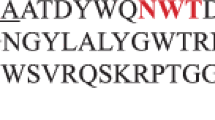Abstract
The isolation, heterologous expression, and characterization of a new, thermostable β-glucanase from Paenibacillus jamilae are described. The bgl26 gene from the P. jamilae Bg1 VKPM B-13 093 strain, which consists of 714 nucleotides, encodes endo-1,3-1,4-β-glucanase (EC 3.2.1.73), which contains 213 amino acids and 24 residues of the putative signal peptide in the N-terminal region. The nucleotide sequence of the bgl26 gene and the amino acid sequence of the mature Bgl26 protein have the greatest homology with the sequences of the Paenibacillus macerans endo-1,3-1,4-β-glucanase (82 and 88%, respectively). A gene fragment encoding the mature protein was expressed in Pichia pastoris. The purified recombinant enzyme Bgl26 was active against barley β-glucan. The optimal pH for the enzyme activity was 7.0, and the optimum temperature range was 40–45°C. The specific β-glucanase activity was at the level of 6650 U/mg of protein; KM and Vmax were equal to 6.4 ± 0.3 mg/mL and 9450.1 ± 471.2 μmol/(min mg), respectively. The recombinant protein Bgl26 was characterized by a high pH and thermal stability, as well as resistance to digestive enzymes. It was also shown that Co2+ ions have a positive effect on enzyme activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
β-Glucans are polysaccharides consisting of D‑glucose monomers linked by the β-1,3- and/or β-1,4-glycosidic bonds. Depending on the type of the glycoside bond, β-glucans can be divided into the following main types: β-1,3-1,4-glucans (barley β-glucans and lichenin), β-1,4-glucan (cellulose) and β-1,3-glucans (laminarin and β-glucan from Euglena gracilis) [1].
β-Glucanases, enzymes that are capable of hydrolyzing β-glucans, are classified according to the type of glucoside bonds they cleave: endo-1,3-1,4-β-glucanases (EC 3.2.1.73), endo-1,3(4)-β-glucanases (EC 3.2.1.6), endo-1,4-β-glucanases (ЕС 3.2.1.4), and endo-1,3-β-glucanases (EC 3.2.1.39).
β-1,3-1,4-Glucans are components of the endosperm cell wall of cereals such as barley, rye, rice, and wheat; they are also found in lichens.
Most of the studied microbial β-1,3-1,4-glucanases belong to GH16 and are produced by bacteria of the Bacillus genus [2–4]. Endo-1,3(4)-β-glucanases are more often components of fungi such as Botryotinia fuckeliana [5], Phaffia rhodozyma [6], Rhizomucor miehei [7], Phanerochaete chrysosporium [8], and Schizosaccharomyces pombe [9].
β-Glucanases are used in industrial processes of combined feed manufacture for monogastric animals and in brewery [10]. In the latter area, the enzymes stimulate the extraction of barley seeds, reduce the amount of wort, and decrease excessive viscosity and sediment formation in beer. In poultry and pig breeding, water-soluble glucan functions as an antinutritional agent. The addition of β-Glucanases to barley-containing feed improve body weight gain in farm animals, reduce the viscosity of the contents of the small intestine, and increase the efficiency of nutrient assimilation [11, 12].
Commercial endo-β-glucanases currently used in production are usually obtained on the basis of enzymes from fungi of the genus Trichoderma [13]. However, the properties of these β-glucanases do not fully meet the requirements for enzymes in feed production and brewing. For example, the thermolability of commercial β‑glucanases has been emphasized [14, 15].
The search for new, highly active β-glucanases with the characteristics necessary for their industrial application is an urgent task.
The goal of the present study was to clone and express β-glucanase from Paenibacillus jamilae Bgl VKPM В-13193 in the expression system of the Pichia pastoris methylotrophic yeast and to study the properties of the recombinant enzyme.
EXPERIMENTAL
Microorganisms, Nutrient Media, and Plasmids
A P. jamilae Bgl strain capable of synthesizing β-glucanase was isolated from a sample of forest soil from Moscow oblast (Russia) and deposited in the All Russia Collection of Industrial Microorganisms (VKPM) National Resource Center under the number В-13 193.
