Abstract
Changes in the concentrations of hydrogen peroxide and cyclic adenosine monophosphate (cAMP) in the roots of seedlings of pea cv. Rondo and its supernodulating Nod3 and anodulating K14 mutants were studied during infection with Rhizobium leguminosarum bv. vicea (strain RCAM 1022) or Pseudomonas syringae pv. pisi (Strain 1845). It was shown that 360 min after infection of pea seedlings of the Rondo variety, the level of endogenous hydrogen peroxide differed slightly from the control. In the roots of Nod3 seedlings, this level significantly decreased, while in the roots of K14 it significantly increased when infected with the 1845 strain, but remained unchanged when exposed to bacteria of the RCAM 1022 strain and young root hairs of Rondo seedlings, while strain 1845 had no effect on this parameter. Both types of bacteria had no effect on the concentration of cAMP in the roots of seedlings of the Nod3 mutant, whereas in K14, under the influence of RCAM 1022, the cAMP level almost doubled, and under the influence of 1845, it decreased. It is assumed that hydrogen peroxide and cAMP may be involved in the formation of supernodulating and nodulating phenotypes of mutants, as well as in the formation of resistance to a specific pathogen, Pseudomonas syringae pv. pisi. It is possible that this phenomenon can be used to diagnose the resistance of newly created mutants and pea varieties to the blight pathogen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Atmospheric nitrogen fixation by plants of the family Fabaceae is carried out in close contact with bacterial micropartners of Rhizobium, Sinorhizobium, and Bradyrhizobium. It is known that the process of nitrogen fixation is under the genetic control of the host plant, thus determining the number of formed nodules, their size and morphology [1]. In this regard, the change in the concentrations of signaling molecules in the cells of the host plant at the early stages of rhizobial infection is of great importance, since secondary messengers are involved in the regulation of the expression of specific genes through signaling cascades. The short-term increase in the concentrations of reactive oxygen and nitrogen species [2, 3], as well as Ca2+ [4] is related to this. From this point of view, pea mutants that differ in nodule formation are a good object for research. However, mutations can cause changes in susceptibility to bacterial pathogens. Since plant signaling systems, in particular, the secondary messenger of the adenylate cyclase signaling system, cyclic adenosine monophosphate (cAMP), are involved in the implementation of resistance–susceptibility mechanisms. [5, 6], their functioning in mutant pea plants is a very important indicator.
The purpose of this work is to study changes in the concentrations of H2O2 and cAMP in the root growth zones of pea seedlings (Pisum sativum) variety Rondo and mutants obtained from this variety, supernodulating, Nod3 and nodulating, K14, upon infection of Rhizobium leguminosarum bv. vicieae (strain RCAM 1022) or Pseudomonas syringae pv. pisi (strain 1845).
METHODOLOGY
The objects of the study were 3-day-old pea seedlings (Pisum sativum), the Rondo variety and supernodulating (Nod3) and nodulating (K14) mutants of this variety, as well as planktonic cultures Rhizobium leguminosarum bv. vicieae; the efficient nitrogen fixation strain RCAM 1022 obtained from the Departmental collection of useful microorganisms for agricultural purposes of the All-Russian Research Institute of Agriculture (ARRIAM); and Pseudomonas syringae pv. pisi (strain 1845), obtained from All-Russian Research Institute of Phytopathology.
Bacteria cultivation. Bacterial cultures were grown in flasks on a liquid medium containing clarified pea decoction, 15 g/L glucose, pH 7.0. The titer of the plankton culture of bacteria was determined on an AIFR-01 Uniplan plate spectrophotometer (ZAO Pikon, Russia) at a wavelength of 655 nm.
Germination of pea seeds. Pea seeds were sequentially sterilized and washed for 5 min in 94% ethanol, 5 min in 5% potassium permanganate solution, and 5 min in 3% hydrogen peroxide. At the final stage, it was washed with sterile water and soaked at 60°C for 4 hours. Seeds were germinated in sterile Petri dishes on moistened filter paper for 3 days in the dark at 23–25°C. The seedlings were washed with a sterile 0.01% nonidet solution (nonionic detergent) to remove exogenous microflora, washed three times with sterile distilled water, and inoculated with one of the types of bacteria in the stationary growth phase.
