Abstract
The ability of rhizosphere bacteria to induce systemic resistance in plants has been widely described in the literature. Various products of rhizobacteria act as signalling molecules, developing stress-like reactions in the plant. At the same time, the accumulation of peroxidase and other reactive oxygen species in the cell plays a major role, and is one of the most important plant defence mechanisms in response to various stressors. Peroxidase is a polyfunctional enzyme involved in both the generation and the utilization of hydrogen peroxide, and its activity in cells is related to plant resistance to phytopathogens and adverse environmental conditions. We measured the levels of free and weakly bound guaiacol-dependent peroxidase in the roots and leaves of wheat plants both inoculated with Pseudomonas extremorientalis PhS1 and Aeromonas media GS4 and uninoculated, under conditions of high temperatures and low humidity, with and without the phytopathogen Bipolaris sorokiniana. Following inoculation, a 1.4–5.2-fold increase in the severity of plants affected with the phytopathogen was observed. Seed bacterization decreased the number of damaged plants both with and without the phytopathogen by 1.2–2.4 and 3.2–4.7 times, respectively. Seed bacterization increased peroxidase activity in leaves and roots with and without the phytopathogen. Correlation analysis showed a statistically significant (P < 0.05) relationship between the increased activity of peroxidase in wheat leaves and roots and the reduced number of affected plants. The strongest relationship was observed between the enzyme activity in wheat roots and a decrease in the disease severity with the phytopathogen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the spread of root rots, especially of wheat and barley, has increased in different agricultural areas across the world including in Europe and Asia (Kumar et al., 2002), Australia (Saad et al., 2021), North and Latin America (Eisa et al., 2013; Kumar et al., 2002), and Western Siberia (Russia) (Toropova et al., 2015). Disturbance of crop rotation, soil acidification, irregular application of organic fertilizers (Toropova et al., 2015), breeding efforts, and irrigation increasing cropping intensity are considered to be among the reasons for this spread (Kumar et al., 2002). The damage caused by root rot diseases is evidenced by a decrease in plant productivity, crop yield, and product quality (Kumar et al., 2002). The species composition of root rot pathogens generally corresponds to certain ecological and geographical areas of crop cultivation and is usually mixed. The most common root rot pathogens are fungi in the genera Fusarium and Bipolaris, with Bipolaris sorokiniana being predominant (Al-Sadi, 2021). Bipolaris sorokiniana causes significant yield losses of 15.5–25% or more (Eisa et al., 2013; Kumar et al., 2002; Saad et al., 2021; Toropova et al., 2015).
Besides root rot disease, B. sorokiniana causes foliar spot blotch, black points on grains, head blight, and seedling blight of wheat and barley (Kumar et al., 2002; Saad et al., 2021). Root and crown infections can be so severe that infected plants wither away without producing any seed. Under favourable conditions spikelets may be affected, causing grain shrivelling (Kumar et al., 2002). The aggressiveness of B. sorokiniana is increased under hot and wet climate conditions (Eisa et al., 2013; Kumar et al., 2002). Thus, spot blotch has become a major production constraint in South Asia’s intensive cropping systems, where nearly 12,000,000 ha are affected (Kumar et al., 2002). Plant damage by this pathogen combined with summer drought leads to a reduction of spikelets and flowers in spring wheat in Western Siberia and can reduce the potential ear grain content by 1.5–1.8 times (Toropova et al., 2015).
Bipolaris sorokiniana is a hemibiotrophic pathogen, the penetration of which into plant tissue contributes to an increase in hydrogen peroxide (H2O2) levels (Kumar et al., 2002). A positive correlation between host susceptibility (to the establishment of spot blotch) and the amount of H2O2 produced in leaf lesions has been reported, and it has been suggested that H2O2 is involved in successful tissue infection (Eisa et al., 2013). Despite this, a rapid response to infection leading to H2O2 accumulation in infected cells at the site of pathogen penetration has been shown to delay the development of the pathogen (Kumar et al., 2002; Plotnikova, 2009).
Cell accumulation of H2O2 and other reactive oxygen species (ROS) is one of the major mechanisms of plant protection as a response to various stressors, including pathogens (Plotnikova, 2009; Rejeb et al., 2014; Maksimov et al., 2015; Choudhary et al., 2016). Thus, when resistant wheat cultivars were infected with brown rust pathogen (also known as wheat leaf rust, Puccinia triticina Erikss.) there was intense generation of superoxide radicals in the stomatal guard cells and leaf mesophyll, suppression of which resulted in pathogen growth and development in the plant tissues (Plotnikova, 2009).
Adverse environmental factors have been found to increase ROS production (Abdelaal et al., 2021; Choudhary et al., 2016; Hasanuzzaman et al., 2020; Huang et al., 2019; Sachdev et al., 2021). Under normal growth conditions, the production of ROS in cells is low, whereas during stress, their rate of production is enhanced (Mittler, 2002; Choudhary et al., 2016). These factors include drought stress and desiccation, salt stress, chilling, heat shock, heavy metals, ultraviolet radiation, air pollutants, mechanical stress, nutrient deprivation, pathogen attack, and high light stress (Mittler, 2002).
Despite available information on the physiological role of ROS generation in plant cells, it is clear that excess levels could kill the cell; however, this does not happen. This phenomenon can be explained by the presence of groups of large enzymes and nonenzymatic compounds with antioxidant properties that neutralize reactive derivatives of molecular oxygen without forming any other toxic substances. Peroxidases play an important role as ROS-detoxication enzymes (Mittler, 2002; Sorahinobar et al., 2016).
Plant peroxidases are multifunctional enzymes with a large number of isoforms (Gill & Tuteja, 2010; Passardi et al., 2005). Due to the abundance of isoforms, and to the highly heterogeneous regulation of their expression, plant peroxidases are involved in a broad range of physiological processes throughout the plant life cycle (Passardi et al., 2005). To begin with, peroxidases have a function in reducing H2O2 to water using different substrates as electron donors (for example, phenols, amines, organic acids, and glutathione). Also, they take part in the processes of growth, development, respiration, nitrogen exchange, mycorrhiza formation, xenobiotic detoxication, phytoalexin synthesis, and lignin and suberin biosynthesis (Passardi et al., 2005; Maksimov et al., 2015). Furthermore, peroxidases may manifest oxidase activity, generating the superoxide anion radical, H2O2 peroxide, and the hydroperoxide radical (Minibayeva et al., 2009).
