuvant to a final concentration of 1.0 mg mL−1 (by protein). For the first immunization, 1.0 mL of this mixture was injected subcutaneously. After 1.5 months the animals received a subcutaneous booster injections of one half of the immunogen dose (emulsified with Freund’s incomplete adjuvant in a final volume of 1.0 mL per animal), and were then injected intravenously with a week interval.
Two booster injections, consisting of subcutaneous (one half dose with incomplete adjuvant) and intravenous (one half dose of immunogen into buffer solution) administration of immunogens were carried out. Animals were bled from the ear vein after each immunization cycle. To obtain the serum, blood samples were left to coagulate for 30–40 min at +37 °C and for 1–2 h at +4 °C, followed by centrifugation at 5000 rpm for 10 min. Supernatant was carefully collected and stored at −20 °C until use. To estimate antibody titer, antisera after each immunization were analyzed by measuring the binding of the fluorescein-labeled antigen by FPIA.
The NP9-OVA and 4AP-BSA conjugates were used to immunize the rabbits in a manner similar to that described in Refs. [16] and [19], respectively.
Synthesis of fluorescent conjugates
Fluorescein thiocarbamyl ethylene diamine (EDF) was synthesized from fluorescein isothiocyanate isomer I as described elsewhere [23]. The fluorescent tracers NP7-EDF and NP9-EDF were synthesized according to the NHS-ester method as described in Ref. [19]. Briefly, NHS (8 mg, 80 μmol) and DCC (8 mg, 40 μmol) were added to a solution of NP7 (4.4 mg, 20 μmol) or NP9 (5 mg, 20 μmol) in 0.2 mL DMFA and the reaction mixture was stirred overnight. Next day the precipitate was removed by centrifugation, 5 mg (10 μmol) EDF was added to the supernatant, and the mixture was stirred for 3 h. The mixture was separated by TLC using dichloromethane–methanol, 4:1 (v/v), as eluent. The main yellow band at R f 0.9 was isolated and stored in methanol at 4 °C. Tracer structures were confirmed by mass-spectrometry data. APCI-MS: NP7-EDF [M+H] 654.8 Da; NP9-EDF [M+H] 682.2 Da.
Tyramine (TA) was conjugated to FITC according to the following procedure. A solution of 4 mg (10 μmol) FITC isomer I was prepared in 0.5 mL methanol containing 10 μL triethylamine. TA (5.4 mg, 40 μmol) was then added and the mixture was stirred overnight at RT. Next day the reaction mixture was applied to a preparative TLC plate using chloroform–methanol, 4:1 (v/v), as eluent. The intense yellow band at R f 0.1 was isolated and stored in methanol at 4 °C.
The tracer concentrations were estimated spectrophotometrically at 492 nm, assuming the absorbance in borate buffer (25 mmol L−1, pH 8) to be the same as for fluorescein (ε=8.78×104 mol−1 L cm−1). The tracer solution was further diluted in 25 mmol L−1 borate buffer and used for FPIA measurements.
Fluorescence polarization immunoassay (FPIA)
Antibody dilution curves were obtained by incubating different antibody concentrations (dilution of antiserum ranged from 1/100 to 1/40,000) with an appropriate amount of NP7-EDF, NP9-EDF or TA-FITC fluorescent conjugate (1.0×10−9 mol L−1) in a total volume of 0.5 mL for 1 min at RT. The fluorescence polarization (P) values, defined according to the formula:
where I v and I h are the vertical and horizontal components of the emitted fluorescence intensities, were measured for each dilution of antiserum. Fluorescence polarization expressed in “milli units” (mP) were used to construct antibody dilution curves.
