Abstract
Tacrolimus (TAC) is highly effective for the prevention of acute organ rejection. However, its clinical use may be challenging due to its large interindividual pharmacokinetic variability, which can be partially explained by genetic variations in TAC-metabolizing enzymes and transporters. The aim of this study was to evaluate the influence of genetic and clinical factors on TAC pharmacokinetic variability in 21 stable pediatric renal transplant patients. This study was nested in a previous Prograf to Advagraf conversion clinical trial. CYP3A5, ABCB1 and two POR genotypes were assessed by real-time PCR. The impact on TAC pharmacokinetics of individual genetic variants on CYP3A5 nonexpressors was evaluated by genetic score. Explicative models for TAC AUC0–24h, Cmax and Cmin after Advagraf were developed by linear regression. The built genetic scores explain 13.7 and 26.5% of the total AUC0–24h and Cmin total variability, respectively. Patients genetic information should be considered to monitorizate and predict TAC exposure.
Similar content being viewed by others
Introduction
Tacrolimus (TAC) is a calcineurin inhibitor highly effective in preventing acute organ rejection after transplantation.1, 2 However, TAC administration is complicated with side effects directly related to its drug blood concentrations such as nephrotoxicity, hypertension, hypercholesterolemia and diabetes mellitus.3, 4 This drug has a high grade of complexity on its use mainly due to its narrow therapeutic index and its high inter and intra subject pharmacokinetic (PK) variability, requiring therapeutic drug monitoring to optimize treatment1, 5, 6 and avoid graft loss and toxicity.7
However, despite therapeutic drug monitoring some transplant recipients experience TAC concentrations above or below the therapeutic range and therefore are either at a greater risk for toxicity (those with higher concentrations) or acute rejection (those with lower concentrations). TAC interindividual PK variability depends on many clinical co-variants such as serum levels of albumin, hematocrit and hemoglobin, concomitant use of drugs, post-operative time, as well as genetic factors.8, 9, 10, 11 TAC presents an extensive hepatic metabolism by cytochrome P450, and its bioavailability is also influenced by the multidrug resistance genotypes (MDR1 or ABCB1).12, 13 Single-nucleotide polymorphisms (SNPs) in the CYP3A5 gene explain 40–50% of TAC dose variability.14 In particular the rs776746 SNP (c.219-237A>G), also referred to as CYP3A5*3 allele, is a consistent predictor of TAC-dosing requirements. Homozygous carriers of the G allele of this SNP (CYP3A5 *3/*3) are referred to CYP3A5 nonexpressers in contrast to CYP3A5*1 carriers (known as CYP3A5 expressers) who show normal protein function and therefore lower trough concentrations related to higher metabolic rates.10, 15, 16, 17, 18, 19, 20, 21, 22 The CYP3A5*3 allele causes an abnormal spliced messenger RNA that results in protein truncation, a decrease of functional CYP3A5 enzyme and reduced TAC-dosing requirements.23, 24, 25
ABCB1 is thought to be responsible for the low oral bioavailability of TAC and is also involved in the distribution of TAC throughout the body and its excretion.14, 26, 27 However, associations between ABCB1 genotype and TAC PKs remain unclear. Some authors describe that there is no association between ABCB1 variations and TAC trough concentrations.28, 29, 30 However other groups found significant differences between patients with different ABCB1 genotypes.27, 31 Up to now, the most studied polymorphism affecting P-glycoprotein expression in human tissue is the silent mutation at position in exon 26 of the gene (c.3435 T>C rs1045642 SNP).32 The CC genotype has been related to a higher expression of P-glycoprotein in the small intestine compared with the TT genotype.33 Polymorphisms in the P450 oxireductase (POR) have been recently demonstrated to modulate the activity of P450 enzymes such as CYP1A2, CYP2C19 and CYP3A.34, 35 POR seems to be essential for CYP-mediated drug oxidation through electron donation. POR is highly polymorphic and more than 100 SNPs have been identified and linked to differential CYP activities. The rs1057868 (c.1508 C>T, POR*28) is the most common variant of the gene, and has been associated to a reduced protein activity in vitro.35 Homozygous POR*28 carriers (TT-expressers) require higher doses of TAC to maintain similar exposure to the drug when compared with wild-type patients (POR*1/*1).36 An association between POR rs2868177 and CYP activity has also been described as it is strongly related to warfarin maintenance dose variations.37
Our main objective was to identify and report the influence of CYP3A5, ABCB1 and two different POR polymorphisms on the PKs of TAC in order to describe a simple method for TAC AUC, Cmax and Cmin prediction in our study population.