Given the physiological roles of peroxidases and their involvement in protective reactions under abiotic and biotic stresses, it is evident that an increase in this enzymatic activity may serve as an indicator of plant resistance to adverse (stress) factors (Maksimov et al., 2015; Minaeva et al., 2018).
It has been established that plant bacterization with plant growth-promoting rhizobacteria (PGPR) strains improves mineral nutrition in plants, has a growth-promoting effect, provides protection against phytopathogens, and enhances resistance to abiotic stresses (Choudhary et al., 2016; Dimkpa et al., 2009; Gupta et al., 2015; Khatoon et al., 2020; Van Loon, 2007).
Studying the microbiological aspects of vermicompost demonstrated that the proportion of PGPR bacteria is significantly increased in worm coprolites, which determined both the growth-promoting properties of vermicompost and its suppression of plant pathogenic organisms. According to several researchers, the application of vermicompost radically changed the soil microbial community structure and significantly suppressed phytopathogenic fungi (Pathma & Sakthivel, 2012; Rajendran Vijayabharathi et al., 2015; Medina-Sauza et al., 2019). Microbiological studies also suggested that vermicompost fungistatic properties could depend on high numbers of Pseudomonas and Bacillus bacteria (Medina-Sauza et al., 2019; Pathma & Sakthivel, 2012). Previously published results of laboratory and field tests of Aeromonas media GS4 and Pseudomonas extremorientalis PhS1 bacteria, isolated from Eisenia fetida coprolites, indicated the possibility of their application to protect plants against root rot pathogens, promoting growth and improving wheat and barley productivity (Tereshchenko et al., 2018).
We also noted the response of free and weakly bound guaiacol-dependent peroxidase of wheat to B. sorokiniana infection in laboratory (model) tests: in the presence of the pathogen the enzyme activity increased. Bacterization with both strains (A. media GS4 and P. extremorientalis PhS1) also increased peroxidase activity in plant leaves and roots, with the greatest differences from non-bacterized plants being observed in wheat roots with the pathogen. We detected a direct link between peroxidase activity in wheat roots and leaf tissues without the pathogen and the feedback between peroxidase activity and plant infestation by the root rot pathogen. In the presence of the phytopathogen, there was a lack of correlation between peroxidase activity in wheat roots and leaves. We discovered the following: if a plant immediately after germination encountered a root rot pathogen, then it significantly increased the peroxidase activity in the roots, decreasing its activity in plant tissues, which played an important role in the development of systemic resistance against the root rot pathogen that penetrated into plants through the roots and root collar (Minaeva et al., 2018).
Moreover, we established that growing non-bacterized wheat plants under abiotic stress (elevated and low temperatures and moisture content) reduced the height of plants, and seed bacterization with P. extremorientalis PhS1 strain increased wheat plant height (by 9–15%) under stress abiotic conditions compared to the non-bacterized plants. Bacterization decreased infestation of wheat seedlings (2.5–4 times) by root rots under unfavourable abiotic conditions compared to non-bacterized plants. We showed that the activity of guaiacol-dependant peroxidase correlated with the development of plant resistance to abiotic stress. In our experiments, plant bacterization resulted in a 2-fold increase in peroxidase activity both in leaves and roots of wheat plants compared to non-bacterized plants (Minaeva et al., 2019).
In light of previous investigations, we hypothesized that bacterization with A. media GS4 and P. extremorientalis PhS1 could have a positive effect on wheat growth and development under simultaneous plant exposure to abiotic (high temperature and lack of moisture) and biotic (pathogen) stressors. At the same time, plant resistance could be associated with the activity of free and weakly bound peroxidase, which previously showed a high correlation with the growth and development parameters of plants and their infestation by root rots.
Thus, the aim of the current research was to evaluate the effect of wheat seed bacterization with A. media GS4 and P. extremorientalis PhS1 strains on the activity of soluble and guaiacol-dependent peroxidase with the B. sorokiniana pathogen in tests at elevated temperatures and in low humidity.
Materials and methods
Objects
The research objects were two bacterial strains (A. media GS4 and P. extremorientalis PhS1), wheat plants (Triticum aestivum L., ‘Iren’ cultivar), and a plant pathogen (B. sorokiniana).
The species of bacterial strains were identified using analysis of the sequences of the variable regions of genes encoding 16S rRNA (Russian National Collection of Industrial Microorganisms, Moscow, Russia). Sequencing results were analysed using the GenBank and RDP2 databases. The phylogenetic tree was constructed using the computer program on the RDP2 website. In the experiments, we used a 24-h liquid bacterial culture grown in 250-ml flasks with a nutrient medium (100 ml) on a shaker (180 rpm, “Biosan ES-20/60”, Latvia) at 28–29 °С. The nutrient medium consisted of fishmeal pancreatic hydrolyzate (18.0 g/l), NaCl (2.0 g/l). The culture density was determined using the colony-forming unit (CFU) method (Scan 300, Interscience, France).
We used the mycelium of B. sorokiniana (Sacc.) Shoemaker fungus as the phytopathogenic load. The pathogen was prepared by culturing in Petri dishes with potato-glucose agar at 22–24°С for 7 days. Bipolaris sorokiniana culture was kindly provided by Professor Margarita Shternshis from the Collection of Agronomy Faculty, Novosibirsk State Agrarian University, Russia.
Experimental procedure
We studied the effect of bacterization on peroxidase activity in wheat leaves and roots in the laboratory using an ecosystem model consisting of the following four links: sand – host plant – bacterial strain – phytopathogenic fungus. The wheat seeds were pretreated with 70% ethanol for 3 min, washed with sterile water, and grown at 18–20 °С in the dark. Once the embryos (1 mm) had appeared, the seeds were bacterized with suspensions of experimental strains at a rate of 104 cells/seed for 20 min. The control seeds were soaked in distilled water. Then, all the seeds were placed in plastic pots (1200 ml, 45 seeds each) filled with coarse sterile river sand (800 ml), and evenly moistened with sterile Knop nutrient solution comprising calcium nitrate (1.0 g/l), monopotassium phosphate (0.25 g/l), potassium nitrate (0.25 g/l), magnesium sulphate (0.125 g/l), and ferric chloride (0.012 g/l) (Minaeva et al., 2019).
Before embedding in the sand agar, strips of the pathogen were placed in rows with wheat seeds. Two types of experiment were carried out: clean agar strips in the tests without the pathogen (to avoid additional influence on both the plant and the bacteria); and agar strips with the fungus mycelium B. sorokoniana in inoculated tests.