The competitive FPIA calibration curves were constructed with the technical mixture of NP chain and ring at the concentrations 0.1, 1, 10, 30, 100, 300, and 1000 μg mL−1. The NP standard (50 μL) was mixed with the labeled antigen and antiserum solution in borate buffer in a final volume of 0.5 mL, and the mP signal was measured. Experimental data were plotted against analyte concentration and a four-parameter equation was used to fit the experimental sigmoidal curve in Origin 6.0 for Windows. The assay limit of detection (LOD) was determined from the calibration curve using the blank signal and the threefold confidence interval, and converted into the analyte concentration.
To compare the calibration curves obtained with different antibodies, both absolute mP and normalized signals calculated as the ratio between the signal for a given analyte concentration to that of zero analyte dose (mPi/mPo) were used. For span test only NP standards at the concentration of 1000 μg mL−1 were used in comparison with zero analyte dose.
Cross-reactivity (CR) data for anti-NP9 and anti-4AP were calculated according to the equation:
where IC50 is the concentration of analyte or cross-reacting compound that inhibits 50% of fluorescent tracer binding. All measurements were performed in static mode in triplicate using disposable borosilicate tubes at 22 °C.
Results and discussion
Design of the haptenic derivatives
Challenges in the production of antibodies against AP are associated with their structural composition. AP are a mixture of isomers differing in the structure of the alkyl chain moiety and the position of the hydroxyphenyl ring. Therefore, successful antibodies against NP should have broad specificity and enable detection of the whole class of alkylphenolic compounds.
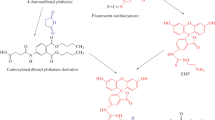
To evaluate the best immunogen, enabling the most sensitive and specific recognition of NP by the resulting antibodies, three different strategies were employed (Fig. 1a). The first relied on mimicking the linear NP isomer structure and utilized ω-(4-hydroxyphenyl)nonanoic (NP9) or ω-(4-hydroxyphenyl)heptanoic (NP7) acids. These derivatives contained a linear alkyl chain with an active terminal carboxyl group that enabled the important antigenic epitopes of the NP, such as the hydroxyphenyl ring, to be kept distant from the protein attachment site.
Chemical structures of the immunogens (a) and fluorescein-labeled antigens (b) used for the development of FPIA for NP. Abbreviations: NP7, ω-(4-hydroxyphenyl)heptanoic acid; NP9, ω-(4-hydroxyphenyl)nonanoic acid; TA, tyramine; Fluor, fluorescein; EDF, fluorescein thiocarbamyl ethylene diamine, FITC, fluorescein isothiocyanate
Another approach was based on 4-aminophenol (4-AP), containing a nitrogen atom adjacent to the benzene ring, with the intention of mimicking a variety of phenolic molecules with different side-chains. The spacer arm between the 4-AP and the carrier protein was obtained via cross-linking with glutaraldehyde. Thus, one might expect that anti-4AP might recognize not only target AP, but also have noticeable cross-reactivity with a variety of phenolic compounds substituted para to the hydroxyl group.
Finally, the third approach implied conjugation of NP (technical mixture of isomers) to the carrier protein by Mannich reaction with formaldehyde (FA). This seems to be the simplest way of synthesizing the immunogen for NP compared with immunogens based on NP7 and NP9 derivatives, which were synthesized in six steps as described elsewhere [21]. In the case of NPFA the natural analyte molecule served as a hapten. However, the shortcoming is that such conjugation via FA provides a very short spacer arm between NP and carrier protein. Therefore, despite the closest mimic of the heterogeneous NP structure expected from the NPFA hapten, the quality of raised antibodies could be low.
The two approaches based on NP9 and 4-AP were described in our earlier papers [16, 19]. The preparation of anti-NP7 and anti-NPFA antibodies is reported in this study for the first time. The antibodies raised against four different immunogens were compared in terms of sensitivity and specificity of FPIA for NP. To produce the fluorescein-labeled antigens different spacer structures were explored (Fig. 1b). TA, NP7 and NP9 derivatives represent the short, middle and long spacers, respectively.
