Abstract
Production of phytases from Pichia kudriavzevii FSMP-Y17 yeast was enhanced by optimization of the fermentation variables under submerged fermentation conditions. Maximum 3.67 U/mL of phytases was produced using 2.5% orange peel flour as a substrate, at pH 5.0 and temperature 50°C, by supplementing fermentation medium with 0.2% (wt/vol) galactose as carbon source and 0.2% (wt/vol) ammonium nitrate as a nitrogen source. Addition of sodium phytate to the production medium, however, did not cause any enhancing effect on phytase production by P. kudriavzevii FSMP-Y17 under submerged fermentation. The yeast enzyme purified to near homogeneity in three steps (ammonium sulphate precipitation, anion exchange chromatography and gel filtration) was found showing thermostability (from 40 to 70°C, with highest activity at 55°C) and stable at acidic pH (from 4.0 to 7.0, with highest activity at pH from 5.0 to 6.0). The purified enzyme was added to the diets of the broilers. The diets enriched with yeast phytase showed increased feed intake in the birds, while food conversion rate was lowered. The phytase supplementation caused better phosphorus retention in the birds consequently resulting in enhanced growth of the broilers. The augmented diets also showed improved egg production and egg quality in the hens.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Phytase (EC 3.1.3.8, inositol hexaphosphate phosphohydrolase) is an enzyme that catalyzes the sequential hydrolysis of phytic acid (myo-inositol 1,2,3,4,5,6)- hexakis (dihydrogen phosphate) to less phosphorylated myo-inositol and phosphoric acid with myo-inositol phosphate formed as intermediate [1]. Phytic acid, as phytate (salt of phytic acid) is a major storage form of phosphorus in the grains and legumes [2]. It accounts for 65–80% of the total phosphorus in the grains [2]. Thus, phytate is an important source of phosphorus in the animal feed. Phytic acid is chemically very stable molecule having high content of phosphate. Ruminants easily digest the phytic acid in their feed with the help of phytases produced by the anaerobic bacteria and fungi present as natural ruminal micro flora in their gut. However, monogastric animals such as poultry, fish and pig are unable to utilize phytate because of lack of gastrointestinal phytases in them. Under normal physiological conditions, phytic acid chelates nutritionally important divalent cations (Ca2+, Zn2+ and Fe2+) thereby rendering them biologically unavailable to the animal [3]. Therefore, the animal feed is supplemented with di-calcium phosphate to meet the nutritional requirements of the animals. On the other hand, this approach stimulates phosphate pollution due to the excretion of phytic acid and unutilized inorganic phosphorus, thereby leading to eutrophication of surface water [4]. Therefore, reduction of phytic acid content of the feeds, via its enzymatic hydrolysis, is essential. Phytases are widespread among all types of organisms including plants, animals, bacteria, fungi and yeasts [5]. Phytases can be broadly categorized into two major classes based on the pH for activity: acid phytases and alkaline phytases. More focus has been concentrated on acidic phytases because of their applicability in the animal feeds and broader substrate specificity than those of the alkaline phytases.
Due to their worldwide use in the feed industry, a wide variety of microbial phytases has been discovered and characterized in the last decade. The acceptance of a new phytase by a feed industry depends on many factors. Three essential characteristics of an “ideal phytase” include their effect in releasing phytate-P in the digestive tract, their thermostability during feed processing and storage, and the commercial production of the enzyme should be economically cheap [6]. Furthermore, the ability of any given phytase to hydrolyze phytate- P in the digestive tract is determined by its enzymatic properties such as catalytic efficiency, substrate specificity, temperature and pH optima, and resistance to the proteolysis [7]. As stomach is the main functional site of the supplemental phytase, a phytase with a low or acidic pH optimum and high resistance to pepsin is certainly desirable. In addition, because dietary ingredients for swine and poultry are often processed through a pelleting machine at 65–80°C with steam, an ideal phytase should be able to withstand high temperature and the steam encountered during this process. Likewise, an enzyme that can tolerate long term storage or transport at ambient temperature is undisputedly attractive. Finally, a phytase won’t be competitive if its productivity and purity is not comparable to relatively inexpensive system [7].