For the first 6 days, we grew plants in a growth chamber (GС-300TLH, Jeio Tech, Korea) with 18–20/14–16 °С (day/night) 16/8-h photoperiods at 200 μmol quanta × m−2 s−1 photosynthetic active radiation (PAR) and a moisture level of 60% of the total water capacity. Starting from the 7th day, we raised the temperature up to 30–31/23–24 °С and reduced the substrate and air moisture levels down to 40%. All stress abiotic factors were used simultaneously as an attempt to simulate an arid climate characterized by high temperatures, as well as low air humidity and soil moisture.
Experiment assessment
After 12 days, we recorded germination and measured the height of each plant and peroxidase activity in the leaves and roots. By assessing the degree of browning of the stem base and the root system on a five-point scale, we registered the intensity of plant infestation by root and near-root rots. On this scale, 0 was defined as the absence of infestation signs and 4 was defined as the death of plants from the disease (Cooke, 2006). As a result, we calculated the root rot incidence and severity in the experiments, where disease incidence refers to the number of plant units that are visibly diseased relative to the total number of plant units assessed in percentage and disease severity is the area of plant tissue that is visibly diseased relative to the total tissue area assessed in percentage (Cooke, 2006).
Detection of enzyme activity
In the experiments, we detected the total activity of guaiacol-dependant peroxidase. A 200 mg sample of fresh tissue of two or three plants was homogenized in 1 ml of cold 0.15 M phosphate buffer (рН 5.4) at 4 °С. Following this the obtained volume was buffered to 25 mL in a volumetric flask and incubated at 4 °С for 10 min. The homogenate was centrifuged at 10,000 rpm (MF48-R, Awel, France) at 4 °С for 10 min. The supernatant was used to detect the enzyme activity spectrophotometrically (Eppendorf AG, Germany): 1.5 mL of 0.15 М K,Na-phosphate buffer (рН 5.4), 0.5 mL of 0.15% H2O2 (Reakhim, Russia), 0.5 mL of 0.05% guaiacol (Sigma, United States) (molar extinction coefficient for tetraguaiacol Е470 = 26.6 mM−1 cm−1), and 0.5 mL of plant extract (with concentrations in the reaction mixture of peroxide and guaiacol of 7.35 and 0.672 mM, respectively) at 25 °С immediately after obtaining the extract. Changes in the adsorption of the reaction solution at 470 nm were recorded for 1 min in the linear portion of the reaction. The enzyme activity was calculated according to Chance and Maehly (1955) considering the molar extinction coefficient of tetraguaiacol and expressed in μmol of guaiacol oxidized within 1 min with 1 g of fresh weight tissue (μmol of guaiacol/(min × g fr wt).
Statistical analysis
The experiments were conducted in three independent biological replications with 40 plants per treatment. The height and infestation were estimated for every plant (the total plant number was 100–110/sample) and expressed as the arithmetic mean with confidence interval using the Student’s t test at the 95% significance level. Statistical significance was estimated by the Student’s t test for 95% significance level. One sample for enzyme activity included leaves and roots of five plants. Measurements were put down three or four times in the average treatment sample of each replication. Thus, each treatment had nine to 12 measurements of the enzyme activity. The data were expressed as the arithmetic mean with standard error. Statistical significance was estimated by the Mann-Whitney U test (P < 0.05). StatSoft STATISTICA 13.0. for Windows 10.0 was used for statistical data processing and graphing.
Results
We evaluated the effect of wheat seed bacterization under high temperature on the height of wheat seedlings, root rot incidence and severity, and the activity of free and weakly-bound guaiacol-dependent peroxidase in plant tissues in model systems with and without the fungal root rot pathogen B. sorokiniana under conditions of high temperature (+32 °C) and low soil moisture and air humidity (40%).
Effect of seed bacterization on the height of wheat seedlings
Figure 1 demonstrates non-bacterized and bacterized wheat plant heights in tests with and without the phytopathogen.
The height of 12-day-old wheat plants under experimental conditions of high temperature (+32 °C) and low soil and air moisture (40%) without pathogen (Path-) and with Bipolaris sorokiniana fungus (Path+); 1 - Without bacterial treatment (control), 2 - Bacterization with Aeromonas media GS4, 3 - Bacterization with Pseudomonas extremorientalis PhS1. The data are expressed as the mean with confidence interval using the Student’s t test (P < 0.05). *Denotes a statistically significant difference from control (P < 0.05)
The obtained data showed a 11–13% (P < 0.05) decrease in plant height in tests with phytopathogen (Path+) compared to those without (Path-). Seed bacterization with A. media GS4 did not affect plant growth; however, the application of P. extremorientalis PhS1 bacteria resulted in a statistically significant 6% increase in wheat height (P < 0.05) in tests both with (Path+) and without (Path-) phytopathogen compared to non-bacterized plants.
Effect of seed bacterization on root rot of wheat seedlings
Table 1 presents data on root rot incidence and severity in the model experiments.
We established that the root rot disease incidence in the test without B. sorokiniana and without bacterization was 27%. The presence of the phytopathogen had no effect on disease incidence in the tests without bacterization. Seed bacterization decreased root rot disease incidence in the test without adding phytopathogen 3.3–10.0 times compared to the control. Wheat bacterization with P. extremorientalis PhS1 decreased this parameter the least. In the test with phytopathogen, seed bacterization with this strain did not affect root rot disease incidence, whereas bacterization with A. media GS4 decreased (P < 0.05) the number of plants with this disease symptoms 1.9 times compared to the corresponding control. Root rot severity in the tests with phytopathogen (Path+) was 1.4–5.2 times higher compared to the tests without phytopathogen (Path-) (Table 1). Seed bacterization also decreased disease severity both with (Path+) and without phytopathogen (Path-) 1.2–2.4 and 3.2–4.7 times, respectively.
Effect of seed bacterization on the peroxidase activity in plant tissues
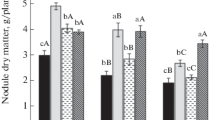
Measuring the peroxidase activity in the aboveground parts of the wheat showed that in the control with phytopathogen (Path+) this enzyme activity was more than 22% higher than in the control without phytopathogen (Path-) (Fig. 2).