Assessment of antibody binding with fluorescent tracers
The principle of FPIA is based on the difference in rotational motion between bound and free fluorescein-labeled tracer [24]. The free tracer is characterized by rapid Brownian movement, and thus has a low polarization value, whereas the tracer bound in the immunocomplex gives a high polarization signal (Fig. 2a). At fixed temperature and viscosity of the solution the fluorescence polarization value is proportional to the size of fluorescent species. When the analyte is present in the sample it will compete with fluorescent tracer for antibody binding sites (Fig. 2b) and the measured polarization will be in a stoichiometric relationship to the amount of free and bound tracer in the sample. PFIA is a homogeneous assay technique, which does not require separation of bound and free label.
Principle of fluorescence polarization fluoroimmunoassay (FPIA). (a) Dependence of the polarization value on molecular size. (b) Competition between the tracer and analyte for antibody binding sites. When the analyte is absent, all tracer molecules will be bound to the antibodies and polarization will be maximum. With increasing analyte concentration polarization will decrease along with a decrease in the amount of bound tracer
Because FPIA is a competitive technique, the lowest tracer concentration should be used for a sensitive assay. For NP7-EDF, NP9-EDF, and TA-FITC, the optimum concentration, corresponding to a total fluorescence intensity approximately ten times higher than the background signal from buffer, was about 1 nmol L−1.
For assessment of antibody affinity toward the labeled antigens, antibody dilution curves after the 5th bleeding were recorded for different antibody–tracer combinations, and antibody titer values were determined (Table 1). As seen from these data, the value of the antibody titer corresponding to 50% tracer binding is dependent on tracer structure. The highest titers for anti-NP9, anti-NP7, anti-4-AP and anti-NPFA were observed with NP9-EDF with the long spacer arm, whereas the lowest values for all antisera were found with TA-EDF. Anti-NP9 had the highest affinity toward NP9-EDF and NP7-EDF as indicated by the titer values (1/2500 and 1/900, respectively), which were at least two times higher than for other antisera, e.g. anti-NP7. Based on the results of antibody binding, NP7-EDF and NP9-EDF were chosen for further experiments, whereas TA-EDF was excluded as the tracer with the lowest affinity.
Assay sensitivity
The assay sensitivity was investigated using NP standard (a technical mixture of NP chain and ring isomers). In the first step, fast screening of antibody affinity toward NP using NP7-EDF and NP9-EDF as labeled antigens was performed by span analysis. This technique enables rapid estimation of the signal dynamic range by comparison of mP signals given by zero analyte dose and high analyte concentration. Antisera raised against NP7 and NPFA, and obtained by immunization of different animals (a total of eight rabbits) were characterized and compared with anti-4AP as described elsewhere [19]. We observed that absolute mP signals were higher for anti-NPFA than for anti-NP7 and anti-4AP, and also for NP9-EDF compared with NP7-EDF (data not shown). However, to estimate the range of the signal span, the normalized signals for each antibody–tracer combination were plotted and compared (Fig. 3). As seen from these data, NP9-EDF resulted in a broader mP range for all the antisera tested. Comparison of anti-NP7 (rabbits 1 and 2) and anti-NP9 (rabbits 3–8) illustrates the different immune response of individual animals and enables selection of the best antisera, providing maximum inhibition of bound tracer signal. On the basis of optimization of immunoreagent structure by the span analysis, NP9-EDF was chosen as a labeled competitor for further experiments; rabbits 2 and 5 were found to result in the broadest signal span among all antisera against NP7 and NPFA, respectively.
