Abstract
The basic function of the tongue in pronouncing diadochokinesis and other syllables is not fully understood. This study investigates the influence of sound pressure levels and syllables on tongue pressure and muscle activity in 19 healthy adults (mean age: 28.2 years; range: 22–33 years). Tongue pressure and activity of the posterior tongue were measured using electromyography (EMG) when the velar stops /ka/, /ko/, /ga/, and /go/ were pronounced at 70, 60, 50, and 40 dB. Spearman's rank correlation revealed a significant, yet weak, positive association between tongue pressure and EMG activity (ρ = 0.14, p < 0.05). Mixed-effects model analysis showed that tongue pressure and EMG activity significantly increased at 70 dB compared to other sound pressure levels. While syllables did not significantly affect tongue pressure, the syllable /ko/ significantly increased EMG activity (coefficient = 0.048, p = 0.013). Although no significant differences in tongue pressure were observed for the velar stops /ka/, /ko/, /ga/, and /go/, it is suggested that articulation is achieved by altering the activity of both extrinsic and intrinsic tongue muscles. These findings highlight the importance of considering both tongue pressure and muscle activity when examining the physiological factors contributing to sound pressure levels during speech.
Similar content being viewed by others
Introduction
Decline in tongue and lip motor function can result from decreased perioral muscle function and brain function due to systemic disease and changes with aging1. Oral diadochokinesis has long been used to assess tongue and lip motor function2 and has been applied for in older patients3,4,5 and patients with neurogenic dysarthria6, amyotrophic lateral sclerosis7, and organic disorders of the tongue8. Oral diadochokinesis, a diagnostic method for oral hypofunction in Japan, involves the repeated pronunciations of three syllables: /pa/, /ta/, and /ka/. In the oral diadochokinesis test, the number of syllables pronounced per second is measured. If any syllable is pronounced less than six times per second, it may indicate reduced lip movement and the anterior and posterior tongue motor functions, or dexterity1. The symbols /t/ and /k/ are international phonetic letters defined by the International Phonetic Association, where /t/ represents a voiceless dental and alveolar plosive and /k/ is a voiceless velar stop9. Decreased tongue-lip motor function test for oral hypofunction measures anterior tongue function with /ta/ pronunciation and posterior tongue function with /ka/ pronunciation.
Oral diadochokinesis utilizes three consonants, /p/, /t/, and /k/, whereas only /a/ is used for vowels. Japanese /a/ is a low, central and /o/ is a back vowel10. In back vowels such as /o/, the tongue is compressed in the anteroposterior direction to elevate the posterior part of the tongue, and the tongue is reported to spread more laterally compared with front vowels11. The posterior part of the tongue contains slower muscle fibers (type I) than the anterior part12, which may make it challenging to perform instantaneous repetitive movements. One study conducted in Japan showed that the mean value of /ka/ in oral diadochokinesis tests in the elderly did not meet the reference value13. Given this, an examination of basic tongue function during pronunciation of the syllables used in oral diadochokinesis and other syllables is needed. We have previously studied an electromyographic method for recording muscle activity during posterior tongue elevation during mastication and swallowing14. This method allows non-invasive and easy evaluation of the function of the posterior tongue. Although this method is not an EMG measurement method developed in the field of Speech-Language Pathology, we thought that this method has the potential to provide new insights.
So, this study aimed to examine the basic function of the tongue during pronunciation of various syllables and at various sound pressure levels. We investigated the effects of sound pressure levels and syllables on tongue pressure and muscle activity using EMG. The null hypothesis was that sound pressure levels and syllables do not affect tongue pressure and muscle activity.
Method of research
This study was conducted per the Declaration of Helsinki of 1975, revised in 2013, and was approved by the Ethics Committee of the Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences and Okayama University Hospital (Approval No.1709–008). This study was performed according to the STROBE guidelines. Written informed consent was obtained from all participants.
Participants
Twenty healthy native Japanese speakers were recruited for the study. The inclusion criteria included: (1) no abnormalities in maxillofacial function, dysphagia, or dysarthria; and (2) provision of written consent after receiving a detailed explanation of the study and demonstration of a clear understanding of their involvement. The exclusion criteria were as follows: (1) diagnosis of temporomandibular disorders after recording the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) questionnaire; (2) dysarthria due to dental or mucosal damage; or (3) periodontal, or psychiatric or pulmonary disease (self-reported). To confirm inclusion and exclusion criteria, DC/TMD questionnaire recording, oral examination, periodontal disease examination, and oral diadochokinesis were performed. Informed consent was obtained from all participants.
Electromyography (EMG) measurement
In this study, muscle activity associated with the movement of the posterior part of the tongue was recorded using the electromyography method described by Manda et al.14. EMG electrodes were placed on both sides of the neck, in the area bordered by the posterior border of the mylohyoid muscle, anterior border of the sternocleidomastoid muscle, and inferior border of the mandible.