Peroxidase activity in 12-day-old leaf tissues of wheat plants during seed bacterization in the tests without pathogen (Path-) and with Bipolaris sorokiniana fungus (Path+); 1 - Without bacterial treatment (control), 2 - Bacterization with Aeromonas media GS4, 3 - Bacterization with Pseudomonas extremorientalis PhS1. The data are expressed as the mean with confidence interval using the Student’s t test (P < 0.05). *Denotes a statistically significant difference from control (P < 0.05)
In general, seed bacterization increased the peroxidase activity in wheat leaves both with (Path+) and without phytopathogen (Path-); at the same time, a statistically significant (P < 0.05) increase in enzyme activity was noted for plants bacterized with A. media GS4 by 64% and 44%, respectively, compared to non-bacterized plants. Bacterization with P. extremorientalis PhS1 also increased the peroxidase activity in leaves by 18% in tests with pathogen and by 39% without it.
We found that the peroxidase activity in wheat root tissues during seed bacterization in tests without phytopathogen (Path-) was statistically significantly higher (P < 0.05) than the enzyme activity in the roots of non-bacterized seedlings: bacterization with A. media GS4 increased the enzyme activity 1.86 times, and that with P. extremorientalis PhS1 2.2 times (Fig. 3). Seed bacterization with B. sorokiniana provided a statistically significant (P < 0.05) increase in the peroxidase activity: by 3 times with A. media GS4 and with P. extremorientalis PhS1 by 90% compared to the corresponding control plants (Fig. 3).
Peroxidase activity in 12-day-old roots of wheat during seed bacterization in the tests without pathogen (Path-) and with Bipolaris sorokiniana fungus (Path+); 1 - Without bacterial treatment (control), 2 - Bacterization with Aeromonas media GS4, 3 - Bacterization with Pseudomonas extremorientalis PhS1. The data are expressed as the mean with confidence interval using the Student’s t test (P < 0.05). *Denotes a statistically significant difference from control (P < 0.05)
It was shown that the activity of free peroxidase in roots was 18.5% higher than in leaves for non-bacterized and non-infected plants.
Correlation between root rot on wheat seedlings and peroxidase activity in plant tissues
Comparison of the same parameters in the leaves and roots of non-bacterized plants with B. sorokiniana revealed the reverse pattern: the peroxidase activity in the roots of infected plants was 12% lower. Moreover, the reaction of enzyme systems to bacterization with pathogen was significantly more important in plant roots than the response of free peroxidase in leaves. Thus, wheat seed treatments with A. media GS4 and P. extremorientalis PhS1 increased the enzyme activity 3.0 times and by 90%, respectively, in roots, and only by 63.6% and 18.2% in leaves of infected plants (Figs. 2 and 3).
Table 2 shows data on the correlation of the studied parameters in plants during the test without phytopathogen. The conducted correlation analysis revealed a statistically significant (P < 0.05) positive correlation (r = 0.55) between the peroxidase activity in wheat roots and leaves. The statistically significant values of correlation coefficients also pointed to a high negative correlation between the peroxidase activity in wheat leaves and the parameters reflecting plant infestation by root rot pathogens: namely, disease incidence and severity (r = −0.62 and − 0.59, respectively). Similarly, a decrease in the root rot disease incidence with an increase in the peroxidase activity is indicated by correlation coefficients between this parameter and the root rot disease incidence and severity (r = −0.33 and − 0.30, respectively), though these values did not reach statistical significance and were inferior to the coefficients obtained for the peroxidase activity in leaf tissues.
Table 3 gives data on the correlation of the studied plant parameters in model systems with B. sorokiniana. We found that the correlation coefficient between the peroxidase activity in wheat leaf and root tissues was statistically insignificant (r = 0.20). In model systems with the phytopathogen, the correlation coefficients between the enzyme activity in leaves and the parameters of plant infestation by root rot pathogens (disease incidence and severity) decreased and lost their statistical significance (r = −0.21 and − 0.33, respectively). Correlation coefficients between the enzyme activity in plant roots and root rot disease incidence and severity, by contrast, increased and become statistically significant (r = −0.58 and − 0.85, respectively).
Table 4 presents generalized correlation coefficients of the studied plant parameters both with (Path+) and without phytopathogen (Path-).
A positive statistically significant (P < 0.05) correlation (r = 0.33) was observed between the activity of guaiacol-dependent peroxidase in wheat leaf tissues. The correlation coefficients for generalized values between the parameters of root rot disease incidence and the peroxidase activity in the seedling leaves were negative but statistically insignificant, whereas those for the parameters reflecting plant disease incidence and the peroxidase activity in roots were negative (r = −0.39 and − 0.45, respectively) and statistically significant (P < 0.05).
Discussion
The effects of abiotic stresses on wheat plants, primarily drought together with high temperature, have been reported to result in significant changes in plant physiology (Kasim et al., 2013; Khalilzadeh et al., 2016; Akter & Islam, 2017). Increased temperatures initially inhibit growth processes in plants eventually resulting in their complete cessation. Furthermore, abiotic stress has been reported to have a profound effect on plant growth and, therefore, to be responsible for serious losses in field yield (Akter & Islam, 2017; Rejeb et al., 2014; Sachdev et al., 2021). Previously, we detected a decrease in plant length under conditions of high temperature and low humidity (Minaeva et al., 2019), and a decrease in wheat length of 2.0–2.5 times compared to plants under optimal conditions. Moreover, biotic stress was an additional challenge inducing a strong pressure on plants and adding to the damage caused by pathogen or herbivore attack (Rejeb et al., 2014). We also demonstrated that the presence of phytopathogen (Path+) in an artificial ecosystem and an increase in seedling infestation by root rots had an additional stress impact on plants, which manifested in a more pronounced decrease in the plant length. This research confirmed that the presence of phytopathogen (B. sorokiniana) in an artificial system under abiotic stress (high temperatures and drought) decreased the height of wheat seedlings (Minaeva et al., 2019).
Globally, numerous research works have studied stress and stress-management mechanisms in plants. However, important questions remain such as how to accelerate stress management in plants and increase their productivity under unfavourable conditions for growth. Plant bacterization with beneficial strains is one such method that is widely used in practice to increase the yield of plants and decrease their disease incidence. Notable among the mechanisms of positive impact of bacteria is their ability to produce biologically active compounds that decrease plant infestation with phytopathogenic microorganisms such as antibiotics, cyanids, and siderophores (Dwivedi & Johri, 2003; Haas & Keel, 2003; Van Loon, 2007; Beneduzi et al., 2012; Hol et al., 2013; Pieterse et al., 2014; Oleńska et al., 2020). Furthermore, rhizosphere bacteria can promote plant growth by increasing the availability of essential nutrients (e.g., nitrogen, phosphorus, and iron), producing plant hormones, vitamins, and other biologically active substances (Van Loon, 2007; Maksimov et al., 2015; Oleńska et al., 2020). The ability of bacteria to induce systemic resistance has been widely documented; however, the mechanisms of its induction are likely to be complex and diverse. These include both direct stimulation of resistance, by increasing the production of salicylates and similar compounds, and activation of enzymes handling oxidative burst (such as peroxidase, catalase, and superoxide dismutase) (Choudhary et al., 2016; Hol et al., 2013; Manikandan & Raguchander, 2014; Oleńska et al., 2020).