Major impediments to the exploitation of phytases are its yield, stability, specificity and the cost of production. Considering the above facts, screening criteria for phytases with better thermostability and desired level of activity have come into greater attention and focus. Moreover, there is need of microorganisms, which produce this enzyme efficiently using cheaper carbon and nitrogen sources. Keeping these significant things in view, the present investigation has been undertaken in which an extracellular phytase was produced from Pichia kudriavzevii FSMP-Y17 under submerged fermentation (SMF) conditions using orange peel as a substrate. The aim of the study was to purify the enzyme to the homogeneity and apply it in the broilers feed to follow their effect on various nutritional parameters.
MATERIALS AND METHODS
Microbial culture. The microbial culture used was P. kudriavzevii FSMP-Y17 yeast isolated from poultry field soil sample (soil collected from poultry farm). The culture was preserved on the MYGP (g/L: malt extract, 3.0; yeast extract 3.0; glucose, 10.0; peptone, 5.0; pH 5.5) agar slants at 30 ± 1°C and preservation was done at 4 ± 1°C in a refrigerator.
Production optimization of phytase. To improve the phytase yield, both nutritional as well as physical parameters were optimized using ‘one variable at a time approach’, under the condition of SMF.
SMF. The culture was grown in 50 mL of Phytase Screening Medium (PSM). The broth (pH 5.5) contained (g/L): glucose, 15.0; Na-phytate, 1.0; NH4NO3, 2.0; KCl, 0.5, MgSO4∙7H2O, 0.5; MnSO4, 0.3; FeSO4∙7H2O, 0.3. Various parameters such as effect of different agro-residue substrates (rice husk, wheat bran, sugarcane bagasse, gram’s covering (outer shell of chickpeas), lemon and orange peel flour), substrate concentration, pH, temperature, and effect of supplements (metal ions, sodium phytate, different carbon and inorganic nitrogen sources) were optimized for maximum phytase production.
Enzyme extraction and assay. The enzyme was extracted by centrifugation of fermentation flask contents under 5590 g for 15 min at 4°C and the supernatant was used as crude enzyme after filtration through Whatman filter paper no. 1 (UK). The phytase activity was determined using method described by Gulati et al. [8]. The reaction mixture consisted of 0.5% (wt/vol) sodium phytate substrate (prepared in 0.2 M sodium acetate buffer, pH 5.5) and appropriately diluted enzyme. The contents were incubated at 50°C for 30 min. Here the enzyme activity assay parameters have been chosen as per the required potential of enzyme to work efficiently in the environment of broiler’s digestive system (as all feed enzymes need to be heat stable to avoid substantial activity loss during pelleting process). Reaction termination was done by adding an equal volume of 15% trichloroacetic acid. For the quantification of the phosphate ions liberated in the reaction, 100 µL of the assay mixture was mixed with 900 µL of 1.0 M H2SO4, 10% ascorbic acid and 2.5% ammonium molybdate (3 : 1 : 0.1) (vol/vol) followed with incubation at 50°C for 20 min. Finally, the absorbance was taken at 820 nm. One enzyme unit (IU) is defined as the amount of enzyme required to hydrolyze 1 mM of substrate (sodium phytate) in 1 min under the assay conditions. The amount of enzyme production was expressed as U/mL.