Materials and methods
This study was nested in a previous Prograf to Advagraf conversion clinical trial in pediatric patients (EudraCT: 2009-017600-89).38
Twenty-one pediatric patients who underwent kidney transplantation and with stable TAC-based immunosuppressive treatment were included in this study (Table 1). All patients were transplanted at La Paz University Hospital in Madrid, which is a reference hospital for pediatric kidney transplantation. Written informed consent was obtained from all subjects or from their legal tutors. All of them continued with its regular TAC doses and were converted from Prograf to Advagraf following a 1:1 (mg: mg) daily dose relation. Twenty-four hours PK profiles at steady state were obtained for Prograf and Advagraf after 7 days administration. For the first 7 days patients received Prograf and then switched to the same dose of Advagraf.
All patients included in the study had stable renal function, followed stable TAC doses over the past 30 days and none of them had changes in co-medications that could modify TAC PKs.
Drug concentration measurement and AUC estimation
Advagraf blood samples were gathered before the beginning of the treatment and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 15 and 24 h after intake. Whole-blood concentrations were analyzed by enzyme immunoassay method made on the DIMENSION RXL platform (Siemens Healthcare, Erlangen, Germany). The lower and upper limits of quantification were 2 and 30 ng ml−1, respectively. The PK data analysis was performed following a non-compartmental model using WinNonlin Pro 2.0 software (Pharsight Corporation, Cary, NC, USA). AUC0–24 was calculated by the trapezoidal rule.
Genotyping assays
Blood samples were collected from each patient and DNA was extracted using a commercial extraction kit QuickGene DNA Whole Blood Kit S on a QuickGene-810 semiautomatic extractor (Fijifilm Corporation, Tokyo, Japan). All subjects were genotyped for CYP3A5*3 c.219-237A>G (rs776746), ABCB1 c.3435C>T (rs1045642) and two SNPs: c.1508C>T (POR*28, rs1057868) and c.188+6405A>G (rs2868177) in the POR gene using commercial RT-PCR Taqman assays following the manufacturer recommendations (Applied Biosystems, Foster City, CA, USA).
Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics 19.0 (SPSS: an IBM company, IBM Corporation, Armonk, NY, USA). The differences between genotype groups for each genetic variant were assessed by the nonparametric Jonckheere–Terpstra trend test. The results were considered significant when P-values were lower than 0.05. No adjustments for multiple comparisons were performed.
Multivariate linear regression was used to assess the impact of clinical, demographic, concomitant medication and genetic covariates on subject variability of dose- and body weight-adjusted Advagraf PK parameters AUC, Cmax and Cmin.
Genetic score
Aiming to increase the sensibility to detect the influence of genetic on PK disposition factors in our population a genetic score was built. In order to evaluate the impact of CYP3A5 SNPs on TAC PK parameters the study sample was divided in two groups: CYP3A5 expressers (*1/*1 or *1/*3) and CYP3A5 nonexpressers (*3/*3). Due to the reduced sample size and the physiological plausibility, the contribution of the genetic variants in ABCB1 and POR on TAC PKs was only evaluated in CYP3A5 nonexpressors. An additive genetic score was built using the analysed genetic variants in ABCB1 (rs1045642) and POR (rs1057868 and rs2868177) genes. Three genetic groups were delimited to build the score: one with no variant alleles, another with one or two and a third group with three variant alleles. The differences between CYP3A5 expressors and nonexpressors, as well as between the three genetic score groups were assessed by the nonparametric Jonckheere–Terpstra trend test.