The P. pastoris strain VKPM Y-2837 (His–) and the pPIC-GAP VKPM В-10 t978 vector were used for expression in the yeast system.
The gene-engineering procedures were carried out with the Escherichia coli strain XL1-Blue (endA1 supE44 thi1 recA1 gyrA96 relA1 lac hsdR17 F' [proAB lacIqZΔM15 Tn10] VKPM В-9838).
LB medium containing 0.5% yeast extract (Dia-M, Russia), 1% trypton (Dia-M), and 1% NaCl (Khimmed, Russia) was used for the cultivation of Escherichia coli XL1 Blue. YPD medium (yeast extract, 1%; trypton, 1.5%; and glucose, 2% (Khimmed)) was the nutrient medium for P. pastoris growth.
Domestic reagents of chemically pure and pure for analysis grades (Khimmed) were also used.
Analysis of Nucleotide and Amino Acid Sequences
The nucleotide and amino acid sequences were analyzed with the BLAST software (http://www.ncbi. nlm.nih.gov/gorf/gorf.html). The SignalP 4.1 Server (http://www.cbs.dtu.dk/services/SignalP/) program was used to search for possible signal sequences. Multiple alignments were performed with the CLUSTAL W (http://www.ebi.ac.uk/clustalw) software. 3D structures were studied on the SWISS-MODEL server (http://swissmodel.expasy.org) with the NCBI database (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi?RID=XGGZ1VF0014&mode=all). The glycosylation sites were detected with the use of the Ne-tNGlyc 1.0 Server (http://www.cbs.dtu.dk/services/NetNGlyc/).
Gene Cloning
The primers Bgl-1 (5'-ATGAAGNAGAANTNTTGGTTRAC-3′) and Bgl-2 (5′-TTATCTTTTTGTGTAACGCANY-3′) were used to amplify the bgl26 gene, which encodes β-glucanase. This gene was sequenced and deposited in GenBank (no. MN053906). It was cloned in the pPIC-GAP vector and expressed in Pichia pastoris.
Construction of Recombinant Expression Plasmid
The DNA fragment encoding the mature Bgl26 protein was amplified via PCR with Pfu DNA-polymerase (Fermentas, Lithuania) and two synthetic primers (BglP.jam-f and BglP.jam-r). The first (5'‑AAAGAATTCGCGGGGAATGTTTTTTGGGAA-3') contains an EcoRI site, and the second (5'-AAAGCGGCCGCTTAATTGCTCGTGTATTTTACС-3') includes a NotI site. The amplified product encoding the mature β-glucanase was cloned in the pPIC-GAP vector, which resulted in the recombinant pPIC-GAP-BglP.jam plasmid. The nucleotide sequence for β-glucanase was inserted into the same reading frame with the vector signal sequence.
Expression of β-Glucanase in P. pastoris and Fermentation with the Yeast Recombinant Strain
The pPIC-GAP–BglP.jam plasmid was linearized via restriction with endonuclease BglII and used to transform the P. pastoris VKPM Y-2837 cells via electroporation (http://tools.thermofisher.com/content/sfs/manuals/pich_man.pdf). The expression cassette was inserted in the AOX1 locus via homologous recombination.
The recombinant clones were grown in YPD medium for 20 h at 30°C and aeration (250 rpm). The cells were then reinoculated into tubes with the YPD medium at a ratio of 1 : 10 and grown for 4 days at 30°C and aeration (250 rpm). Glucose (2%) was added every 24 h. The enzyme activity in the CL was assessed after fermentation. The clone with the highest β-glucanase activity was selected for further study.
Purification of Recombinant β-Glucanase
The CL was prewashed of low-molecular components of the medium on a VivaFlow ultrafiltration unit (Sartorius, Germany) with a membrane module cut-off of 10 kDa. The protein was purified via ion-exchange chromatography. The obtained retentate was loaded on a column of SP-Sepharose in HiTrapQ FF (GE Healthcare, GB) equilibrated with 20 mM Tris-HCl buffer, pH 8.6. The elution was carried out with a sodium chloride step gradient; the target protein was released from the column at a NaCl concentration of 0.25 M. The protein content was measured with the Bradford method [15].