The EMG system used in this study consisted an analog signal processor and a differential amplification integrated hybrid circuit (NB-6201HS; Nabtesco Co., Tokyo, Japan), including a high-pass (10 Hz) and low-pass filter (1000 Hz). A differential electrode with a center-to-center distance of 8 mm was used, consisting of three disposable Ag/AgCl surface electrodes (SMP-300, Metz, Blumberg, Germany). The electrodes and cables were fixed to the skin using a thin biocompatible adhesive tape (Cathereep FS 1010; Nichiban Corporation, Tokyo, Japan). The EMG signals were recorded using a digital recorder (IC RR-XS455; Panasonic Co., Osaka, Japan) at a sampling rate of 22.05 kHz and stored on a digital (SD) memory card. The obtained EMG data were down-sampled to 100 Hz after offline full-wave rectification.
Tongue pressure measurement
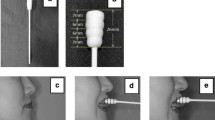
The tongue pressure measurement method was based on previous studies that measured tongue pressure by placing pressure sensors on the palate15,16. We used a pressure sensor (PSM-1KAB, Kyowa Electronic Instruments Co., Ltd., Tokyo, Japan) to measure tongue pressure during pronunciation (Fig. 1A). The sensor had a 3.5-mm diameter, 0.65-mm-thick sensor part, and a 0.25-mm diameter cable. To waterproof the sensor, the intraoral insertion site was covered with Parafilm (Parafilm; Bemis Flexible Packaging, Chicago, IL, USA) and placed on the palate with sheet denture stabilizer (Touch Correct II, Shionogi Corp., Osaka, Japan). The cables were routed out of the mouth through the oral vestibule to avoid interfering with tongue movements and connected to a PC. The sensors were affixed to the left and right sides of the palate. Their position was determined by having the test participants pronounce /ka/, /ko/, /ga/, and /go/ and visually confirming the position where the posterior part of the tongue contacted the palate. Prior to the pronunciation task, it was confirmed that the pressure waveform data were obtained when the test sounds were pronounced. Tongue pressure data was recorded at 100 Hz and synchronized offline using a trigger switch with EMG data.
Experimental procedure
The velar stops, /k/ and /g/, were used as test sounds. The vowels used were /a/, used in oral diadochokinesis, and /o/, a back vowel that involves raising the back part of the tongue, and the four syllables employed were, /ka/, /ko/, /ga/, and /go/. To examine the impact of tongue pressure and EMG on sound pressure level, the participants were instructed to pronounce each syllable 10 times at 70, 60, 50, and 40 dB. All measurements were performed in a shield room with the participant’s head fixed to a headrest in a Frankfurt plane position, parallel to the ground. To account for the potential influence of fatigue, we provided a one-minute rest period between each task. Furthermore, the order of the tasks was randomized. A digital sound-level meter (TESTO816; Testo SE & Co., Titisee–Neustadt, Germany) was placed 1 m away from the participant’s mouth, parallel to the ground, and the baseline of the sound-level meter was confirmed to be 20 ± 5 dB before starting each experimental task (Fig. 1B). The values displayed on the sound-level meter were fed back to the participants, who were instructed to pronounce at the specified sound pressure level. Prior to the experimental tasks, participants were trained to produce each syllable at the specified sound pressure levels (70, 60, 50, and 40 dB) using a sound-level meter. During the measurements, the participants were provided with real-time feedback from the sound-level meter and instructed to match the target sound pressure level for each production. This procedure was implemented to minimize the potential for errors in producing the syllables at different sound pressure levels.
Data analyses
Tongue pressure and EMG data during pronunciation were output as CSV files and synchronized offline using MATLAB 9.9.0.1857802 R2020b Update 7 (MathWorks, Natick, MA, USA) based on the examiner’s trigger signal. The peak values of tongue pressure and muscle activity for each syllable and sound pressure level were analyzed. The EMG data were normalized with reference to the highest muscle activity observed during pronunciation and expressed as percentages.
Statistical analysis
We used G*Power 3.1.1417 to determine the appropriate sample size. Our previous study has found a moderate correlation between the force to elevate the posterior tongue and the amount of muscle activity14. Based on this study, we expected a somewhat stronger association between tongue pressure, EMG, and sound pressure level and set the effect size at 40% to account for participant recruitment and test feasibility. The results revealed that at least 16 subjects were required to achieve 95% statistical power at a significance level of 0.05. Syllables, tongue pressure data, EMG activity data, and sound pressure levels were used for statistical analysis. The normality of the data was examined using the Shapiro–Wilk test. As normality was not found in some of the data, the following statistical analysis procedures were performed.
First, Spearman's rank correlation coefficient was used to assess the association between sound pressure level, tongue pressure, and EMG. Next, mixed-effects models were constructed to evaluate the effect of syllable and sound pressure on tongue pressure and muscle activity. In the mixed-effects models, the syllables were treated as fixed effects with “ka” set as the reference category, and the sound pressure levels were treated as fixed effects with 40 dB set as the reference category. Subjects were treated as random effects. After fitting the mixed-effects models, pairwise comparisons between sound pressure levels were conducted, and the Bonferroni correction was applied to account for multiple comparisons.