Model building
Two different linear regression models were built. One model including all subjects and a second model for CYP3A5 nonexpressors. Genetic and nongenetic (race, body weight, body height, age, sex, body mass index, body surface area (BSA), hemoglobin, hematocrit, albumin and concomitant treatments) covariates were tested in a step-wise manner. Covariates removal was also performed in a step by step way. To evaluate the bias and precision of our model we calculated dose-/weight-adjusted AUC0–24h and dose-/weight-adjusted Cmin (predicted values) in our patients and we compared them with the real ones, determining mean error, mean absolute error and mean absolute error as a percentage of the real value.
All models were built using dose/weight AUC0–24h, Cmax and Cmin with logarithmic transformation in order to assure a normal distribution of dependant variables.
Same procedures were followed for Prograf before the conversion to Advagraf.
Results
Study population characteristics
Twenty-one stable kidney-transplanted children aged between 4 and 17 years were included in the study. Most of them were Caucasian males. Study population characteristics are shown in Table 1. Allelic frequencies for CYP3A5, ABCB1 and POR SNPs in our study population are also shown in Table 1. All genetic variants are in Hardy–Weinberg equilibrium.
Pharmacokinetic and pharmacogenetic results
For this analysis we selected AUC0–24h, Cmax and Cmin as the main PK parameters adjusted by daily dose administered and body weight.
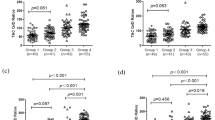
As shown in Table 2 and Figure 1 the univariate analysis found significant differences in AUC0–24h, Cmax and Cmin between different genotypes of CYP3A5 (CYP3A5*3, rs776746). CYP3A5 nonexpressors (CYP3A5*3/*3, n=17) presented a Cmax, AUC0–24h and Cmin 72, 119 and 210% higher than expressors (CYP3A5*1/*1 or *1/*3). No other significant differences were observed.
Pharmacokinetic parameters in CYP3A5 (CYP3A5*1*1 or*1/*3 and CYP3A5*3/*3) genotypes (n=21). (a) Dose-/weight-adjusted AUC0–24h. (b) Dose-/weight-adjusted Cmax. (c) Dose-/weight-adjusted Cmin.
In order to evaluate the contribution of the genetic variants in ABCB1 and POR genes we selected the 17 CYP3A5 nonexpressors (CYP3A5*3/*3) and built a three-group additive genetic score. The genetic score groups includes subjects with 0 (group 1, reference), 1 or 2 (group 2) SNPs in POR, and 3 (group 3) variants in ABCB1 and POR genes. Mean values for TAC PK parameters for these three groups are shown in Table 3. Group 2 (carriers of 1 or 2 POR genes variant alleles) have a Cmax, AUC0–24h and Cmin 43, 27 and 11% higher than subjects in group 1, respectively (Table 3 and Figure 2). The Cmax, AUC0–24h and Cmin observed in group 3 are 94, 82 and 68% higher than subjects without variant alleles (group 1), respectively (Table 3). Comparing the AUC0–24h, Cmax and Cmin values of each group we can find significant differences between them (P=0.018, P=0.037 and P=0.018, respectively) (Table 4).
Pharmacokinetic parameters by genetic score (n=17). (a) Dose-/weight-adjusted AUC0–24h. (b) Dose-/weight-adjusted Cmax. (c) Dose-/weight-adjusted Cmin.