Span (0 and 1 mg mL−1) analysis using two fluorescein-labeled tracers (NP7-EDF and NP9-EDF) and nine antisera (anti-NP7, anti-4AP and anti-NPFA from different rabbits). Black bars correspond to zero analyte dose response (mP), gray to response from 1 mg mL−1 analyte and NP7-EDF, and white to response from 1 mg mL−1 analyte and NP9-EDF. Numbers on x-axis correspond to: 1, Anti-NP7-BSA (rabbit 1); 2, Anti-NP7-BSA (rabbit 2); 3, Anti-NPFA-STI (rabbit 3); 4, Anti-NPFA-STI (rabbit 4); 5, Anti-NPFA-STI (rabbit 5); 6, Anti-NPFA-STI (rabbit 6); 7, Anti- NPFA-BSA (rabbit 7); 8, Anti-NPFA-BSA (rabbit 8); 9, Anti-4AP-BSA. Fluorescence polarization value for unbound tracer is 60 mP
FPIA calibration curves for NP were recorded with different antisera (anti-NP9, anti-NP7, anti-NPFA (rabbit 5) and anti-4-AP) in a combination with NP9-EDF fluorescent tracer. Figure 4a shows fluorescence polarization values plotted against analyte concentration. These signals were obtained at different antibody concentrations and the upper plateau varies within 120–170 mP, which hampers correct comparison of the calibration curves. Indeed, the curves obtained for anti-NP7, anti-NPFA and anti-4AP are practically indistinguishable in the concentration range 30–1000 μg mL−1. For this reason, the normalized signals were calculated (Fig. 4b), with the inherent mP values of the tracer and mP in the absence of analyte taken into account (the equation is given in the legend to Fig. 4b). As seen from the data presented in Fig. 4b, the best competition between NP and labeled antigen, and thus the most sensitive NP detection, is achieved by using anti-NP9 and NP9-EDF. Anti-NPFA resulted in a less sensitive assay, despite the fact that for NP9 the immunogen mimics only linear NP isomers whereas NPFA utilizes an entire mixture of branched NP isomers. Analytical characteristics of FPIA based on different antisera are listed in Table 2. As seen from these data, LOD values obtained by using anti-NP9 and anti-NPFA are equal, though a higher slope was observed for anti-NP9 antiserum. (It should be noticed that LOD is commonly used as a measure of immunoassay sensitivity [25]). The least sensitive assay was obtained with anti-NP7, when the calibration curve had an LOD of 51 mg L−1 and IC50 above 1000 mg L−1. This finding was surprising, because the chemical structures of NP9 and NP7 are homologous. Perhaps such a dramatic discrepancy between the features of antibodies raised against NP9 and NP7 illustrate the importance of immune response of an individual animal and also the length of the spacer arm of the immunogen. The latter assumption is in accordance with previous data based on the use of p-(hydroxyphenyl)propionic acid as an immunizing hapten. In this case the resulting antibodies did not recognize NP (data not published). Therefore, only the haptens with long spacers such as NP9 should be used for production of successful antibodies against NP.
FPIA calibration curves for NP using NP9-EDF tracer in combination with anti-NP7, anti-NP9, anti-4AP, and anti-NPFA antisera. (a) Absolute mP values are plotted against NP concentration. (b) The normalized signals, expressed as (B−F)/(B 0−F), where B and B 0 are the fluorescence polarization values (mP) obtained for analyte solution and blank sample, respectively, and F is the background signal in the absence of antibody, are shown. Experimental data were fitted with a four-term logistic equation: y=(A−D)/[1+(x/C)B]+D, where A and D are the maximum and minimum signals, B is the slope of the sigmoid, and C is the analyte concentration resulting in 50% inhibition of tracer binding (IC50)
Cross-reactivity studies
To evaluate the specificity of developed assays, anti-NP9, anti-NP7, anti-4-AP and anti-NPFA were tested for their cross-reactivity (CR) with related compounds, including AP, APEO, their metabolites and other para-substituted phenolic compounds (Table 3). The data presented show that all antisera can discriminate NP among such structurally similar compounds as 4-tert-octylphenol, APEO and NP2EC (CR values are below 100%). However, discrimination among these compounds was not equal for all antisera tested. Anti-NP7 had the lowest CR values for related alkylphenols and their metabolites (CR=5–23%) whereas anti-NPFA was characterized by the most profound recognition of these compounds (CR=23–78%). This might be because NPFA hapten is the closest mimic of the heterogeneous structure of NP itself and its metabolites and analogs.