Enzyme purification and characterization. The crude enzyme produced under optimized fermentation conditions was subjected to three steps purification. The ammonium sulphate precipitation was done to five cuts off (0–20, 20–40, 40–60, 60–80 and 80–90% saturation) at 4°C. The precipitated proteins were dissolved in minimum amount of 0.2 M acetate buffer (pH 5.5) and the solution was subsequently dialyzed against 0.2 M acetate buffer (pH 5.5) for 24 h at 4°C (by changing buffer repeatedly after every 6 h). The concentrated enzyme was subjected to the anion exchange chromatography. The charged DEAE-cellulose (equilibrated with 0.5 N HCl and 0.5 N NaOH), suspended in 0.1 M acetate buffer (pH 4.8), was packed in a glass column (2 × 42 cm) and equilibrated with 0.2 M acetate buffer (pH 5.0). The sample (dialyzed solution) was passed through the column at a flow rate of 1 mL/min with 0.2 M acetate buffer (pH 5.0), followed by a linear gradient of 0–1 M NaCl in the same buffer. The major fractions possessing phytase activity were pooled, concentrated by sucrose treatment and were further subjected to gel filtration chromatography using Sephadex G-100 column (prepared by packing 75 × 1.6 cm glass column with 8 g of Sephadex G-100 swollen by suspending in distilled water followed with washing with 0.1 M acetate buffer, pH 4.8).
The partially purified enzyme was characterized by studying the effect of the pH (using different buffers with pH from 2.0 to 10.0) and temperature (20–90°C) on the activity and the stability of the enzyme. In addition, the effect of different cations (Na+, K+, Ca2+, Hg2+, Mg2+, Cu2+, Zn2+, Ba2+, Fe2+, and Mn2+), detergents (SDS, Triton X-100, Tween 80), metal chelator (EDTA) and inhibitor (β-mercaptoethanol) was studied on the enzyme activity. The shelf life of the enzyme was tested at different temperatures, i.e., 50, 35 and 4°C.
Applications of yeast phytase in broilers. To study the application of phytase in the broilers feed, experimental mixtures containing various diets were designed according to the broiler organisms. Total number of broilers present in the farm, selected for experiment was divided into three groups having 10 broilers in each group. One group has been fed with diet supplemented with the Pichia kudriavzevii FSMP-Y17 enzyme, another group has been fed with diet supplemented with commercial phytase, and third group had the diet without phytase (control). Composition of the formulated diets has been shown in Table 1. In the broiler organisms, different parameters such as weight gain, feed conversion ratio (FCR), phosphorus retention (determined by the continuous observation of phosphorus content in the fecal matter of broilers) and dropping phosphorus content were compared in the commercial diet containing commercial phytase, commercial diet supplemented with the P. kudriavzevii FSMP-Y17 enzyme and control without any phytase. Fecal samples were ashed and P was determined spectrophotometrically (UV-visible spectrophotometer, UV-1900 Shimadzu Corp., Japan) at 680 nm [9]. The effect of phytase containing diets was also analyzed on the productivity of broilers in terms of egg production, specific gravity, broken egg ratio, shelf life of egg and yolk index. The effect of different diet mixture has been studied on the broilers, that are in egg laying age and the studies were caried for 2 weeks as per given in previous studies.
CALCULATIONS
where Feed Consumed is amount of Feed consumed by an individual broiler bird, and Gain in Body Weight is the weight gain in that individual broiler after consuming the feed.
where Phosphorus retained is the phosphorus content that retained in the broilers body, which is calculated by subtracting (phosphorus content in feces) from (Phosphorus intake).
RESULTS AND DISCUSSION
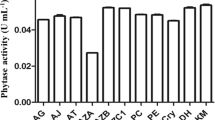
Optimization of phytase production under SMF conditions. Effect of substrate concentration. Phytase is an inducible enzyme and produced only in the presence of sodium phytate. The concentration of sodium phytate is a crucial factor for the growth and phytase induction. Our findings demonstrated that orange peel flour was the best substrate for maximum phytase production by P. kudriavzevii FSMP-Y17 yielding 1.95 U/mL enzyme under SMF (Table 2). The effect of different concentrations (1–5%) of this selected substrate on the enzyme production showed that 2.5% (wt/vol) concentration was best for maximum phytase (2.14 U/mL) production (Table 2). The high production of phytase in the case of orange peel flour may be due to some inducing factors like presence of sufficient phytate and carbon sources that accelerate the synthesis of the enzyme. Increased phytase production (521 ± 28.16 U/gds) by RSM attained with 5-g wheat bran supplemented with 2% mannitol, 0.5% ammonium sulfate was reported by Kumari and Bansal [10]. The production of phytase using Aspergillus niger NCIM 563 under SMF conditions was optimized using protein rich chickpea flour by Shah et al. [11]. The highest phytase production (208.30 ± 0.22 U/gds) was achieved using wheat bran as cheap agro-industrial substrate for SSF by Kumari and Bansal [12].