Electrophoresis of Proteins in PAG
The procedure was performed in 12% PAG in the presence of SDS in a Mini-Protean Tetra Cell chamber for vertical electrophoresis (Bio-Rad Laboratories, United States), first for 1 h at 50 V and then for 2–3 h at 150 V. The proteins were stained with 0.1% Coumassie blue R-250 (Dia-M).
Assessment of Enzyme Activity
The conventional procedure was performed in a total volume of 100 μL with the mixing of a 1% substrate solution (50 μL) in 0.5 M Tris-HCl buffer, pH 7.0, with a β-glucanase-containing sample (50 μL), followed by incubation at 40°C for 10 min. The reducing sugars were measured via SDS with glucose as a standard [16].
The unit of the enzyme activity (U) was equal to the amount of β-glucanase required for the conversion of 1 μmol of reducing sugars per min.
The substrate specificity was determined via measurement of the activity with barley β-glucan (Megazame, United States), lichenin (Serva, Germany), β‑1,3-glucan from Euglena gracilis (Sigma, United States), or birch xylane (CMC) (Khimmed) as substrates.
Characteristics of Recombinant Bgl26
The pH optimum of the activity was determined via incubation of the purified Bgl26 protein with barley β‑glucan in the following buffer solutions: 0.5 M glycine (pH 2.0–3.0), 0.5 M acetate (pH 4.0–6.0) or 0.5 M Tris-HCl (pH 7–9).
The effect of the pH level on Bgl26 stability was assessed in the same buffer systems in a pH range of 2–9. The temperature optimum was determined via standard analysis of the activity in a temperature interval of 30–80°C.
The thermal stability was determined via measurement of the residual enzyme activity after incubation for 10 min at 70, 80, or 90°C in the presence of SDS.
The enzyme activity in the buffer with the addition of appropriate ions or other substances (1 mM) was analyzed to determine the effect of metal ions and other compounds on β-glucan hydrolysis.
KM and Vmax of the recombinant Bgl26 were determined with the Lineweaver–Burk method and assessment of the enzyme activity at 40°C in 0.5 M Tris-HCl buffer, pH 7.0, with barley β-glucan (2.5–10 mg/mL) as a substrate.
The resistance to proteolytic enzymes was studied via 30 min of Bgl26 incubation with 0.1% pepsin (at pH 2.0) and 0.1% trypsin (pH 7.0) at 37°С, followed by estimation of the β-glucanase residual activity.
RESULTS AND DISCUSSION
The Bgl strain can grow with barley β-glucan as the sole carbon source and can secrete an active β-glucanase. Analysis of the 16S rRNA gene nucleotide sequence (GenBank, no. MN053905) identified this strain as Paenibacillus jamilae.
The β-Glucanases from P. jamilae have not been studied previously; therefore, in this work, we focused on the gene sequences of a phylogenetically related species, Paenibacillus polymyxa. Degenerate primers designed on the basis of conservative segments of the available gene sequences of various P. polymyxa strains that supposedly encode endo-1,3-1,4-β-glucanases were used to clone the β-glucanase gene.
Analysis of the DNA fragment produced by PCR showed that it was a 174-bp coding region of the bgl26 gene. The gene translation product, Bgl26 protein, has a size of 237 aa.
Analysis of the nucleotide (bgl26) and amino-acid (Bgl26) sequences highlighted their homology with the sequences of β-glucanases from Paenibacillus macerans (82 and 88%, respectively) [17], Bacillus subtilis (74 and 77%) [18] and Bacillus licheniformis (73 and 79%) (GenBank, no. AF546871.1). The amino-acid sequences of all three homologous enzymes are characteristic of GH16 (https://www.ncbi.nlm.nih.gov/Structure/cdd/cddsrv.cgi).
The data obtained with the SignalP 4.1 program allowed the determination of a putative signal sequence consisting of 24 N-terminal amino acids. Thus, the sequence of the mature Bgl26 protein was established (Fig. 1).
Alignment of amino-acid sequences of Bgl26 and its related enzyme: Bgl26—β-glucanase from Paenibacillus jamilae, P. macerans—β-glucanase from Paenibacillus macerans. The possible Bgl26 signal sequence is underlined; putative N-glycosylation sites are in bold. Yellow color designates the GH16conservative motif; amino acids of the active site and Ca2+ binding site are marked in green and red, respectively. Asterisks indicate identical amino acids in all sequences in the alignment; (:) and (.) designate conservative and semiconservative amino acid substitutions, respectively.