Our previous studies have shown that wheat plant bacterization with A. media GS4 and P. extremorientalis PhS1 reduces the consequences of abiotic stress (low and high temperatures, high humidity, and drought) (Minaeva et al., 2019). Also, we demonstrated decreased root rot infestation of wheat both under abiotic stress and under optimal conditions with a pathogen (Path+) in the model system (Minaeva et al., 2018).
During these studies, when there was a simultaneous effect of both biotic and abiotic stresses, we established that seed bacterization with P. extremorientalis PhS1 could decrease the impact of stress on plants and increase seedling length. Moreover, the effectiveness of bacterization was the same both with (Path+) and without (Path-) pathogen. The increase in the seedling length under the effect of these bacteria was statistically significant (P < 0.05) compared to non-bacterized plants. At the same time, there was no significant change in the given parameter of plant growth under bacterization with A. media GS4. Pseudomonas bacteria, which are the most widespread inhabitants of the plant rhizosphere, have been applied to promote the growth of various agricultural crops (Beneduzi et al., 2012; Gupta et al., 2015; Maksimov et al., 2015; Choudhary et al., 2016). Aeromonas bacteria, although not the most widespread soil and rhizosphere inhabitants, are also notable control agents of soil phytopathogens (Khatoon et al., 2020; Lutz et al., 2020). A. media improved suppressiveness of compost against Puthium ultimum in cress (Lepidium sativum L.), and the largest effect was observed in compost with a low suppressiveness (Oberhaensli et al., 2017).
We determined the experimental effectiveness of wheat bacterization according to the decrease in root rot disease incidence and severity in tests with and without phytopathogen. The seed surface includes a zone of increased microbial activity called the spermosphere (Lemanceau et al., 2017), consisting of microflora that are neutral or beneficial for plants, as well as phytopathogens. Seed infestation by phytopathogens decreases due to the use of chemical compounds (such as pesticides and solutions of heavy metals), ionizing radiation, and other activities that can both negatively affect the viability of the embryo and be fatal to the studied bacteria. Therefore, despite seed disinfection, we did not observe a total absence of seed infestation by root rot pathogens in our experiment. This can be confirmed by infestation of non-bacterized plants by root rot pathogens in tests without phytopathogen (Path-). It should be noted that under conditions of drought and high temperatures, there was no significant increase in the number of plants infected with root rots by B. sorokiniana, which is a causative agent of this disease (Table 1). Infestation of non-bacterized plants did not statistically significantly differ both with (Path+) and without (Path-) phytopathogen. The absence of phytopathogen (Path-) impact on the non-bacterized plants could have been due to unfavourable conditions for development. Moisture plays a decisive role in the propagation and reproduction of micromycetes (Kumar et al., 2002). A lack of sufficient soil moisture inhibits fungal development, formation of conidia, and so on. In spite of the known increased aggressivity of this pathogen under hot climate and drought conditions, one of the possible outcomes of multiple stress exposure is that plants that are able to defend themselves from one stress can become more resistant to others. This phenomenon is called cross-tolerance, and indicates that plants have a powerful regulatory system that allows them to adapt quickly to the changing environment (Rejeb et al., 2014).
Seed bacterization in the tests without phytopathogen (Path-) significantly (P < 0.05) decreased wheat root rot incidence and severity. Root rot disease incidence and severity under bacterization showed a statistically significant (P < 0.05) decrease compared to untreated control plants. With the phytopathogen present, there was no statistically significant decrease in these parameters. Only seed bacterization with A. media resulted in a statistically significant decrease in root rot incidence and severity. The obtained effect may have been due to the fact that under high temperatures (+30–31 °С) A. media bacteria were better adapted than P. extremorientalis bacteria. The optimal temperature of A. media and P. extremorientalis bacteria is 25–26 °C. When studying the physiological and biochemical properties of bacterial strains, we found A. media GS4 growth at temperatures of 25–28 °C and 41 °C but not at +4 °C, P. extremorientalis PhS1 growth at was observed at +4 °C and 25–28 °C but not at 41 °C. Egamberdieva and Kucharova (2009) reported that P. extremorientalis TSAU6 maintained vitality at 37 °C, but the effect of these bacteria on shoot and root growth, as well as the dry matter of wheat, survival in the rhizosphere, and antagonistic activity in in vitro tests, were confirmed at 12–30 °C. Ivanova et al. (2002) discovered that the temperature range for growth of P. extremorientalis KMM 3447T was 4–35 °C, with an optimum temperature of 25 °C; no growth was detected at 42 °C. Thus, in our experiments, A. media appeared to propagate faster in the rhizosphere of wheat, and to enter into a more successful direct competition with the phytopathogen for habitat and root metabolites of plants.
When plants are affected by root rots, it is these structures, and not stems or leaves, that initially come into contact with the phytopathogenic fungus; therefore, a separate assessment of the activity of oxidative stress enzymes in the roots and the aboveground parts of plants is of scientific interest. By assessing the activity of free and weakly bound guaiacol-dependent peroxidase, we found that, in general, bacterization significantly increased the enzyme activity in wheat leaves both with (Path+) and without (Path-) phytopathogen in the system.
Without bacterization, there was an increase in the peroxidase activity in wheat leaves with (Path+) the phytopathogen in tests, which confirmed data that peroxidase activity may indicate both stress and systemic resistance in plants. As peroxidases are protective proteins, they can be, on the one hand, enzymes quenching oxidative-burst reactions, and, on the other hand, some are responsible for producing peroxide during oxidative bursts in response to the action of an elicitor from pathogenic fungi.
In the current study, despite the activation of these enzymes in plant leaves, we observed no increase in their activity in roots. This fact, as well as no decrease in plant infestation by root rot pathogens in tests without bacterization, allows us to conclude that an increased peroxidase activity can most likely be due to quenching oxidative burst.