Effect of pH. The pH of the production medium plays a significant role in the production of different metabolites. In the present study, the effect of various pH (from 3.5 to 8.0) was tested in order to enhance the yield of the phytase. It can be seen from Table 2 that the maximum phytase activity of 2.29 U/mL was obtained at pH 5.0 with P. kudriavzevii FSMP-Y17. This may be due to the fact that yeast strain grows best at acidic pH. It seems that phytase from the isolate needed an acidic or neutral environment to be active. The considerably higher phytase production was obtained using rice bran as solid substrate (17.8–28.6 U/mL) at pH 5.0 by Sandhya et al. [13]. Investigations by Qasim et al. [14] revealed that optimal productivity of phytase was achieved using wheat bran at pH 5.0. Maximum phytase production under SSF supplemented with wheat bran at pH 5.0 was obtained for Bacillus sp. HCYL03 isolated from soil by Sardar et al. [15].
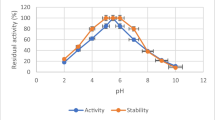
Effect of temperature. Incubation temperature affects various metabolic processes such as protein denaturation, enzymatic inhibition, promotion or inhibition of production of a particular metabolite, cell death, etc. Thus, the effect of temperature ranging from 30 to 60°C was studied on the phytase production by P. kudriavzevii FSMP-Y17. It was observed that maximum 2.31 U/mL phytase was produced by the yeast at 50°C (Table 2). Different microorganisms require different temperature for the enzyme synthesis and the utilization of the substrate. When temperature is raised, the kinetic energy of the substrate and enzyme molecules increases resulting in an increase in the number of collisions per unit time between the enzyme and its substrate. An increase in the temperature beyond the optimum value caused reduction in the catalytic rate of the phytase, which could be due to the denaturation of the enzyme rendering it inactive Some other scientists reported highest levels of phytase synthesis at 30°C for Aspergillus tubingensis SKA strains using wheat bran as substrate and 45°C for Thermoascus aurantiacus [14]. Maximum phytase production was observed at 45°C and pH 5.0 by Bacillus sp. HCYL03 [15]. The effect of different temperatures (20–90°C) on activity and stability of purified phytase from P. kudriavzevii FSMP-Y17 is presented in Fig. 1. This enzyme showed a temperature range of 45–65°C for the best activity (>80%) and the optimum temperature of 55°C for phytase activity was observed. There was an increase in phytase activity with increase in temperature up to 55°C, and a subsequent decrease in activity with further increase in temperature (at 80°C the residual phytase activity remained 48%). The stability (>80%) of the purified phytase was achieved in the range of 40–70°C.
Effect of sodium phytate supplementation. The effect of sodium phytate (Sigma-Aldrich, USA) on phytase yield was studied by adding this substrate at different concentrations (0–1%) in the production medium. No inductive effect of sodium phytate was observed at any concentration. The highest enzyme production by P. kudriavzevii FSMP-Y17 was obtained in the medium containing orange peel flour (2.34 U/mL) as phytate-rich substrate. This finding suggests the orange peel flour as an economical substrate for the production of microbial phytase. Similar results were obtained by Wodzinski and Ullah [16], Sano et al. [17] and Quan et al. [18] who observed an inhibitory effect of phytate on phytase synthesis by Aspergillus ficuum, Arxula adeninivorans and Candida kruseii, respectively. On the other hand, the synthesis of phytase in some Aspergillus species, Schwanniomyces castellii, and Bacillus subtilis has been shown to be stimulated by phytate [19–21].