The technique of multiple alignments of amino-acid sequences based on the information resources of the NCBI database, analysis of the expected 3D model of the Bgl26 protein predicted by the SWISS-MODEL server, and consideration of the structure of the most closely related endo-1,3-1,4-glucanase from Paenibacillus macerans [19] made it possible to identify the amino acids of the active site and of the conservative motif of GH16 (Fig. 1).
The mature Bgl26 protein has traits that are characteristic of the GH16 endo-1,3-1,4-β-glucanase family (EC 3.2.1.73): the occurrence of the conservative EIDIE motif and a domain including two 7-chained antiparallel β-sheets that, adjoining each other, form a compact β-sandwich with a jelly-like structure [20].
The plasmid pPIC-GAP, which allows highly efficient, constitutive expression of the heterologous gene under the control of the GAP promoter, was used to express the bgl26 gene in P. pastoris.
The nucleotide sequence encoding the mature Bgl26 protein region was amplified via PCR with the BglP.jam-f and BglP.jam-r primers (see Experimental section) and cloned in a single reading frame with the α-factor signal sequence. Thus, the expression plasmid pPIC-GAP-Bgl26 was constructed.
The expression plasmid was linearized and used to transform P. pastoris cells. Of the 233 positive clones, the transformant Bgl26-117 demonstrated the highest β-glucanase activity.
Fermentation with the Bgl26–117 clone was carried out in a 500-mL flask for 120 h. The culture was sampled (1 mL) every 24 h. The results of SDS-PAGE showed that the concentration of the recombinant Bgl26 protein increased with time. The protein secretion measured by β-glucanase activity also increased. The enzyme activity reached 580 U/mL by the end of fermentation.
The recombinant enzyme was purified via anion-exchange chromatography. SDS-PAGE showed that the MW of the purified Bgl26 was about 30 kDa (which is higher than the theoretically predicted value of 24.1 kDa), and the protein was represented by a double band (Fig. 2). We believe that this was a result of posttranslational modifications, i.e., glycosylation, of the protein in the yeast cells. Amino-acid sequencing (NetNGlyc 1.0 Server) showed that the protein contains three potential N-glycosylation sites, Asn-X-Ser/Thr [21].
The enzyme activity with barley β-glucan was assessed at each stage of Bgl26 purification. The purified enzyme had a high specific activity of 6650 U/mg protein (Table 1). This value is only inferior to the specific activity of the bacterial Fibrobacter succinogenes β-glucanase (10 800 U/mg of protein) [22].
The recombinant Bgl26 characteristics were studied with barley β-glucan as a substrate.
The kinetic parameters were analyzed under optimal conditions: a pH of 7.0 and a temperature of 40°C. KM and Vmax were equal to 6.4 ± 0.3 mg/mL and 9450.1 ± 471.2 μmol/(min mg), respectively.
The pH optimum of the recombinant β-glucanase activity was shown to be 7.0 (Fig. 3a). The enzyme functioned in a pH range of 4–9 and retained more than 50% activity at a pH of 4.7–7.8. Inactivation of the recombinant β-glucanase was observed at pH ≤4.
The temperature optimum of the enzymatic activity was proven to lie in the interval of 40–45°C; an activity of 80% or higher was observed in the temperature range of 30–68°С (Fig. 3b).
The effect of the pH level on Bgl26 stability was studied via enzyme incubation in buffer solutions with different pH values at 37°C for 30 min. The enzyme was stable in a wide pH range; more than 70% of activity was preserved after incubation at a pH of 3.0–8.0 (Fig. 4a).
The recombinant β-glucanase was resistant to high temperatures. The residual activity after heating at 70, 80, and 90°C for 10 min was equal to 100, 70, and 67.5%, respectively (Fig. 4b).
The protein also showed resistance to digestive enzymes: after treatment with pepsin and trypsin in an appropriate buffer at 37°C for 30 min, the enzyme retained 88 and 91% of its activity, respectively (Fig. 4c).
The specific activity of the purified enzyme Bgl26 with various substrates was measured at 40°C and a pH of 7.0 for10 min (Table 2).