For other para-substituted phenolic compounds (4-aminophenol, 4-hydroxybenzoic acid, 4-chlorophenol, 2,4-dimethylphenol, 2,4-dinitrophenol, 2-amino-4-chloropenol) the most noticeable cross-reactivity was found for anti-NP7. All other antisera recognized these compounds to a minor extent, with the exception of anti-NP and such bis-substituted phenols as 2,4-dinitrophenol and 2-amino-4-chlorophenol. Considering the heterologous structure of 4-AP hapten (containing a nitrogen atom in the vicinity of the benzene ring) and CR observed for 2,4-dinitrophenol and 2-amino-4-chlorophenol, one might suppose that anti-4-AP recognizes a specific “electronic shape” of these compounds by interaction of π electrons with the aromatic ring which, perhaps, resembles that of the 4-AP hapten. A similar trend has been reported for anti-paracetamol antibodies [26].
Summarizing the results of assay specificity studies, anti-NPFA antiserum could potentially be used for group-specific detection of both AP and APEO, because of the relatively high CR for these compounds and low CR for other phenolic compounds. Anti-NP7 was shown to recognize both AP/APEO and other tested compounds to approximately the same extent and could thus be used to determine a broad range of phenolic substances.
Conclusions
A variety of immunogens designed to mimic different NP structural fragments were used for production of antibodies and compared in this study. Immunogens based on 4-AP and NPFA rely on a simple one-step reaction whereas the others, NP9 and NP7, were obtained as a result of six-step chemical synthesis. The most sensitive FPIA was observed for anti-NP9 antibodies, which mimicked the linear NP isomer structure. The assay was characterized with an LOD value of 7 mg L−1 and IC50 of 53 mg L−1. However, the complexity of multistep synthesis required for NP9 makes this hapten design strategy inconvenient and complicated to implement. Comparable sensitivity (LOD 7 and IC50 67 mg L−1) was obtained with antibodies raised against NPFA immunogen, which could be easily synthesized by Mannich reaction with formaldehyde. The CR pattern for this antiserum shows it is capable of group-specific detection of AP and APEO with minor recognition of other phenolic compounds. Therefore, one could recommend this hapten design strategy for production of antibodies against AP and APEO.
Besides traditional enzyme immunoassay, FPIA is an on-going trend in environmental analysis [27] which offers the advantages of speed, simplicity and reproducibility of analysis [28]. The sensitivity reached by FPIA for NP developed in this work is sufficient for the preliminary screening of industrial wastewater effluents, where NP concentration can reach mg L−1 or even g L−1 values. With regard to analysis of environmental water samples, NP have been reported to be toxic to aquatic organisms at concentrations of 0.18–5 mg L−1 [29]. Taking into account that water samples can be analyzed by FPIA without any pretreatment steps, the developed assays could potentially be applied for screening detection of NP in environmental samples above the threshold level. Such application is important for rapid identification of NP contaminated sites. To improve NP assay sensitivity we suggest application of anti-NPFA antibodies based on one-step immunogen synthesis via Mannich reaction for a development of ELISA and other immunoanalytical techniques. Our previous work [16] showed that the sensitivity of ELISA based on the same antibody is 1–2 orders of magnitude better than that of the FPIA technique. Therefore, ELISA based on anti-NPFA antibodies has broad specificity for detection of AP and APEO and might thus find wide application for environmental control of endocrine-disrupting chemicals.