The titer of the plankton culture of bacteria was 0.1 × 108. Inoculation was carried out for 5 or 360 min, after which the roots of the seedlings were separated from the pea, washed in a sterile 0.01% nonidet solution to remove weakly bound bacteria, and then washed three times in sterile water.
A feature of legumes is that it is not the entire root of seedlings that is susceptible to rhizobia, but only its individual sections, which differ in the degree of formation of root hairs, that is, the presence of rudiments, young or mature root hairs on them. In this work, based on the literature data [7] and our own studies using light microscopy, a primary root 35–40 mm long was divided into five sections differing in the degree of hair formation: I, apical meristem (2 mm from the tip); II, a zone without root hairs (2–7 mm); III, the zone containing rudiments of root hairs (7–12 mm); IV, the zone of young root hairs (12–17 mm); V, the zone of formed root hairs (17–22 mm from the root tip), and the separate epicotyl, zone VI [8].
Determination of the intensity of bacterial adhesion. After incubation with bacteria, the roots of the seedlings were cut into segments in accordance with the indicated zones, and each sample was triturated in sterile water, after which a scattered seeding was performed on Petri dishes with an agar nutrient medium similar in composition to the liquid medium for cultivating each type of bacteria. After 3 days, colony-forming units (CFUs) were counted, reflecting the number of bacteria adhering to each root zone. In the experiments, ten seedlings were used.
Determination of H2O2 and cAMP concentrations. The endogenous H2O2 level was determined by the FOX method based on the color change of xylenol orange [9]. The cAMP concentration in pea root segments was determined by ELISA [8]. Endogenous H2O2 and cAMP concentrations were expressed as µmol/mg protein or nmol/mg, respectively. The protein concentration was determined by the Bradford method; BSA was used to construct a calibration curve. All experiments were carried out in 3 biological replications, determination of the level of cAMP and H2O2 used 8 analytical repetitions. On the graphs, the results are presented as a percentage of the control, which served as uninfected plants.
The experimental results were statistically processed using the SigmaPlot 12.3 program. The graphs show the means and errors of the mean (SE). The significance of differences between the groups of values was calculated using the Duncan test at P ≤ 0.05. Significance of differences was determined between the values for the Rondo variety and each of the mutants. Significantly different values on the graphs are marked with asterisks.
RESULTS AND DISCUSSION
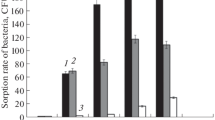
The interaction of plants with any type of microorganism begins with adhesion [10]. The experiments showed that both after 5 and after 360 min the most intense adhesion of bacteria occurred in the zones of mature and old root hairs, and was higher in RCAM 1022 rhizobia (Fig. 1). At the same time, the highest level of adhesion of bacteria of this species was retained on the roots of the seedlings of the Nod3 and K14 mutants, but not on the roots of the seedlings of the original Rondo variety. The intensity of adhesion of the 1845 strain also differed significantly in seedlings of the variety and mutants. After 5 min of coincubation, the adhesion of this bacterial species on the roots of pea seedlings of the Rondo variety was insignificant, whereas in Nod3 seedlings it was 6–8 times higher, and on K14 roots it was 2–3 times higher. After 360 min, adhesion in all parts of the root of Rondo seedlings was already completely absent, while on the roots of Nod3 it still increased, and in K14 it remained approximately at the level recorded after 5 min of coincubation (Fig. 1).
The adhesion intensity Rhizobium leguminosarum bv. vicieae (1) and Pseudomonas syringae pv. pisi (2) on the roots of pea seedlings (zones I–V) of the Rondo variety (a, b) and its mutants Nod3 (c, d) and K14 (e, f) through 5 (a, c, e) and 360 (b, d , f) min after inoculation. *Differences are statistically significant when comparing the Rondo variety (control) with Nod3 or K14 mutants according to the Duncan test, n = 3, m ± SE; R ≤ 0.05.