The data in Table 2 show that Bgl26 is capable of hydrolyzing barley β-glucan and lichenin but not β‑1,3-glucan and CMC. This substrate specificity is characteristic of endo-1,3-1,4-β-glucanases (EC 3.2.1.73). However, the extremely low activity of Bgl26 with lichenin as a substrate, which is only 1.5% of the maximal level, is uncharacteristic for this class of enzymes. The substrate specificity of the recombinant β-glucanases needs further study.
The enzymatic activity was also analyzed in the presence of metal ions, EDTA and SDS (Table 3). Co2+, Mg2+, Fe3+, Ca2+, Li+, and EDTA had a positive effect on the β-glucanase activity, increasing it by 161.4, 21, 31.6, 15.8, 14, and 23.9%, respectively. Ni2+, Cu2+, and SDS decreased enzyme activity by 31.6, 28.1, and 22.8%, respectively. The addition of other metal ions had no significant effect on the activity of the recombinant β-glucanase.
Co2+ ions had the greatest effect on β-glucanase activity, increasing it by more than 2.5 times. An increase in glycosylhydrolase activity in the presence of cobalt ions, though not exceeding 15%, was reported in [23, 24]. It is also known that calcium ions activate this enzyme class via stabilization of the structure of β-glucanase molecules, which makes them more heat-resistant [25, 26]. A binding site for calcium ions was identified in the P. macerans β-glucanase; it is located in the peripheral loop area of the enzyme molecule [27].
Comparison of the amino-acid sequences in the calcium-binding sites of P. macerans and P. jamilae β-glucanases (Fig. 1) showed that Bgl26 has the following substitutions: 34S/N, 35Y/G, 38P/S, 39S/G, and 40T/A. The substitutions at positions 35, 38, and 40 are the most significant, since they are responsible for a substantial modification in the conformation and size of the metal-binding area. These substitutions may give cobalt ions the ability to stimulate recombinant enzyme activity. However, this proposition requires further study; the functions of amino-acid residues in the aforementioned positions should be analyzed via site-directed mutagenesis.
CONCLUSIONS
Thus, the properties of a new, highly active endo-1,3-1,4-β-glucanase from the P. jamilae strain Bg1 VKPM В-13193 in the P. pastoris expression system were studied.
The recombinant protein possesses a high specific activity, kinetic parameters characteristic of a high-active enzyme, high thermal stability, and resistance to digestive enzymes. The temperature optimum of the Bgl26 activity is 40–45°С, the pH optimum is 7.0, and the activity remains high over a wide range of pH and temperature values. The enzyme is capable of efficiently hydrolyzing barley β-glucan, the main nonstarch polysaccharide of the raw material for feed production and brewing.
The identified properties indicate that the studied enzyme is promising for the creation on its basis of a yeast recombinant producer of β-glucanase for industrial use.
REFERENCES
McCarthy, T., Hanniffy, O., Savage, A.V., and Tuohy, M.G., Catalytic properties and mode of action of three endo-β-glucanases from Talaromyces emersonii on soluble β-1,4-and β-1,3; 1,4-linked glucans, Int. J. Biol. Macromol., 2003, vol. 33, no. 1 (3), pp. 141–148. https://doi.org/10.1016/S0141-8130(03)00080-1
Ekinci, M.S., McCrae, S.I., and Flint, H.J., Isolation and overexpression of a gene encoding an extracellular beta-(1,3-1,4)-glucanase from Streptococcus bovis JB1, Appl. Environ. Microbiol. 1997, vol. 63, no. 10, pp. 3752–3756.