References
Yadetie F, Arukwe A, Goksoyr A, Male R (1999) Sci Total Environ 233:201–210
Soto AM, Justicia H, Wray JW, Sonnenschein C (1991) Environ Health Perspect 92:167–173
Blackburn MA, Waldock MJ (1995) Water Res 29:1623–1629
Heemken OP, Reincke H, Stachel B, Theobad N (2001) Chemosphere 45:245–259
Tsuda T, Suga K, Kaneda E, Ohsuga M (2000) J Chromatogr B 746:305–309
Tsuda T, Takino M, Kojima M, Harada H Muraki K (1999) J Chromatogr B 723:273–279
Farre M, Barcelo D (2003) Trends Anal Chem 22:299–310
Meesters R, Schröder HF (2002) Anal Chem 74:3566–3574
Ding WH, Tzing SH (1998) J Chromatogr A 824:79–90
Takasu T, Iles A, Hasebe K (2002) Anal Bioanal Chem 372:554–561
Careri M, Elviri M, Mangia A (2001) J Assoc Off Anal Chem 84:1383–1392
Marquez N, Bravo B, Ysambertt F, Chavez G, Subero N, Salager JL (2003) Anal Chim Acta 477:293–303
Matsunaga T, Ueki F, Obata K, Tajima H, Tanaka T, Takeyama H, Goda Y, Fujimoto S (2003) Anal Chim Acta 475:75–83
Yakovleva J, Lobanova AY, Michura IV, Formanovsky AA, Franek M, Zeravik J, Eremin SA (2002) Anal Lett 35:2279–2294
Fujita M, Ike M, Goda Y, Fujimoto S, Toyoda Y, Miyagawa K (1998) Environ Sci Technol 32:1143–1146
Franek M, Zeravik J, Eremin SA, Yakovleva J, Badea M, Danet A, Nistor C, Ocio N, Emnéus J (2001) Fresenius J Anal Chem 371:456–466
Ohkuma H, Abe K, Ito M, Kokado A, Kambegawa A, Maeda M (2002) Analyst 127:93–97
Meulenaer B, Baert K, Lanckriet H, Hoed V, Huyghebaert A (2002) J Agric Food Chem 50:5273–5282
Yakovleva J, Zeravik J, Michura IV, Formanovsky A, Franek M, Eremin SA (2003) Int J Environ Anal Chem 83:597–607
Samsonova JV, Rubtsova MY, Franek M (2003) Anal Bioanal Chem 375:1017–1019
Michura IV, Formanovsky AA, Nikitin AO, Yakovleva JN, Eremin SA (2000) Mendeleev Commun 5:193–194
Hirose K, Akizawa T, Yoshioka M (1998) Anal Chim Acta 365:129–135
Nistor C, Oubina A, Marco MP, Barcelo D, Emnéus J (2001) Anal Chim Acta 426:185–195
Nasir MS, Jolley ME (1999) Comb Chem High T SCR 2:177–190
Ekins RP, Chu FW (1991) Clin Chem 37:1955-1967
Bennett AP, Gallacher G, Landon J (1987) Ann Clin Biochem 24:374–384
Schneider RJ (2003) Anal Bioanal Chem 375:44–46
Eremin SA, Smith DS (2003) Comb Chem High T SCR 6:257–266
Thiele B, Gunter K, Schwunger MS (1997) Chem Rev 97:3247–3272
Acknowledgments
The authors thank Dr Milan Franek (Veterinary Research Institute, Brno, Czech Republic) for providing us with polyclonal anti-NP9 antibodies and Dr Emilio Benfenati (Mario Negri Institute of Pharmacological Research, Milan, Italy) for his valuable help with APCI mass spectra. This work was supported by INTAS grant “Simple Methods for Determining Potent Xenobiotics in Water and Foodstuffs” (project No. 00–00870) and INCO-Copernicus grant “Development of New Express Enzyme Immunotechniques for Pesticides and Surfactants Monitoring in Water” (project ICA 2–1999–10032).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yakovleva, J.N., Lobanova, A.Y., Shutaleva, E.A. et al. Express detection of nonylphenol in water samples by fluorescence polarization immunoassay. Anal Bioanal Chem 378, 634–641 (2004). https://doi.org/10.1007/s00216-003-2307-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-003-2307-1