Explicative models
A multivariate linear regression was used to evaluate the contribution of genetic and nongenetic factors to daily dose and body weight-adjusted Cmax, AUC0–24h and Cmin variability. In the model including the whole-study population (n=21) the three retained covariates were: CYP3A5 rs776746 SNP and Deflazacort and methylprednisolone co-medications. CYP3A5 nonexpressors (CYP3A5*3/*3) showed increased TAC PK parameters and CYP3A5 genotype explained 39.6, 20.6 and 59.5% of AUC0–24h, Cmax and Cmin variability, respectively (Table 5). Deflazacort and methylprednisolone co-medications were related to decreased TAC PK parameters. Deflazacort explained 20.9 and 19.5% of AUC0–24h and Cmax variability, respectively, and methylprednisolone explained 14.3% of the AUC0–24h variability (Table 5).
A second model was built for the subgroup of CYP3A5 nonexpressors (CYP3A5 *3/*3, n=17) in order to assess the effect of the genetic variants in ABCB1 and POR genes through a genetic score. Genetic variants explained 13.7% of total AUC0–24h variability. On the other hand, Deflazacort and methylprednisolone co-medications explained 32 and 11.1% of total AUC0–24h variability. The genetic score was the only significant covariate in case of Cmin explaining 26.5% of its variability. None of the genetic and nongenetic covariates included in this study were found to explain a significant part of Cmax variability.
Model evaluation
Using the developed models for daily dose and body weight-adjusted Cmax, AUC0–24h and Cmin the mean predicted absolute error for each model was calculated. The mean predicted absolute error expressed as percentage for Cmax, AUC0–24h and Cmin is shown in Table 5. Similar results were found for the Prograf formulation and are presented in Supplementary Material.
Discussion
Optimization of therapeutic strategies through individual genetic information can maximize therapeutic efficacy and reduce adverse drug reactions,39, 40 thus contributing to the development of personalized medicine.
TAC shows a large interindividual variation in oral bioavailability ranging from 4 to 89%.7 A significant amount of this variability is explained by genetic variants affecting the drug metabolizing enzymes CYP3A4/5, genes regulating their activity as well as those genetic variants in cellular transporters as ABCB1.10, 11, 13, 23, 41
The aim of this project was to explore the impact of genetic and clinical factors in the exposure to TAC in kidney-transplanted children. As far as we know this is the first study assessing the joint influence of CYP3A5 (rs776746), POR (rs1057868 POR*28 and rs2868177) and ABCB1 (rs1045642) in the disposition of TAC after the administration of both Prograf and Advagraf. Advagraf results are reported in the main body of the article. Information about the Prograf study is reported in the Supplementary Material.
In a first univariate analysis with all 21 patients (Table 2) we found that the only genetic variant significantly related to TAC disposition is the rs776746 SNP (CYP3A5*3 allele) affecting all three PK parameters analysed (dose weight-adjusted AUC0–24, Cmax and Cmin) and showing a clear gene–dose response. This confirms the major impact of this polymorphism in the disposition of TAC. This fact has been well described in the literature.10, 11, 13, 15, 16, 17, 19, 20
We therefore performed a multivariate analysis including all the patients (n=21) and found that CYP3A5*3 genotype explains 40, 21 and 60% of variability in daily dose and body weight-adjusted AUC0–24h, Cmax and Cmin, respectively, in stable renal transplant children receiving one-daily TAC formulation (Advagraf) (Table 5). Similar results were found for Prograf (see Supplementary Table 1S to Supplementary Table 3S presented in Supplementary Material). These findings are in agreement with previous published results by de Jonge et al.42 in adult stable Prograf-treated renal allograft recipients, where CYP3A5*3 (rs776746) explains more than 29% of the TAC dose requirements. The well-known CYP3A activity inducers Deflazacort and methylprednisolone were the only nongenetic covariates retained in the model. Deflazacort and methylprednisolone explain 21 and 14% of variability in adjusted AUC0–24h, respectively (Table 5). Only Deflazacort was retained in the case of Cmax, explaining 20% of its variability. Cmin variability was not influenced by these co-medications. No other clinical, biochemical or demographic variables explain a significant part of TAC exposure variability. Some authors have described that hematocrit could explain up to 14 and 11% of TAC clearance and dose-adjusted AUC0–12h, respectively.42 The discrepancy found in our study may be due to the reduced sample size and low variability in the hematocrit values among our patients (CV%=9.4%).