Teng, D., Wang, J.H., Fan, Y., et al., Cloning of β-1, 3-1, 4-glucanase gene from Bacillus licheniformis EGW039 (CGMCC 0635) and its expression in Escherichia coli BL21 (DE3), Appl. Microbiol. Biotechnol., 2006, vol. 72, no. 4, pp. 705–712. https://doi.org/10.1007/s00253-006-0329-2
Huang, H., Yang, P., Luo, H., et al., High-level expression of a truncated 1,3-1,4-β-D-glucanase from Fibrobacter succinogenes in Pichia pastoris by optimization of codons and fermentation, Appl. Microbiol. Biotechnol., 2008, vol. 78, no. 1, pp. 95–103. https://doi.org/10.1007/s00253-007-1290-4
Martinez, M.J., Reyes, F., Lahoz, R., and Perez-Leblic, M.I., Lytic enzymes in autolysis of Botrytis cinerea,FEMS Microbiol. Lett., 1983, vol. 19, nos. 2–3, pp. 157–160. https://doi.org/10.1111/j.1574-6968.1983.tb00532.x
Bang, M.L., Villadsen, I., and Sandal, T., Cloning and characterization of an endo-β-1,3 (4)glucanase and an aspartic protease from Phaffia rhodozyma CBS 6938, Appl. Microbiol. Biotechnol., 1999, vol. 51, no. 2, pp. 215–222.
Boyce, A. and Walsh, G., Production, purification and application-relevant characterisation of an endo-1,3 (4)-β-glucanase from Rhizomucor miehei,Appl. Microbiol.Biotechnol., 2007, vol. 76, no. 4, pp. 835–841. https://doi.org/10.1007/s00253-007-1058-x
Kawai, R., Igarashi, K., Yoshida, M., et al., Hydrolysis of β-1,3/1,6-glucan by glycoside hydrolase family 16 endo-1,3 (4)-β-glucanase from the basidiomycete Phanerochaete chrysosporium,Appl. Microbiol. Biotechnol., 2006, vol. 71, no. 6, pp. 898–906. https://doi.org/10.1007/s00253-005-0214-4
Martín-Cuadrado, A.B., Del Dedo, J.E., De Medina-Redondo, M., et al., The Schizosaccharomyces pombe endo-1,3-β-glucanase Eng1 contains a novel carbohydrate binding module required for septum localization, Mol. Microbiol., 2008, vol. 69, no. 1, pp. 188–200. https://doi.org/10.1111/j.1365-2958.2008.06275.x
Anderson, M.A. and Stone, B.A., A new substrate for investigating the specificity of β-glucan hydrolases, FEBS Lett., 1975, vol. 52, no. 2, pp. 202–207. https://doi.org/10.1016/0014-5793
Almirall, M. and Esteve-Garcia, E., In vitro stability of a β-glucanase preparation from Trichoderma longibrachiatum and its effect in a barley based diet fed to broiler chicks, Anim. Feed Sci. Technol., 1995, vol. 54, nos. 1–4, pp. 149–158. https://doi.org/10.1016/0377-8401(94)00757-Z
Choct, M., Enzymes supplementation of poultry diets based on viscous cereals, in Enzymes Farm Animal Nutrition, Bedford, M.R. and Partridge, G.G., Eds., Wallingford, UK: CAB Int., 2001, pp. 145–160.
Community Register of Feed Additives Pursuant to Regulation (EC) No. 1831/2003, Appendixes 3 and 4, Annex: List of Additives. European Commission 2007. http://ec.europa.eu/food.
Vahjen, W. and Simon, O., Biochemical characteristics of non-starch polysaccharide hydrolyzing enzyme preparations designed as feed additives for poultry and piglet nutrition, Arch. Anim. Nutrit., 1999, vol. 52, no. 1, pp. 1–14.
Bradford, M.M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding, Anal. Biochem., 1976, vol. 72, pp. 248–254.
Miller, G.L., Use of dinitrosalicylic acid reagent for determination of reducing sugar, Anal. Chem., 1959, vol. 31, no. 3, pp. 426–428.
Borriss, R., Buettner, K., and Maentsaelae, P., Structure of the beta-1,3-1,4-glucanase gene of Bacillus macerans: homologies to other beta-glucanases, Mol. Gen. Genet., 1990, vol. 222, nos. 2–3, pp. 278–283.
Wolf, M., Geczi, A., Simon, O., and Borriss, R., Genes encoding xylan and β-glucan hydrolysing enzymes in Bacillus subtilis: characterization, mapping and construction of strains deficient in lichenase, cellulase and xylanase, Microbiology, 1995, vol. 141, no. 2, pp. 281–290.
Hahn, M., Olsen, O., Politz, O., et al., Crystal structure and site-directed mutagenesis of Bacillus macerans endo-1,3- 1,4-glucanase, J. Biol. Chem., 1995, vol. 270, no. 7, pp. 3081–3088.