Effect of carbon sources. The effect of different carbon sources on the production of phytase was evaluated under SMF by P. kudriavzevii FSMP-Y17. The results revealed that the phytase yield was increased to 3.19 U/mL in the presence of galactose as an additional carbon source. When the effect of various concentrations of galactose was tested, an increase in activity was obtained with 0.2% galactose i.e., 3.34 U/mL. However, an increase in concentration beyond 0.2% resulted in a decrease in the enzyme activity. This might be due to the interruption of yeast budding by metabolic products generated at higher concentration of the carbon sources. Similar to our results, Sano et al. [17] observed that galactose favored both growth and phytase production in Saccharomyces cerevisiae CY and A. adeninivorans, respectively. Galactose and molasses also enhance phytase production in S. castellii and in A. niger St-6 [20, 22]. The 0.3% dextrose, showed the best phytase production and enzyme activity is the case of Aspergillus fumigatus NF191 [23]. Glucose was proved to be the best carbon source for enhanced phytase production from A. awamori NRC- F18 V [24].
Nitrogen source is very essential component for the growth and the enzyme production by the microorganisms. Therefore, different nitrogen sources like ammonium nitrate, ammonium sulphate, ammonium dihydrogen phosphate, beef, tryptone, urea and peptone were evaluated for their effect on phytase production by P. kudriavzevii FSMP-Y17. It may be depicted from the results that ammonium nitrate showed stimulatory effect on the phytase production by the yeast (3.55 U/mL), while all other nitrogen sources were found inhibitory. On studying the effect of different concentrations of ammonium nitrate, it was observed that 0.2% (wt/vol) ammonium nitrate was suitable for maximal phytase yield (3.67 U/mL). Similar to our results, Ramachandran et al. [25] and Tahir et al. [22] also observed the highest growth and phytase production in the presence of ammonium nitrate by Rhizopus spp. and A. niger St-6, respectively. Other workers have reported maximum phytase production in the presence of ammonium sulphate (2.0%) by S. castellii [20]. According to one of a previous investigation, the optimal productivity of phytase was achieved using wheat bran supplemented with 0.5% (NH4)2SO4 as nitrogen source for Aspergillus tubingensis SKA [14]. Ammonium acetate was proved to be the best nitrogen source for enhanced phytase production from A. awamori NRC- F18 [24].
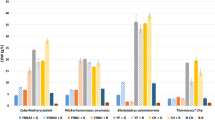
Effect of metal ions. Many enzymes require the presence of metal ions to express their catalytic activity completely. Hence, it is important to know the kind of ions and their concentrations in achieving maximal reaction efficiency [26]. The effect of different monovalent and divalent cations (5 mM) such as chlorides of Ca2+, Ba2+, Na+, K+ and sulphates of Fe2+, Zn2+, Mn2+, Cu2+ and Mg2+ on phytase production was assessed. No significant effect was observed on phytase production by P. kudriavzevii FSMP-Y17 for any metal ion except calcium showing an inhibitory effect on the enzyme production. From these observations, it can be concluded that phytase from P. kudriavzevii FSMP-Y17 did not require metal ions for its activity. In a study by Casey and Walsh [27], the phytase activity in Rhizopus oligosporus was found to be unaffected or moderately stimulated by a range of metal ions. On the other hand, Pichia rhodanensis was found to function >50% of its maximal rate at 1 M NaCl [28]. Activity of the enzyme produced from C. kruseii WZ-001 was inhibited by Co2+, Cd2+, Ba2+ and Hg2+ while Pb2+ and Cu2+ improved the phytase production of the strain [18]. Soni et al. [29] reported that A. niger NCIM 563 produced two different extracellular phytases in which Phy II was strongly inhibited by Ag+, Hg2+ (1 mM) metal ions and Phy I was partially inhibited under SMF. The thermal stability of the purified recombinant enzyme phy(ycE) from Escherichia coli was drastically improved in the presence of calcium ions (Ca2+) [30]. The results of a previous study showed that the effect of different divalent metal ions on phytase activity was observed for Zn2+ > Cu2+ > Fe2+ > Ca2+ [31].