Recently CYP3A4*22 has been described as a determinant genetic factor influencing TAC total exposure, as this variant increases the formation of the non-functional CYP3A4 splice variant.3, 14, 42, 43 We did not evaluate these variants as we considered that its low frequency (minor allele frequency=0.025) made it very unlikely to be found in our cohort due to the number of patients included (we would require at least twice the patients to find more than 1 carrier). We therefore assumed that all patients were wildtype for CYP3A4 (CYP3A4*1/*1) for the interpretation and evaluation of our results.
The influence of ABCB1 polymorphisms on TAC PKs has been extensively investigated; however the results are still controversial.27, 30 Rong et al.44 describe that ABCB1 variants have no effect on TAC exposure, whereas some others found that patients homozygous for allele C (rs1045642) would require higher daily doses of TAC to obtain levels into the therapeutic range when compared with the T allele carriers.26 In our determinations we haven’t found any significant difference between the different ABCB1 (rs1045642) genotypes (Table 2).
POR is a membrane-bound coenzyme that functions as an electron donor for the CYP enzymes, therefore genetic variability in this gene may be related to CYP3A enzymatic activity variations. De Jongue et al.36 and Gijsen et al.45 found that POR*28 T allele carriers had significantly higher TAC dose requirements in CYP3A5 expressors (CYP3A5*1 carriers) but not in CYP3A5 nonexpressers (CYP3A5*3/*3) However, Elens et al.41 found that POR*28 homozygosity (CC) was related to a significant higher CYP3A4 activity in CYP3A5 nonexpressors for TAC metabolism. We found no significant differences in dose weight-adjusted AUC0–24, Cmax or Cmin in patients with different POR*28 genotypes (P=0.178, P=0.512 and P=0.388, respectively) but like Elens et al.41 we did find an increasing trend in these parameters in POR*28 carriers (Table 2). Recently, an intronic polymorphism (rs2868177) in POR gene was related to higher warfarin maintenance dose needs (mutated patients require lower doses)37 but in a univariate analysis we found no significant relation between the TAC PK parameters and the different POR rs2868177 genotypes (Table 2).
Figure 1 shows there is a great variability in the TAC PK parameters dose-adjusted AUC0–24 and Cmax mainly in CYP3A5 nonexpressors (CYP3A5*3/*3). In addition, due to the majority of CYP3A5 nonexpressors in Caucasian population we decided to study this variability only in this group of patients (n=17).
To increase the sensibility of our statistical analysis we developed an additive genetic score using the analysed genetic variants in ABCB1 (rs1045642) and POR (rs1057868 and rs2868177) and we found significant differences in TAC PK parameters between different ABCB1 and POR genotypes (Table 4 and Figure 2).
By applying the built genetic score in a multivariate analysis we found that this variable explains 14 and 27% of the daily dose and body weight-adjusted AUC0–24h and Cmin, variability, respectively (Table 5). The concomitant administration of Deflazacort and methylprednisolone explained 32 and 11% of daily dose and body weight-adjusted AUC0–24h variability (Table 5).
To our knowledge, this is the first multivariate model developed, for pediatric population with kidney transplant, for daily dose and body weight-adjusted TAC PK parameters. The mean absolute error of all models is lower than 28% indicating that if these results are replicated in another independent pediatric population the models could be used in clinics to improve TAC exposure prediction.
Similar results have been found for the Prograf formulation (Supplementary Material). This study confirmed that CYP3A5 genetic variants have a major effect in TAC-dosing requirements, with CYP3A5 genotype explaining ~21% of Cmax and 60% of Cmin variability. Nonetheless, our results show that some previously reported SNPs in ABCB1 (rs1045642) and POR (rs1057868 and 2868177) may explain residual variability in response to TAC in CYP3A5 nonexpressors and shouldn't be underestimated when evaluating TAC exposure.