In the current experiments, seed bacterization significantly (P < 0.05) increased the peroxidase activity both in the leaves and the roots of plants. Moreover, the highest peroxidase activity was detected in tests with the least plant infestation by root rot pathogens, which may indicate the effect of bacteria on the mechanisms involved in the induction of systemic resistance in plants. To date, numerous research articles and reviews have demonstrated and substantiated the effect of bacteria on the induction of systemic resistance in various plants. Manikandan and Raguchander (2014) demonstrated that in tomato plants pretreated with liquid formulation of P. fluorescence (PF1) and challenged with Fusaruim oxysporum f. sp. lycopersici, there was an increase in the enzyme activities (namely, phenylalanine ammonia lyase, peroxidase, polyphenoloxidase, catalase and β-1 3-glucanases) compared to pathogen-inoculated and untreated healthy controls. Burkhanova et al. (2017) showed that a reduction of the degree of Septoria nodorum blotch development on wheat leaves under the influence of Bacillus spp. was accompanied by the suppression of catalase activity, as well as by an increase in peroxidase activity and H2O2 content. Dukare and Paul (2021) established that Pseudomonas sp. NS 1 and Bacillus sp. NS 22 in an in planta assay made the pigeon pea plant more resistant to Fusarium udum through the induction of systemic defence in host tissue by improving the status of different defence enzymes (that is, phenylalanine ammonia lyase, polyphenol oxidase, and peroxidase) and phenolics. Hernández-Esquivel et al. (2020) discovered that both lipopolysaccharides of Azospirillum brasilense Sp245, a PGPR, and Pseudomonas aeruginosa PAO1, a pathogenic bacterium, caused increased plant growth and peroxidase activity in wheat seedlings. Moreover, lipopolysaccharides from the pathogenic bacterium generated higher peroxidase increments.
Van Loon (2007) on gene expression, including those involved in plant protection, and showed an ambiguous effect of PGPR on their activation in the roots and leaves of different plants. A number of the reviewed papers indicated an increase in expression in leaves, whereas there was no change at the site of inoculation (roots). Other studies indicated that expression could be enhanced in both roots and leaves during bacterial inoculation. Thus, the effect of rhizobacteria on the expression of resistant genes seems to be determined by both the species of the organisms involved in the interaction and the conditions under which it occurs.
The analysis of the peroxidase activity in response to B. sorokiniana infestation showed a good reaction of enzymatic oxidation systems of wheat plants and confirmed research results that have demonstrated a significant response of oxidative stress enzymes to root rot pathogens. According to some authors, the peroxidase activity may be used as a marker of plant resistance to pathogens (Reuveni, 1995; Van Lelyveld, 2008; Eisa et al., 2013; Minaeva et al., 2018).
Previously, we described the relationship between the peroxidase activity in wheat root and leaf tissues without pathogen in the system, and the feedback between the peroxidase activity in wheat roots and leaves and parameters reflecting the susceptibility of plants to root rot disease (Minaeva et al., 2018).
Also, we noted that the presence of the phytopathogen in tests significantly altered the relationship between the peroxidase activity in wheat root and leaf tissues. The correlation coefficient between these parameters became negative but not statistically significant, whereas without phytopathogen it was significant and positive. When a plant encountered an aggressive root rot pathogen immediately after germination, it changed strategy in favour of a significant increase in the peroxidase activity in roots, reducing the activity in leaf tissues. Since root rot pathogens, including B. sorokiniana, penetrate the plant through the roots and root collar, such alteration of activity may play a decisive role in the induction of systemic resistance of plants to this group of diseases (Minaeva et al., 2018).
In this research, when the phytopathogens associated with normal spermosphere microflora interacted with plants under high temperatures and drought, we also observed stronger correlation coefficients between the peroxidase activity in plant leaves and the parameters of infestation by root rot pathogens (disease incidence and severity). At the same time, the relationship between the peroxidase activity in roots without pathogen in the system also remained at a lower but statistically significant level. In contrast to our previous results (without abiotic stress), the effect of abiotic and biotic stress increased the relationship (i.e., the correlation index was greater) between the increase in the peroxidase activity in plant roots and the decrease in wheat disease incidence, while the relationship between the peroxidase activity in leaves and disease incidence decreased but did not completely disappear.
These results supported the finding that the interaction of factors can change a plant’s response to stress. Moreover, this response can either enhance or reduce the negative effects of stress. Our experiment showed that the interaction of factors decreased plant infestation by root rot pathogens; however, the decrease in disease incidence did not result in the complete disappearance of negative stress effects. Yet, the activity of guaiacol-dependent peroxidase can be recommended to assess the degree of impact of both abiotic and biotic stresses on plants, as well as the effectiveness of using bacterial strains to reduce negative effects.
References
Abdelaal, K., AlKahtani, M., Attia, K., Hafez, Y., Király, L., & Künstler, A. (2021). The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology, 10, 520. https://doi.org/10.3390/biology10060520
Akter, N., & Islam, M. R. (2017). Heat stress effects and management in wheat. A review. Agronomy for Sustainable Development, 37, 37. https://doi.org/10.1007/s13593-017-0443-9
Al-Sadi, A. M. (2021). Bipolaris sorokiniana-induced black point, common root rot, and spot blotch diseases of wheat: A review. Frontiers in Cellular and Infection Microbiology, 11, 584899. https://doi.org/10.3389/fcimb.2021.584899
Beneduzi, A., Ambrosini, A., & Passaglia, L. M. P. (2012). Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genetics and Molecular Biology, 35, 1044–1051.
Burkhanova, G. F., Veselova, S. V., Sorokan’, A. V., Blagova, D. K., Nuzhnaya, T. V., & Maksimov, I. V. (2017). Strains of Bacillus ssp. regulate wheat resistance to Septoria nodorum Berk. Applied Biochemistry and Microbiology, 53(3), 346–352. https://doi.org/10.1134/S0003683817030048
Chance, B., & Maehly, A. C. (1955). Assays of catalases and peroxidases. In S. P. Colowick & N. O. Kaplan (Eds.), Methods in enzymology (Vol. II, pp. 764–775). Academic Press.
Choudhary, D. K., Kasotia, A., Jain, S., Vaishnav, A., Kumari, S., Sharma, K. P., & Varma, A. (2016). Bacterial-mediated tolerance and resistance to plants under abiotic and biotic stresses. Journal of Plant Growth Regulation, 35, 276–300. https://doi.org/10.1007/s00344-015-9521-x
Cooke, B. M. (2006). Disease assessment and yield loss. In B. M. Cooke, D. G. Jone, & B. Kaye (Eds.), The epidemiology of plant diseases (2nd ed., pp. 43–80). Springer.