Close contact with bacteria causes activation of signaling systems in plants [11].
It should be noted that the initial concentrations of endogenous hydrogen peroxide and cAMP in the roots and epicotyl of pea seedlings differed significantly in the Rondo variety and its mutants, Nod3 and K14 (Table 1). Moreover, the concentration of endogenous hydrogen peroxide in the organs of Nod3 seedlings exceeded the level in Rondo seedlings by 3–5 times, while in K14 seedlings it was only 2.0–2.5 times (Table 1). At the same time, the level of endogenous cAMP in both Nod3 and K14 seedlings exceeded the same parameter in Rondo seedlings by 20–25 times (Table 1).
Coincubation of seedlings with both types of bacteria led to a change in the concentrations of endogenous hydrogen peroxide and cAMP. In Rondo seedlings, after 5 min of interaction with rhizobia, the level of hydrogen peroxide increased to the greatest extent in zones II and III, while decreasing slightly below the control in the epicotyl (Fig. 2). Under the influence of strain 1845 the concentration of this signal molecule increased compared to the control almost to the same extent in all root zones, but to a lesser extent than when exposed to the RCAM 1022 strain.
The impact of infection Rhizobium leguminosarum bv. vicieae (1) or Pseudomonas syringae pv. pisi (2) on the concentration of endogenous hydrogen peroxide in root regions (I–V, E-epicotyl) of pea seedlings of the Rondo variety (a, b) and its mutants Nod3 (c, d) and K14 (e, f) after 5 (a, c, e) and 360 (b, d, f) min after inoculation. * Differences are statistically significant when comparing Rondo (control) with Nod3 or K14 mutants according to Duncan’s test, n = 3, m ± SE; R ≤ 0.05. ** Control—H values2O2 from seedlings incubated in water.
After 360 min of co-incubation with RCAM 1022, the hydrogen peroxide level dropped below the control in the zones of primordia and young root hairs (zones II and III), remaining almost at the level of 100% in other parts of the root and epicotyl. Under the influence of strain 1845, the concentration of H2O2 remained at the level of 120% in all parts of the root and epicotyl. In Nod3 pea seedlings, after 5 and 360 min of coincubation with both types of bacteria, there was a significant decrease in the level of H2O2 from 50 to 70% of the control in all zones, while in the epicotyl this was close to 100% (Fig. 2). In the roots of K14 seedlings, after 5 min of infection with the same bacterial species, the level of H2O2 significantly decreased, but to a lesser extent, remaining at the level of 100% in some zones (IV and V) and the epicotyl (Fig. 2). After 360 min, under the influence of strain RCAM 1022, the level of H2O2 remained close to control in all parts of the root and epicotyl, while strain 1845 induced a significant increase in this parameter in all root zones and epicotyl (Fig. 2).
Infection also affected the change in the endogenous concentration of cAMP in the roots of pea seedlings. Inoculation with rhizobia of the root of Rondo pea seedlings for 5 min caused a consistent slight increase in the level of cAMP in zones I–V, while in the epicotyl this indicator remained at the control level. Under the influence of strain 1845, the cAMP concentration changed in a similar way (Fig. 3). After 360 min, infection with the RCAM 1022 strain caused the most significant increase in the level of cAMP in the zones of primordia and young root hairs (zones II–III), while its concentration remained at the control level in zones IV and V and the epicotyl.
The impact of infection Rhizobium leguminosarum bv. vicieae (1) or Pseudomonas syringae pv. pisi (2) on the concentration of endogenous cAMP in the root regions (I–V, E-epicotyl) of seedlings of pea cv. Rondo (a, b) and its mutants Nod3 (c, d) and K14 (e, f) after 5 (a, c, e) and 360 (b, d, f) min after inoculation. * Differences are statistically significant when comparing the Rondo variant as a control with the Nod3 variant or the K14 variant according to Duncan’s test, n = 3, m ± SE; R ≤ 0.05. ** When calculating endogenous cAMP concentrations, the concentration of endogenous cAMP from seedlings incubated in water served as a control.