Gaiser, O.J., Piotukh, K., Ponnuswamy, M.N., et al., Structural basis for the substrate specificity of a Bacillus 1,3-1,4-β-glucanase, J. Mol. Biol., 2006, vol. 357, no. 4, pp. 1211–1225. https://doi.org/10.1016/j.jmb.2006.01.014
Cereghino, J.L. and Cregg, J.M., Heterologous protein expression in the methylotrophic yeast Pichia pastoris,FEMS Microbiol. Rev., 2000, vol. 24, no. 1, pp. 45–66. https://doi.org/10.1111/j.1574-6976.2000.tb00532.x
Wen, T.N., Chen, J.L., Lee, S.H., et al., A truncated Fibrobacter succinogenes 1,3-1,4-β-D-glucanase with improved enzymatic activity and thermotolerance, Biochemistry, 2005, vol. 44, no. 25, pp. 9197–9205. https://doi.org/10.1021/bi0500630
Bai, Y., Wang, J., Zhang, Z., et al., A novel family 9 β‑1,3 (4)-glucanase from thermoacidophilic Alicyclobacillus sp. A4 with potential applications in the brewing industry, Appl. Microbiol. Biotechnol., 2010, vol. 87, no. 1, pp. 251–259. https://doi.org/10.1007/s00253-010-2452-3
Teng, D., Fan, Y., Yang, Y.L., et al., Codon optimization of Bacillus licheniformis β-1,3-1,4-glucanase gene and its expression in Pichia pastoris,Appl. Microbiol. Biotechnol., 2007, vol. 74, no. 5, pp. 1074–1083. https://doi.org/10.1007/s00253-006-0765-z
Furtado, G.P., Ribeiro, L.F., Santos, C.R., et al., Biochemical and structural characterization of a β-1,3-1,4-glucanase from Bacillus subtilis 168, Process Biochem., 2011, vol. 46, pp. 1202–1206.
Masilamani, R., Sharma, OP., Muthuvel, S.K., and Natarajan, S., Cloning, expression of β-1,3-1,4 glucanase from Bacillus subtilis SU40 and the effect of calcium ion on the stability of recombinant enzyme: in vitro and in silico analysis, Bioinformation, 2013, vol. 9, no. 19, p. 958. https://doi.org/10.6026/97320630009958
Welfle, K., Politz, O., Borriss, R., Misselwitz, R., and Welfle, H., Individual amino acids in the N-terminal loop region determine the thermostability and unfolding characteristics of bacterial glucanases, Protein Sci., 1996, vol. 5, no. (11), pp. 2255–2265.
ACKNOWLEDGMENTS
The work was carried out on the equipment of the Multipurpose Scientific Installation of the All-Russia Collection of Industrial Microorganisms of the Kurchatov Institute National Resource Center (GOSNIIgenetika).
Funding
The work was financially supported by the Ministry of Science and Higher Education of the Russian Federation (Unique Project Identifier RFMEFI60717X0179).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
This article does not contain any studies involving animals performed by any of the authors.
This article does not contain any studies involving human participants performed by any of the authors.
Additional information
Translated by I. Gordon
Abbreviations: aa—amino acid residue(s); CL—culture liquid; CMC—carboxymethylcellulose; DNS—3,5-dinitrosalicylic acid; DNS method—method for xylanase activity measurement with DNS; EDTA—ethylendiamine tetraacetic acid; GH—glycosyl hydrolase; GH16—glycosyl hydrolase family 16; LB medium—lysogeny-broth medium; PAGE—polyacrylamide gel electrophoresis; PCR—polymerase chain reaction; SDS—sodium dodecyl(lauryl)sulfate; YPD medium—medium containing yeast extract, peptone, and dextrose.
Rights and permissions
About this article
Cite this article
Borshchevskaya, L.N., Gordeeva, T.L., Kalinina, A.N. et al. Expression of the β-Glucanase Gene from Paenibacillus jamilae Bg1 in Pichia pastoris and Characteristics of the Recombinant Enzyme. Appl Biochem Microbiol 56, 854–860 (2020). https://doi.org/10.1134/S0003683820080025
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683820080025