Dimkpa, C., Weinand, T., & Asch, F. (2009). Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant, Cell and Environment, 32, 1682–1694. https://doi.org/10.1111/j.1365-3040.2009.02028.x
Dukare, A., & Paul, S. (2021). Biological control of fusarium wilt and growth promotion in pigeon pea (Cajanus cajan) by antagonistic rhizobacteria, displaying multiple modes of pathogen inhibition. Rhizosphere, 17, 100278.
Dwivedi, D., & Johri, B. N. (2003). Antifungals from fluorescent pseudomonads: Biosynthesis and regulation. Current Science, 85(12), 1693–1703.
Egamberdieva, D., & Kucharova, Z. (2009). Selection for root colonising bacteria stimulating wheat growth in saline soils. Biology and Fertility of Soils, 45, 563–571. https://doi.org/10.1007/s00374-009-0366-y
Eisa, M., Chand, R., & Joshi, A. K. (2013). Biochemical and histochemical traits: A promising way to screen resistance against spot blotch (Bipolaris sorokiniana) of wheat. European Journal of Plant Pathology, 137, 805–820. https://doi.org/10.1007/s10658-013-0290-8
Gill, S. S., & Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48(12), 909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gupta, G., Parihar, S. S., Ahirwar, N. K., Snehi, S. K., & Singh, V. (2015). Plant growth promoting Rhizobacteria (PGPR): Current and future prospects for development of sustainable agriculture. Journal of Microbial & Biochemical Technology, 7, 2. https://doi.org/10.4172/1948-5948.1000188
Haas, D., & Keel, C. (2003). Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant disease. Annual Review of Phytopathology, 41, 117–153. https://doi.org/10.1146/annurev.phyto.41.052002.095656
Hasanuzzaman, M., Borhannuddin Bhuyan, M. H. M., Zulfiqar, F., Raza, A., Mohsin, S. M., Mahmud Jubayer, A., Masayuki, F., & Fotopoulos, V. (2020). Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants, 9, 681. https://doi.org/10.3390/antiox9080681
Hernández-Esquivel, A. A.,·Castro-Mercado, E., & García-Pineda, E (2020). Comparative effects of Azospirillum brasilense Sp245 and Pseudomonas aeruginosa PAO1 lipopolysaccharides on wheat seedling growth and peroxidase activity. Journal of Plant Growth Regulation. Advance online publication. https://doi.org/10.1007/s00344-020-10241-x.
Hol, W. H., Bezemer, T. M., & Biere, A. (2013). Getting the ecology into interactions between plants and the plant growth-promoting bacterium Pseudomonas fluorescens. Frontiers in Plant Science, 4, 81. https://doi.org/10.3389/fpls.2013.00081
Huang, H., Ullah, F., Zhou, D.-X., Yi, M., & Zhao, Y. (2019). Mechanisms of ROS regulation of plant development and stress responses. Frontiers in Plant Science, 10, 800. https://doi.org/10.3389/fpls.2019.00800
Ivanova, E. P., Gorshkova, N. M., Sawabe, T., Hayashi, K., Kalinovskaya, N. I., Lysenko, A. M., Zhukova, N. V., Nicolau, D. V., Kuznetsova, T. A., Mikhailov, V. V., & Christen, R. (2002). Pseudomonas extremorientalis sp. nov., isolated from a drinking water reservoir. International Journal of Systematic and Evolutionary Microbiology, 52, 2113–2120. https://doi.org/10.1099/ijs.0.02197-0
Kasim, W. A., Osman, M. E., Omar, M. N., Abd El-Daim, I. A., Bejai, S., & Meijer, J. (2013). Control of drought stress in wheat using plant-growth-promoting bacteria. Journal of Plant Growth Regulation, 32, 122–130. https://doi.org/10.1007/s00344-012-9283-7
Khalilzadeh, R., Sharifi, R. S., & Jalilian, J. (2016). Antioxidant status and physiological responses of wheat (Triticum aestivum L.) to cycocel application and bio fertilizers under water limitation condition. Journal of Plant Interactions, 11(1), 130–137. https://doi.org/10.1080/17429145.2016.1221150
Khatoon, Z., Huang, S., Rafique, M., Fakhar, A., Kamran, M. A., & Santoyo, G. (2020). Unlocking the potential of plant growth-promoting rhizobacteria on soil health and the sustainability of agricultural systems. Journal of Environmental Management, 273, 111118. https://doi.org/10.1016/j.jenvman.2020.111118
Kumar, J., Schäfer, P., Hückelhoven, R., Langen, G., Baltruschat, H., Stein, E., Nagarajan, S., & Kogel, K. (2002). Bipolaris sorokiniana, a cereal pathogen of global concern: Cytological and molecular approaches towards better control. Molecular Plant Pathology, 3(4), 185–195.
Lemanceau, P., Barret, M., Mazurier, S., Mondy, S., Pivato, B., Fort, T., & Vacher, C. (2017). Plant communication with associated microbiota in the spermosphere, rhizosphere and phyllosphere. In G. Becard (Ed.), How plants communicate with their biotic environment. Advances in botanical research (Vol. 82, pp. 102–133). https://doi.org/10.1016/bs.abr.2016.10.007
Lutz, S., Thuerig, B., Oberhaensli, T., Mayerhofer, J., Fuchs, J. G., Widmer, F., Freimoser, F. M., & Ahrens, C. H. (2020). Harnessing the microbiomes of suppressive composts for plant protection: From metagenomes to beneficial microorganisms and reliable diagnostics. Frontiers in Microbiology, 11, 1810. https://doi.org/10.3389/fmicb.2020.01810
Maksimov, I. V., Veselova, S. V., Nuzhnaya, T. V., Sarvarova, E. R., & Khairullin, R. M. (2015). Plant growth promoting bacteria in regulation of plant resistance to stress factors. Russian Journal of Plant Physiology, 62, 715–726.