Strain 1845 also induced an increase in the level of this signal molecule, but it was less intense than that for strain RCAM 1022 and almost identical in all parts of the root and epicotyl (Fig. 3). Infection of the root of Nod3 seedlings with strain RCAM 1022 for 5 min had almost no effect on the initial level of cAMP concentration. After 360 min, this indicator remained practically at the level of the control in all parts of the root. However, strain 1845 led to a significant decrease in the level of cAMP in all parts of the root after 5 min; at the same time, after 360 min, the level of cAMP approached the control. Infection of the roots of seedlings of the K14 mutant for 5 min with both bacterial species had almost no effect on the change in the level of cAMP in the root regions. However, after 360 min, under the influence of strain RCAM 1022, its value increased by almost 2 times in almost all parts of the root (Fig. 3). In contrast, coincubation with strain 1845 led to a significant decrease in the level of cAMP in the same parts of the root.
In nature, microorganisms are able to adhere and form biofilms on any surface. According to modern concepts, adhesion of rhizobia on the roots of legumes is nonspecific and does not depend on their symbiotic properties [12]. It is difficult to agree with this, since we have previously shown that rhizobia strains that are not effective in nitrogen fixation, but are highly competitive, demonstrated significantly lower adhesion on the roots of Rondo seedlings [13]. It is likely that adhesion at an early stage of infection is nonspecific, but passes into a specific stage at the stage of partner recognition. Bacteria can take part in the adhesion process exopolysaccharides, type IV and V fimbriae, various adhesins and flagellins [14]. Such determinants, which are necessary at the initial stage of adhesion, are virulence factors and are present in almost all types of gram-negative bacteria, regardless of specialization [15, 16]. Apparently, increased adhesion of rhizobia after 360 min of coincubation indicates transition to a specific irreversible stage of this process, in which both some plant structures and metabolites and bacterial exometabolites take part. It is believed that Nod factors, which are lipochitooligosaccharides and not only species-specific, but also strain-specific, are among the fundamental components that determine the specificity of nitrogen-fixing symbiosis [17]. At the same time, plant lectins take an active part in this process. It was shown that the pea lectin gene introduced into white clover provided good adhesion. Rhyzobium leguminosarum on its roots, although this type of microsymbiont never infects clover in nature [18]. In addition, overexpression of the lectin moiety of legume lectin-like receptor protein kinase can lead to a supernodulation phenotype [19]. Despite the fact that the corresponding studies were not carried out in the work, based on the literature data [18, 19], it can be assumed that the difference in the intensity of adhesion of rhizobia on the roots of pea seedlings of the Rondo variety and the Nod3 and K14 mutants is to a certain extent associated with the qualitative composition of the isolated them lectins. This can also be confirmed by the fact that the intensity of adhesion strain 1845 was similar in both mutants, and in Rondo seedlings was completely absent after 360 min, which indicates that this process is nonspecific for this bacterial species.