References
Scott LJ, McKeage K, Keam SJ, Plosker GL . Tacrolimus: a further update of its use in the management of organ transplantation. Drugs 2003; 63: 1247–1297.
First MR . Tacrolimus based immunosuppression. J Nephrol 2004; 17 (Suppl 8): S25–S31.
Kershner RP, Fitzsimmons WE . Relationship of FK506 whole blood concentrations and efficacy and toxicity after liver and kidney transplantation. Transplantation 1996; 62: 920–926.
Laskow DA, Vincenti F, Neylan JF, Mendez R, Matas AJ . An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: a report of the United States Multicenter FK506 Kidney Transplant Group. Transplantation 1996; 62: 900–905.
Felipe CR, Silva HT, Machado PG, Garcia R, da Silva Moreira SR, Pestana JO . The impact of ethnic miscegenation on tacrolimus clinical pharmacokinetics and therapeutic drug monitoring. Clin Transplant 2002; 16: 262–272.
Taylor AL, Watson CJ, Bradley JA . Immunosuppressive agents in solid organ transplantation: mechanisms of action and therapeutic efficacy. Crit Rev Oncol Hematol 2005; 56: 23–46.
Venkataramanan R, Swaminathan A, Prasad T, Jain A, Zuckerman S, Warty V et al. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet 1995; 29: 404–430.
Undre NA, van Hooff J, Christiaans M, Vanrenterghem Y, Donck J, Heeman U et al. Low systemic exposure to tacrolimus correlates with acute rejection. Transplant Proc 1999; 31: 296–298.
Kuypers DR, Claes K, Evenepoel P, Maes B, Coosemans W, Pirenne J et al. Time-related clinical determinants of long-term tacrolimus pharmacokinetics in combination therapy with mycophenolic acid and corticosteroids: a prospective study in one hundred de novo renal transplant recipients. Clin Pharmacokinet 2004; 43: 741–762.
Mourad M, Mourad G, Wallemacq P, Garrigue V, Van Bellingen C, Van Kerckhove V et al. Sirolimus and tacrolimus trough concentrations and dose requirements after kidney transplantation in relation to CYP3A5 and MDR1 polymorphisms and steroids. Transplantation 2005; 80: 977–984.
Mourad M, Wallemacq P, De Meyer M, Brandt D, Van Kerkhove V, Malaise J et al. The influence of genetic polymorphisms of cytochrome P450 3A5 and ABCB1 on starting dose- and weight-standardized tacrolimus trough concentrations after kidney transplantation in relation to renal function. Clin Chem Lab Med 2006; 44: 1192–1198.
Anglicheau D, Verstuyft C, Laurent-Puig P, Becquemont L, Schlageter MH, Cassinat B et al. Association of the multidrug resistance-1 gene single-nucleotide polymorphisms with the tacrolimus dose requirements in renal transplant recipients. J Am Soc Nephrol 2003; 14: 1889–1896.
Roy JN, Barama A, Poirier C, Vinet B, Roger M . Cyp3A4, Cyp3A5, and MDR-1 genetic influences on tacrolimus pharmacokinetics in renal transplant recipients. Pharmacogenet Genomics 2006; 16: 659–665.
Tang JT, Andrews LM, van Gelder T, Shi YY, van Schaik RH, Wang LL et al. Pharmacogenetic aspects of the use of tacrolimus in renal transplantation: recent developments and ethnic considerations. Expert Opin Drug Metab Toxicol 2016; 12: 555–565.
Thervet E, Anglicheau D, King B, Schlageter MH, Cassinat B, Beaune P et al. Impact of cytochrome p450 3A5 genetic polymorphism on tacrolimus doses and concentration-to-dose ratio in renal transplant recipients. Transplantation 2003; 76: 1233–1235.