Application of yeast phytases in broilers feed. Effect of phytase on weight and feed efficiency. The phytase produced by P. kudriavzevii FSMP-Y-17 was supplemented in broilers’ diet and its effect was studied for 2 weeks on the percent weight gain, daily feed intake and feed conversion efficiency (FCR). Phytase supplementation showed improved growth in the broiler birds (Table 4). The overall weight gain in the yeast phytase fed groups (66.8%) was higher than that of the control (51.0%) and commercial phytase fed groups (60.5%). Therefore, FCR in them was lesser (1.895) than that of the control (2.509) and commercial fed groups (2.100). Moreover, an increase in the feed intake was observed with the increase in the amount of phytase enzyme in the diets.
Effect of phytase on the productivity (egg production and egg quality) of broilers. The P. kudriavzevii FSMP-Y17 phytase-supplemented diets showed increased egg production (1.12) compared to diets containing commercial enzyme (0.96) and those without any enzyme (0.88) (Table 4). In addition, the weight of the eggs produced by the birds fed with the diet containing yeast phytase was more (56.15 g) than those fed with diets with commercial enzyme (52.05 g) and without any enzyme supplement (49.23 g) (Table 4). Similar results were obtained by Ciftci et al. [32] while studying the effect of phytase supplementation on the egg production and egg weight in the poultry.
The effect of the phytase supplementation in the diet was also studied on the quality of the eggs produced by the broiler birds determined as the specific gravity of eggs, egg shell thickness, egg shell weight, broken egg to total egg ratio, pigmentation on egg surface, shelf life of eggs and yolk index. The P. kudriavzevii FSMP-Y17 phytase-supplemented diet showed highest value of specific gravity (1.085), followed by diets containing commercial phytase (1.085) and control diets (1.080). Similar results have been recorded during studies conducted previously. Likewise microbial phytase supplementation to the diets of older hens was proved to improve production performance, extend the peak laying period, and alter the egg quality parameters [33]. Phytase supplementation at 0.12% non-phytate phosphorus level, was proved to be the optimum for Swarnadhara breeders to maintain egg production and egg quality [34]. The egg shell weight, yolk index and the shelf life of the egg (A-grade) were the highest in the eggs produced by the birds fed with P. kudriavzevii FSMP-Y17 phytase. No pigmentation was observed on the egg surface. Moreover, the broken egg: total egg ratio was the lowest in broilers birds fed with yeast enzyme (Table 4).
Effect of phytase on phosphorus content. The supplementation of broilers birds feed with the yeast phytase resulted in better retention of phosphorus in the birds. The birds fed with the yeast phytase exhibited 75.12% phosphorus retention compared to 75.03% in the commercial enzyme fed groups and 30.97% in the control (without phytase enzyme) (Table 5). Further, a decrease in phosphorus excretion in the poultry feces was seen upon feeding with a diet supplemented with the yeast phytase (206.48 mg/g dry matter) compared to that in the commercial enzyme fed groups (224.50 mg/g) and the control (679.16 mg/g). From the observations, it can be seen clearly that the retention of crude ash by using phytase supplemented diet (8.53%) was better compared to the diet amended with commercial enzyme (9.62%) and that without any enzyme (10.24%) (Table 5). These results indicated increased availability of mineral components in the yeast phytase supplemented diet. Similar results were observed by Vohra et al. [35] in poultry birds, who reported lesser excretion of ash in the phytase-supplemented groups.
* *
Optimization of cultural conditions during SMF enhanced the phytase production in P. kudriavzevii FSMP-Y17 significantly. The characteristics of the extracellular phytase such as thermostability, acid stability and longer shelf life make it suitable for its application in the broilers feed. The addition of the yeast phytase in the diet of the broiler birds augmented the nutritive value of the feed by making phytate phosphorus available to the birds. The broilers showed an improvement in the weight gain. No additional phosphorus supplementation of the diet was required. The birds showed higher phosphorus retention, significant reduction in P excretion, high minerals retention, enhanced productivity and quality of the eggs. Therefore, the enzyme produced by P. kudriavzevii FSMP-Y17 gave results better or comparable to the commercial phytase even at lower dose. The study showed that this phytase can be used as an efficient diet supplement in the broilers feed.