Close contact of pea seedling roots with bacteria was accompanied by significant rearrangements in plant cell signaling. Despite the fact that the generation of ROS in plants is induced by both symbiotic nitrogen fixers and pathogens [20, 21], the purpose, as well as the duration of the rise and the amplitude of the concentrations of these signaling molecules, are different under the influence of various types of microorganisms [22]. According to the literature data, at the initial stages of pea interactions with mutualistic bacteria, a single peak of ROS generation was observed, including H2O2, which was stronger and longer than when infected with pathogens [23]. Such an effect was observed in experiments on seedlings of the Rondo variety during inoculation with RCAM 1022 or 1845 strains within 5 min. According to the literature data, at the early stages of interaction, rhizobia exhibit the properties of pathogens, suppressing plant immunity with their virulence factors [23]. In bacterial pathogenesis, hydrogen peroxide in plants performs protective functions and its concentration increases in two phases separated in time [24]. Interestingly, in both mutants, the change in the level of hydrogen peroxide in the root zones after a short-term exposure to both rhizobia and the pathogen was almost the same and fundamentally differed from that in Rondo seedlings. Thus, already at the earliest stages of interaction with both mutualists and pathogens, rearrangements in the signaling systems of pea mutants are evident. These differences were more pronounced after 360 min of interaction when this process passed into a specific stage, accompanied by mutual recognition of partners. According to the literature data a decrease in the level of hydrogen peroxide in the zones of adhesion and penetration of rhizobia (zones of primordia and young root hairs, zones II–III) should promote increased colonization and invasion of rhizobia into these root regions [25], which occurs in seedlings of the Rondo variety pea. In this case, hydrogen peroxide, along with a signaling role, performs the functions of an antibacterial agent, distributing the infectious load over the root zones. Strain 1845, despite the lack of adhesion at up to 360 min on the roots of Rondo seedlings, was able to induce a slight oxidative burst due to secreted toxins, in particular syringomycin. This exometabolite is able to influence the operation of calcium ion channels in the plant cell membrane [26], which can lead to modulation of the work of pro- and antioxidant enzymes, thereby affecting the level of endogenous hydrogen peroxide in the tissues of pea seedlings.
However, in the Nod3 and K14 mutants, the dynamics of endogenous H2O2 in the root zones is fundamentally different not only from that in Rondo, but also between themselves. It can be assumed that a significant decrease in the level of H2O2 in all root zones of seedlings of the Nod3 mutant after 360 min of coincubation with the strain RCAM 1022 is one of the causes of the supernodulating phenotype. However, the same decrease in the level of H2O2 under the influence of strain 1845, probably indicates a decrease in local nonspecific immunity in root tissues. This is confirmed by the level of this signaling molecule in the epicotyl, where it was close to the control. This indicates the absence of systemic activation of nonspecific defense reactions in seedlings of the Nod3 mutant. In contrast, in the K14 mutant, under the influence of rhizobia, the concentration of H2O2 remained almost at the control level throughout the experiment, which is also not typical for legume–rhizobium symbiosis [20]. At the same time, under the influence of strain 1845, a significant increase in the concentration of H2O2 occurred, which affected all root zones and epicotyl. Considering that the intensity of adhesion of this pathogen varies across root zones, it can be assumed that a systemic reaction develops in this case, whose cause may be PAMPs (Patogen-Aassociated Molecular Patterns), including flagellar and other structural proteins of the pathogen, as well as its toxins (syringotoxin, coronatin, syringoline, etc.); these are secreted into the incubation medium [27] and reach the required concentration only by 360 min.
The dynamics of cAMP concentration upon infection of the roots of pea seedlings of the Rondo variety and both mutants fundamentally differed from the dynamics of endogenous hydrogen peroxide (Figs. 2, 3). Based on the literature data [28] and comparing the dynamics of this second messenger in seedlings of Rondo and mutants, it can be assumed that endogenous cAMP is involved in the regulation of symbiosis efficiency at the initial stages of the interaction. At the stage of short-term interaction (5 minutes), this is likely due to PAMPs, which are characteristic of both types of bacteria [15, 16]. Plants use a variety of receptor molecules for recognition of PAMPs, including lectins, which perform the functions of receptors, as well as complexes of receptor kinases on the cell surface. This enhances the host defense response, although the effect seems to be attenuated in mutualistic symbiosis, since Nod factors are able to suppress defense responses through direct or indirect interaction with the lectin receptor kinase (LecRK) [12].