Zheng H, Webber S, Zeevi A, Schuetz E, Zhang J, Bowman P et al. Tacrolimus dosing in pediatric heart transplant patients is related to CYP3A5 and MDR1 gene polymorphisms. Am J Transplant 2003; 3: 477–483.
Goto M, Masuda S, Kiuchi T, Ogura Y, Oike F, Okuda M et al. CYP3A5*1-carrying graft liver reduces the concentration/oral dose ratio of tacrolimus in recipients of living-donor liver transplantation. Pharmacogenetics 2004; 14: 471–478.
MacPhee IA, Fredericks S, Tai T, Syrris P, Carter ND, Johnston A et al. The influence of pharmacogenetics on the time to achieve target tacrolimus concentrations after kidney transplantation. Am J Transplant 2004; 4: 914–919.
Tsuchiya N, Satoh S, Tada H, Li Z, Ohyama C, Sato K et al. Influence of CYP3A5 and MDR1 (ABCB1) polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplantation 2004; 78: 1182–1187.
Zheng H, Zeevi A, Schuetz E, Lamba J, McCurry K, Griffith BP et al. Tacrolimus dosing in adult lung transplant patients is related to cytochrome P4503A5 gene polymorphism. J Clin Pharmacol 2004; 44: 135–140.
Macphee IA, Fredericks S, Mohamed M, Moreton M, Carter ND, Johnston A et al. Tacrolimus pharmacogenetics: the CYP3A5*1 allele predicts low dose-normalized tacrolimus blood concentrations in whites and South Asians. Transplantation 2005; 79: 499–502.
Numakura K, Satoh S, Tsuchiya N, Horikawa Y, Inoue T, Kakinuma H et al. Clinical and genetic risk factors for posttransplant diabetes mellitus in adult renal transplant recipients treated with tacrolimus. Transplantation 2005; 80: 1419–1424.
Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet 2001; 27: 383–391.
Lamba JK, Lin YS, Schuetz EG, Thummel KE . Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev 2002; 54: 1271–1294.
Op den Buijsch RA, Christiaans MH, Stolk LM, de Vries JE, Cheung CY, Undre NA et al. Tacrolimus pharmacokinetics and pharmacogenetics: influence of adenosine triphosphate-binding cassette B1 (ABCB1) and cytochrome (CYP) 3A polymorphisms. Fundam Clin Pharmacol 2007; 21: 427–435.
Ayrton A, Morgan P . Role of transport proteins in drug absorption, distribution and excretion. Xenobiotica 2001; 31: 469–497.
Yu X, Xie H, Wei B, Zhang M, Wang W, Wu J et al. Association of MDR1 gene SNPs and haplotypes with the tacrolimus dose requirements in Han Chinese liver transplant recipients. PLoS One 2011; 6: e25933.
Santoro A, Felipe CR, Tedesco-Silva H, Medina-Pestana JO, Struchiner CJ, Ojopi EB et al. Pharmacogenetics of calcineurin inhibitors in Brazilian renal transplant patients. Pharmacogenomics 2011; 12: 1293–1303.
Shi XJ, Geng F, Jiao Z, Cui XY, Qiu XY, Zhong MK . Association of ABCB1, CYP3A4*18B and CYP3A5*3 genotypes with the pharmacokinetics of tacrolimus in healthy Chinese subjects: a population pharmacokinetic analysis. J Clin Pharm Ther 2011; 36: 614–624.
Kim IW, Noh H, Ji E, Han N, Hong SH, Ha J et al. Identification of factors affecting tacrolimus level and 5-year clinical outcome in kidney transplant patients. Basic Clin Pharmacol Toxicol 2012; 111: 217–223.
Hawwa AF, McKiernan PJ, Shields M, Millership JS, Collier PS, McElnay JC . Influence of ABCB1 polymorphisms and haplotypes on tacrolimus nephrotoxicity and dosage requirements in children with liver transplant. Br J Clin Pharmacol 2009; 68: 413–421.