Manikandan, R., & Raguchander, T. (2014). Fusarium oxysporum f. sp. lycopersici retardation through induction of defensive response in tomato plants using a liquid formulation of Pseudomonas fluorescens (Pf1). European Journal of Plant Pathology, 140(3), 469–480. https://doi.org/10.1007/s10658-014-0481-y
Medina-Sauza, R. M., Álvarez-Jiménez, M., Delhal, A., Reverchon, F., Blouin, M., Guerrero-Analco, J. A., Cerdán, C. R., Guevara, R., Villain, L., & Barois, I. (2019). Earthworms building up soil microbiota, a review. Frontiers in Environmental Science, 7, 81. https://doi.org/10.3389/fenvs.2019.00081
Minaeva, O. M., Akimova, E. E., Tereshchenko, N. N., & Zyubanova, T. I. (2019). Effect of bacterization with Aeromonas media GS4 and Pseudomonas extremorientalis PhS1 on wheat seedlings under different abiotic conditions. Tomsk State University journal of biology, 45, 128–141. https://doi.org/10.17223/19988591/45/7
Minaeva, O. M., Akimova, E. E., Tereshchenko, N. N., Zyubanova, T. I., Apenysheva, M. V., & Kravets, A. V. (2018). Effect of Pseudomonas bacteria on peroxidase activity in wheat plants when infected with Bipolaris sorokiniana. Russian Journal of Plant Physiology, 65(5), 717–725. https://doi.org/10.1134/S1021443718040052
Minibayeva, F., Kolesnikov, O., Chasov, A., Beckett, R. P., Lüthje, S., Vylegzhanina, N., Buck, F., & Böttger, M. (2009). Wound-induced apoplastic peroxidase activities: Their roles in the production and detoxification of reactive oxygen species. Plant, Cell & Environment, 32, 497–508. https://doi.org/10.1111/j.1365-3040.2009.01944.x
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science, 7(9), 405–410.
Oberhaensli, T., Hofer, V., Tamm, L., Fuchs, J. G., Koller, M., Herforth-Rahmé, J., Maurhofer, M., & Thuerig, B. (2017). Aeromonas media in compost amendments contributes to suppression of Pythium ultimum in cress. Acta Horticulturae, 1164, 353–360. https://doi.org/10.17660/ActaHortic.2017.1164.45
Oleńska, E., Małek, W., Wójcik, M., Swiecicka, I., Thijs, S., & Vangronsveld, J. (2020). Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Science of the Total Environment, 743, 140682. https://doi.org/10.1016/j.scitotenv.2020.140682
Passardi, F.,·Cosio, C.,·Penel, C.,·& Dunand C. (2005). Peroxidases have more functions than a Swiss army knife. Plant Cell Reports, 24, 255–265. https://doi.org/10.1007/s00299-005-0972-6.
Pathma, J., & Sakthivel, N. (2012). Microbial diversity of vermicompost bacteria that exhibit useful agricultural traits and waste management potential. Springerplus, 1, 26. https://doi.org/10.1186/2193-1801-1-26
Pieterse, C. M. J., Zamioudis, C., Berendsen, R. L., Weller, D. M., van Wees, S. C. M., & Bakker, P. A. H. M. (2014). Induced systemic resistance by beneficial microbes. Annual Review of Phytopathology, 52, 347–375.
Plotnikova, L. Y. (2009). The involvement of reactive oxygen species in defense of wheat lines with the genes introgressed from Agropyron species contributing the resistance against brown rust. Russian Journal of Plant Physiology, 56, 181–189. https://doi.org/10.1134/S102144370902006X
Rejeb, I. B., Pastor, V., & Mauch-Mani, B. (2014). Plant responses to simultaneous biotic and abiotic stress: Molecular mechanisms. Plants, 3(4), 458–475. https://doi.org/10.3390/plants3040458
Reuveni, R. (1995). Biochemical markers for disease resistance. In R. P. Singh & U. S. Singh (Eds.), Molecular methods in plant pathology (pp. 99–114). Lewis Publishers Boca Raton, Fla
Saad, A., Macdonald, B., Martin, A., Knight, N. L., & Percy, C. (2021). Comparison of disease severity caused by four soil-borne pathogens in winter cereal seedlings. Crop & Pasture Science, 72(5), 325–334. https://doi.org/10.1071/CP20245
Sachdev, S., Ansari, S. A., Ansari, M. I., Fujita, M., & Hasanuzzaman, M. (2021). Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants, 10, 277. https://doi.org/10.3390/antiox1002027
Sorahinobar, M., Niknam, V., Ebrahimzadeh, H., Soltanloo, H., Moradi, B., & Bahram, M. (2016). Lack of association between fusarium graminearum resistance in spike and crude extract tolerance in seedling of wheat. European Journal of Plant Pathology, 144, 525–538. https://doi.org/10.1007/s10658-015-0792-7
Tereshchenko, N., Akimova, E., Minaeva, O., Kravets, A., & Zyubanova, T. (2018). Presowing with bacteria improved the productivity and resistance to fungal root pathogen in wheat and barley. In Z. Tadele (Ed.), Grasses as food and feed (pp. 153–167). IntechOpen. https://doi.org/10.5772/intechopen.80084
Toropova, E. Y., Kirichenko, A. A., Stetsov, G. Y., & Suhomlinov, V. Y. (2015). Soil infections of grain crops with the use of the resource-saving technologies in Western Siberia, Russia. Biosciences, Biotechnology Research Asia, 12(2). https://doi.org/10.13005/bbra/1761
Van Loon, L. C. (2007). Plant responses to plant growth-promoting rhizobacteria. European Journal of Plant Pathology, 119, 243–254.
Vijayabharathi, R., Sathya, A., & Gopalakrishnan, S. (2015). Plant growth-promoting microbes from herbal vermicompost. In D. Egamberdieva, S. Shrivastava, & A. Varma (Eds.), Plant-growth-promoting Rhizobacteria (PGPR) and medicinal plants. Soil biology (Vol. 42, pp. 71–88). Springer. https://doi.org/10.1007/978-3-319-13401-7_4
Acknowledgments
The authors are grateful to Alexander V. Kartashov, Cand. Sci. (Biol.), Senior Researcher and Yury V. Ivanov, Cand. Sci. (Biol.), Senior Researcher, K.A. Timiryazev Institute of Plant Physiology of the Russian Academy of Sciences (Moscow, Russia) for their advice and assistance in developing methodology and presenting research results.
Funding
The reported study was funded by Russian Foundation for Basic Research, project number 20–34-90065.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Minaeva, O.M., Zyubanova, T.I., Akimova, E.E. et al. Effect of seed bacterization on peroxidase activity in wheat plants when infected with Bipolaris sorokiniana under high temperature and low moisture. Eur J Plant Pathol 164, 79–91 (2022). https://doi.org/10.1007/s10658-022-02540-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-022-02540-8