The very high level of cAMP in the zones of primordia and young root hairs of Rondo seedlings 360 min after inoculation with the strain RCAM 1022, and, conversely, the absence of the reaction of the adenylate cyclase signaling system in the root growth zones of the Nod3 mutant, is noteworthy. According to the literature data, in the supernodulating mutant of beans, the level of cAMP in the roots during infection Bradyrhizobium japonicum remained at the control level for several days, while an artificial increase in its concentration had a negative effect on the formation of nodules [28]. Taking the fact into account that in the nodule-free mutant this indicator exceeded the control by only 40–60%, it can be assumed that when interpreting the results the intensity of changes in the cAMP level and the stage of the infectious process should be taken into account. At 360 min, adhesion passes into a specific stage and the penetration of rhizobia begins, accompanied by induction sym plant genes [18]. It is known that the activation of certain genes depends on the concentration of second messengers that transmit signals to specific transcription factors [29]. In addition, it was previously shown that with a significant increase in the endogenous cAMP level the concentration of endogenous hydrogen peroxide in the roots of pea seedlings of the Rondo variety decreases significantly [5]. Such an inverse relationship, which is clearly traced on the roots of seedlings of varietal peas, is not very clearly expressed in the roots of seedlings of its mutants, which may be one of the reasons for super- or no nodulation. This appears to be quite possible, since supernodulation is under the control of the root [30], and the most pronounced changes in cAMP concentrations are also observed in this organ.
During rhizobial infection the change in the level of endogenous cAMP in the root of pea seedlings is aimed at the formation of mutualistic relationships, while under the influence of a pathogen it can be an indicator of resistance to Pseudomonas syringae pv. pisi. The literature data indicate that changes in the level of cAMP in plants upon infection with specific pathogens indicate the degree of their resistance [31]. It can be assumed that Rondo peas are the most resistant to Pseudomonas syringae pv. pisi, since the level of cAMP in all parts of the root and epicotyl significantly exceeded the readings in the control, in contrast to the mutants.
Thus, mutations aimed at regulating the number of nodules in Nod3 and K14 affected intracellular signaling, significantly increasing the concentrations of endogenous H2O2 and cAMP, as well as the immune response of plants when interacting with pathogens. In the seedlings of these pea mutants, the intensity of adhesion of rhizobia and phytopathogenic bacteria changed and the modulation of the levels of endogenous hydrogen peroxide and cAMP most likely indicated a change in the resistance of these plants to a specific pathogen, Pseudomonas syringae pv. pisi. It is possible that this phenomenon can be used in the future to diagnose the resistance of newly created mutants and pea varieties to the blight pathogen Pseudomonas syringae pv. pisi.
REFERENCES
Vlasova, E.Yu., Sidorova, K.K., Glyanenko, M.N., and Mishchenko, T.M., Vavilov. Zh. Genet. Sel., 2012, vol. 16, no. 4/2, pp. 879–886.
Nanda, A.K., Andrio, E., Marino, D., Pauly, N., and Dunand, C., J. Integr. Plant Biol., 2010, vol. 52, no. 2, pp. 195–204.
Torres, M.A., Physiol. Plant., 2010, vol. 138, no. 4, pp. 414–429.
Ma, W., Qi, Z., Smigel, A., Walker, R.K., Verma, R., Gerald, A., and Berkowitz, G.A., Proc. Natl. Acad. Sci. U. S. A., vol. 106, no. 49, pp. 20995–21000.
Lomovatskaya, L.A., Kuzakova, O.V., Goncharova, A.M., and Romanenko, A.S., Russ. J. Plant Physiol., 2020, vol. 67, no. 3, pp. 435–442. https://doi.org/10.1134/S1021443720020107
Suzuki, N. and Katano, K., Front. Plant Sci., 2018, vol. 9, p. 490.
Makarova, L.E. and Nurminskii, V.N., Tsitologiya, 2005, vol. 47, no. 6, pp. 519–525.
Lomovatskaya, L.A., Kuzakova, O.V., Romanenko, A.S., and Goncharova, A.M., Russ. J. Plant Physiol., 2018, vol. 65, no. 4, pp. 588–597.
Galletti, R., Denoux, C., Gambetta, S., Dewdney, J., De Lorenzo, A. F.M., and Ferrari, S., Plant. Physiol., 2008, vol. 148, pp. 1695–1706.
Zvyagintsev, D.G., Bab’eva, I.P., and Zenova, G.M., Moscow: Mosk. Gos. Univ., 2005.
Bleau, J.R. and Spoel, S.H., Plant Physiol., 2021, vol. 186, pp. 53–65.