Lopez-Montenegro Soria MA, Kanter Berga J, Beltran Catalan S, Milara Paya J, Pallardo Mateu LM, Jimenez Torres NV . Genetic polymorphisms and individualized tacrolimus dosing. Transplant Proc 2010; 42: 3031–3033.
Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA 2000; 97: 3473–3478.
Murakami M . Rupture of renal arterial aneurysm in a pregnant patient. Masui 1993; 42: 1367–1370.
Agrawal V, Huang N, Miller WL . Pharmacogenetics of P450 oxidoreductase: effect of sequence variants on activities of CYP1A2 and CYP2C19. Pharmacogenet Genomics 2008; 18: 569–576.
de Jonge H, Metalidis C, Naesens M, Lambrechts D, Kuypers DR . The P450 oxidoreductase *28 SNP is associated with low initial tacrolimus exposure and increased dose requirements in CYP3A5-expressing renal recipients. Pharmacogenomics 2011; 12: 1281–1291.
Zhang X, Li L, Ding X, Kaminsky LS . Identification of cytochrome P450 oxidoreductase gene variants that are significantly associated with the interindividual variations in warfarin maintenance dose. Drug Metab Dispos 2011; 39: 1433–1439.
Carcas-Sansuan AJ, Espinosa-Roman L, Almeida-Paulo GN, Alonso-Melgar A, Garcia-Meseguer C, Fernandez-Camblor C et al. Conversion from Prograf to Advagraf in stable paediatric renal transplant patients and 1-year follow-up. Pediatr Nephrol 2014; 29: 117–123.
Eichelbaum M, Ingelman-Sundberg M, Evans WE . Pharmacogenomics and individualized drug therapy. Annu Rev Med 2006; 57: 119–137.
Meyer UA . Pharmacogenetics - five decades of therapeutic lessons from genetic diversity. Nat Rev Genet 2004; 5: 669–676.
Elens L, Hesselink DA, Bouamar R, Budde K, de Fijter JW, De Meyer M et al. Impact of POR*28 on the pharmacokinetics of tacrolimus and cyclosporine A in renal transplant patients. Ther Drug Monit 2014; 36: 71–79.
de Jonge H, de Loor H, Verbeke K, Vanrenterghem Y, Kuypers DR . In vivo CYP3A4 activity, CYP3A5 genotype, and hematocrit predict tacrolimus dose requirements and clearance in renal transplant patients. Clin Pharmacol Ther 2012; 92: 366–375.
Elens L, Capron A, van Schaik RH, De Meyer M, De Pauw L, Eddour DC et al. Impact of CYP3A4*22 allele on tacrolimus pharmacokinetics in early period after renal transplantation: toward updated genotype-based dosage guidelines. Ther Drug Monit 2013; 35: 608–616.
Rong G, Jing L, Deng-Qing L, Hong-Shan Z, Shai-Hong Z, Xin-Min N . Influence of CYP3A5 and MDR1(ABCB1) polymorphisms on the pharmacokinetics of tacrolimus in Chinese renal transplant recipients. Transplant Proc 2010; 42: 3455–3458.
Gijsen VM, van Schaik RH, Soldin OP, Soldin SJ, Nulman I, Koren G et al. P450 oxidoreductase *28 (POR*28) and tacrolimus disposition in pediatric kidney transplant recipients—a pilot study. Ther Drug Monit 2014; 36: 152–158.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the The Pharmacogenomics Journal website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Almeida-Paulo, G., Dapía García, I., Lubomirov, R. et al. Weight of ABCB1 and POR genes on oral tacrolimus exposure in CYP3A5 nonexpressor pediatric patients with stable kidney transplant. Pharmacogenomics J 18, 180–186 (2018). https://doi.org/10.1038/tpj.2016.93
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2016.93
- Springer Nature Limited