REFERENCES
Kumar, V., Yadav, A.N., Verma, P., Sangwan, P., Saxena, A., Kumar, K., et al., Int. J. Biol. Macromol., 2017, vol. 98, pp. 595–609. https://doi.org/10.1016/j.ijbiomac.2017.01.134
Walters, H.G., Coelho, M., Coufal, C.D., and Lee, J.T., J. Appl. Poult. Res., 2019, vol. 28, no. 4, pp. 1210–1225. https://doi.org/10.3382/japr/pfz087
Dersjant-Li, Y., Awati, A., Schulze, H., and Partridge, G., J. Sci. Food Agric., 2015, vol. 95, no. 5, pp. 878–896. https://doi.org/10.1002/jsfa.6998
Gutiérrez-Arenas, D.A., Cuca-García, M., Méndez-Rojas, M.A., Pro-Martínez, A., Becerril-Pérez, C.M., Mendoza-Álvarez, M.E., et al., Animals, 2021, vol. 11, no. 10, p. 2773. https://doi.org/10.3390/ani11102773
Jatuwong, K., Suwannarach, N., Kumla, J., Penkhrue, W., Kakumyan, P., and Lumyong, S., Front. Microbiol., 2020, vol. 11, p. 493778. https://doi.org/10.3389/fmicb.2020.00188
Shanmugam, G., Int. J. Curr. Microbiol. App. Sci., 2018, vol. 7, no. 3, pp. 1006–1013. https://doi.org/10.20546/ijcmas.2018.703.120
Rizwanuddin, S., Kumar, V., Naik, B., Singh, P., Mishra, S., Rustagi, S., et al., J. Agric. Food Res., 2023, p. 100559. https://doi.org/10.1016/j.jafr.2023.100559
Gulati, H.K., Chadha, B.S. and Saini, H.S., Folia Microbiol., 2007, vol. 52, pp. 491–497. https://doi.org/10.1007/BF02932109
AOAC International, Official Methods of Analysis, Washington: AOAC International, DC: 2005, 18th ed.
Kumari, N. and Bansal, S., Biomass Convers. Biorefin., 2023, vol. 13, no. 9, pp. 8339–8349. https://doi.org/10.1007/s13399-021-01672-x
Shah, P.C., Kumar, V.R., Dastager, S.G., and Khire, J.M., AMB Express, 2017, vol. 7, no. 1, pp. 1–11. https://doi.org/10.1186/s13568-017-0370-9
Kumari, N. and Bansal, S., Biotechnol. Lett., 2021, vol. 43, pp. 865–879. https://doi.org/10.1007/s10529-020-03069-8
Sandhya, A., Sridevi, A., Suvarnalatha, D., and Narasimha, G., Pharm. Lett., 2015, vol. 7, pp. 148–153.
Qasim, S.S., Shakir, K.A., Al-Shaibani, A.B., and Walsh, M.K., Food Nutr. Sci., 2017, vol. 8, no. 7, p. 733. https://doi.org/10.4236/fns.2017.87052
Sardar, R., Asad, M.J., Ahmad, M.S., and Ahmad, T., Braz. Arch. Biol. Technol. 2022, vol. 65. https://doi.org/10.1590/1678-4324-2022210307
Wodzinski, R.J. and Ullah, A.H.J., Adv. Appl. Microbiol., 1996, vol. 42, pp. 263–302. https://doi.org/10.1016/S0065-2164(08)70375-7
Sano, K., Fukuhara, H. and Nakamura, Y., Biotechnol. Lett., 1999, vol. 21, pp. 33–38. https://doi.org/10.1023/A:1005438121763
Quan, C., Zhang, L., Wang, Y., and Ohta, Y., J. Biosci. Bioeng., 2001, vol. 92, no. 2, pp. 154–160. https://doi.org/10.1016/S1389-1723(01)80217-6
Yamada, K., Minoda, Y., and Yamamoto, S., Agric. Biologic. Chem., 1968, vol. 32, no. 10, pp. 1275–1282. https://doi.org/10.1080/00021369.1968.10859223
Segueilha, L., Lambrechts, C., Boze, H., Moulin, G., and Galzy, P., J. Ferment. Bioeng., 1992, vol. 74, no. 1, pp. 7–11. https://doi.org/10.1016/0922-338X(92)90259-W
Kerovuo, J., Lauraeus, M., Nurminen, P., Kalkkinen, N., and Apajalahti, J., Appl. Environ. Microbiol., 1998, vol. 64, no. 6, pp. 2079–2085. https://doi.org/10.1128/AEM.64.6.2079-2085.1998
Tahir, A., Mateen, B., Saeed, S., and Uslu, H., Micol. Apl. Int., 2010, vol. 22, no. 2, pp.51–57.