Tsyganova, A.V., Brewin, N.J., and Tsyganov, V.E., Cells, 2021, vol. 10, no. 1050, pp. 1–32.
Kuzakova, O.V., Lomovatskaya, L.A., Goncharova, A.M., and Romanenko, A.S., Russ. J. Plant Physiol., 2019, vol. 66, no. 5, pp. 712–717.
Bhuvaneswari, T.V., Turgeon, B.G., and Bauer, W.D., Plant Physiol., 1980, vol. 66, no. 6, pp. 1027–1031.
Seregina, N.V., Chestnova, T.V., Zherebtsova, V.A., and Khromushin, V.A., Vestn. Nov. Med. Tekhnol., 2008, no. 4, pp. 75–77.
Tsyganova, A.V. and Tsyganov, V.E., Usp. Sovrem. Biol., 2012, vol. 132, no. 2, pp. 211–222.
Vershinina, Z.P., Lavina, A.M., and Chubukova, O.B., Biomika, 2020, vol. 12, no. 1, pp. 27–49. https://doi.org/10.31301/2221-6197.bmcs.2020-3
Zhukov, V.A., Rychagova, T.S., Shtark, O.Yu., Borisov, A.Yu., and Tikhonovich, I.A., Ekol. Genet., 2008, vol. 6, no. 4, pp. 12–19.
Babosha, A.V., Zh. Obshch. Biol., 2008, vol. 69, no. 5, pp. 379–396.
Peleg-Grossman, S., Melamed-Book, N., and Levine, A., Plant Signal. Behav., 2012, vol. 7, no. 3, pp. 409–415.
Hawkins, J.P. and Oresnik, I.J., Front. Plant Sci., 2022. https://doi.org/10.3389/fpls.2021.796045
Bleau, J.R. and Spoel, S.H., Plant Physiol., 2021, vol. 186, pp. 53–65. https://doi.org/10.1093/plphys/kiaa088
Gourion, B., Berrabah, F., Ratet, P., and Stacey, G., Trends Plant Sci., 2015, vol. 20, no. 3, pp. 186–194.
Bolwell, G.P., Bindschedler, L.V., Blee, K.A., Butt, V.S., Davies, D.R., Gardner, S.L., and Minibayeva, F., J. Exp. Bot., 2002, vol. 53, no. 372, pp. 1367–1376.
Cárdenas, L., Martínez, A., Saánchez, F., and Quinto, K., Plant J., 2008, vol. 56, pp. 802–813.
Takemoto, J.Y., Zhang, L., Taguchi, N., Tachikawa, T., and Miyakawa, T., Microbiology, 1991, vol. 137, no. 3, pp. 653–659.
Ichinose, Y., Taguchi, F., and Mukaihara, T., J. Gen. Plant Pathol., 2013, no. 79, pp. 285–296.
Terakado, J., Fujihara, S., and Yoneyama, T., Soil Sci. Plant Nutr., 2003, vol. 49, no. 3, pp. 459–462.
Xu, R., Guo, Y., Peng, S., Liu, J., Li, P., Jia, W., and Zhao, J., Biomolecules, 2021, vol. 1, p. 688.
Sidorova, K.K. and Shumnyi, V.K., Sib. Ekol. Zh., 1999, no. 3, pp. 281–288.
Sabetta, W., Vandelle, E., Locato, V., Costa, A., Cimini, S., Moura, A.B., Luoni, L., Graf, A., Viggiano, L., De Gara, L., Bellin, D., and Blanco, E., Plant J., 2019, vol. 98, pp. 590–606.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Lomovatskaya, L.A., Zakharova, O.V., Goncharova, A.M. et al. The Influence of Bacterial Mutualists and Phytopatogenes on Changes in the Concentrations of cAMP and H2O2 in Pea Seedless of the Rondo Varieties and its Anodulating and Supernodulating Mutants. Appl Biochem Microbiol 59, 216–222 (2023). https://doi.org/10.1134/S0003683823020084
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683823020084