Gangoliya, S.S., Gupta, R.K., and Singh, N.K., Indian J. Exp. Biol., 2015, vol. 53, pp. 350–355.
Elkhateeb, Y.A. and Fadel, M., Open Microbiol. J., 2022, vol. 16, no. 1, e2202160. https://doi.org/10.2174/18742858-v16-e2202160
Ramachandran, S., Roopesh, K., Nampoothiri, K.M., Szakacs, G., and Pandey, A., Process Biochem., 2005, vol. 40 no. 5, pp. 1749–1754. https://doi.org/10.1016/j.procbio.2004.06.040
Aguilar, C.N., Rodríguez, R., Gutiérrez-Sánchez, G., Augur, C., Favela-Torres, E., Prado-Barragan, L.A., et al., Appl. Microbiol. Biotechnol., 2007, vol. 76, pp. 47–59. https://doi.org/10.1007/s00253-007-1000-2
Casey, A. and Walsh, G., J. Biotechnol., 2004, vol. 110, no. 3, pp. 313–322. https://doi.org/10.1016/j.jbiotec.2004.03.001
Nakamura, Y., Fukuhara, H. and Sano, K., Biosci. Biotechnol. Biochem., 2000, vol. 64, no. 4, pp. 841–844. https://doi.org/10.1023/A:1016850121719
Soni, S.K., Magdum, A. and Khire, J.M., World J. Microbiol. Biotechnol., 2010, vol. 26, pp. 2009–2018. https://doi.org/10.1007/s11274-010-0385-8
Yao, M.Z., Lu, W.L., Chen, T.G., Wang, W., Fu, Y.J., Yang, B.S., et al., Ann. Microbiol., 2014, vol. 64, no. 3, pp. 1123–1131. https://doi.org/10.1007/s13213-013-0751-5
Liu, C., Yang, C., Yang, Q., Wang, J., and Liu, Y., Acta Agric. Scand., B: Soil Plant Sci., 2021, vol. 71, no. 2, pp. 112–123. https://doi.org/10.1080/09064710.2020.1856917
Ciftci, M., Dalkilic, B., and Azman, M.A., Int. J. Poult. Sci., 2005, vol. 4, no. 10, pp. 758–760. https://doi.org/10.1155/2015/867014
Sun, H.Y. and Kim, I.H., J. Poultry Sci., 2021, vol. 58, no. 3, pp. 171–176. https://doi.org/10.2141/jpsa.0200001
Sreedhara, J.N., Ram, J., Malathi, V., Gopinath, C.R., and Prabhu, T.M., J. Exp. Zool. India, 2023, vol. 26, no. 1, pp. 527–531.
Vohra, A., Rastogi, S.K. and Satyanarayana, T., World J. Microbiol. Biotechnol., 2006, vol. 22, pp. 553–558. https://doi.org/10.1007/s11274-005-9070-8
Funding
University Research Scholarship (URS) awarded to A. Mittal by Kurukshetra University, Kurukshetra (India).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, P., Mittal, A., Gupta, V. et al. Concomitant Production and Utilization of Thermoacidic Phytases from Pichia kudriavzevii FSMP-Y17 in Broilers Feed. Appl Biochem Microbiol 60, 871–879 (2024). https://doi.org/10.1134/S0003683823602883
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683823602883