Results
One participant with a history of TMD was excluded; therefore, 19 healthy adults (10 men and 9 women; mean age 28.2 years; range: 22–33 years) were included. No participants mentioned fatigue during the experiment. The results of the evaluation of the association between sound pressure level, tongue pressure, and EMG are presented in Table 1. Spearman's rank correlation coefficient showed significant positive correlations between sound pressure level and EMG, sound pressure level and tongue pressure, and tongue pressure and EMG. Compared to the correlation coefficients between sound pressure and tongue pressure (0.30, p < 0.01) and between sound pressure and EMG (0.42, p < 0.01), the correlation coefficient between tongue pressure and EMG was small (0.14, p < 0.05). In our previous study, we found a moderate correlation between tongue pressure and muscle activity during simple elevation movements of the posterior tongue. However, in the current study, although there was a significant correlation between tongue pressure and muscle activity, the correlation coefficient was very small. Therefore, considering the possibility that tongue pressure and muscle activity may be influenced by different factors, we decided to analyze them as separate outcomes.
The result of mixed-effects model analyses to evaluate the effects of syllable and sound pressure on tongue pressure and muscle activity are presented in Tables 2 and 3. Regarding the effects on tongue pressure, sound pressure showed a significant positive effect (coefficient = 0.051, p < 0.01). In contrast, syllable type did not significantly affect tongue pressure (p > 0.05). The random effect of subjects was significant, indicating the importance of considering individual differences. Concerning the effects on muscle activity, sound pressure showed a significant positive effect (coefficient = 0.0057, p < 0.01). Regarding syllable type, “ko” (coefficient = 0.048, p = 0.013) showed a significant positive effect on muscle activity, while “ga” (coefficient = − 0.011, p = 0.569) and “go” (coefficient = 0.034, p = 0.081) did not have significant effects. The random effect of subjects was also significant, indicating the importance of considering individual differences.
After evaluating the effects of sound pressure level on tongue pressure and muscle activity using mixed-effects models, pairwise comparisons between sound pressure levels were performed, and the Bonferroni correction was applied (Tables 4 and 5). For tongue pressure, there was no significant difference between sound pressure levels 50 dB and 40 dB (corrected p = 1.00) or between 60 and 50 dB (corrected p = 0.21). However, a statistically significant difference in tongue pressure was observed between sound pressure levels 70 dB and 60 dB (corrected p < 0.01). Overall, sound pressure level was found to have a statistically significant effect on tongue pressure (p < 0.01).
For muscle activity, there was no significant difference between sound pressure levels 50 dB and 40 dB (corrected p = 0.24) or between 60 and 50 dB (corrected p = 0.42). However, a statistically significant difference in muscle activity was observed between sound pressure levels 70 dB and 60 dB (corrected p < 0.01). Overall, sound pressure level was found to have a statistically significant effect on muscle activity (p < 0.01).
These results reveal that sound pressure has a significant positive effect on both tongue pressure and muscle activity, with particularly high sound pressure levels (70 dB) showing a significantly higher influence compared to other levels. Furthermore, while syllable type does not significantly affect tongue pressure, “ko” have significant positive effects on EMG. Additionally, the significant random effects of subjects in both tongue pressure and muscle activity suggest the importance of considering individual differences.
Discussion
Tongue pressure and sound pressure levels
In Japanese conversational speech, the average sound pressure level 1 m in front of the lips is reported to be 60.5 dB18. Referring to this report, 40, 50, 60, and 70 dB pronunciations were used as tasks in this study. As a result, tongue pressure at 70 dB pronunciation, which is higher than Japanese average sound pressure level, was found to be higher than that at the other sound pressure levels. Vocal intensity depends on the source intensity and radiation efficiency, as speech is generated at the glottis, filtered by the vocal tract, including the tongue, and radiated through the mouth1920. It has also been reported that, during consonant pronunciation, the tongue completely or almost completely constricts the vocal tract12,21. Based on these previous reports and the results of this study, we suggest that at a sound pressure level of 70 dB, the tongue and soft palate were closed strongly with increased contact pressure to control the increased expiratory sound pressure level due to increased glottal flow. On the other hand, there was no significant difference in tongue pressure at sound pressure levels below 60 dB, that is, at or below Japanese conversational speech. This suggests that the contact pressure required for the closure of the pharynx by the soft palate and tongue may remain relatively constant at these levels. These results suggest that when evaluating tongue dexterity using pronunciation, especially when evaluating it separately from tongue strength, it is necessary to pronounce at a sound pressure level below conversational speech level. Furthermore, we believe that these results can also be used to discuss environmental sounds when measuring oral diadochokinesis. Although oral diadochokinesis examinations are not typically performed during active dental treatments, it is important to consider the potential impact of environmental noise on these assessments. As studies have reported that the noise level during dental treatments is approximately 70 dB25,26, and vocal behavior is affected in environments with noise levels of approximately 70 dB22,23,24, it is crucial to pay attention to environmental noise when constructing the setting for oral diadochokinesis examinations.
EMG and sound pressure levels
We previously reported a significant positive correlation between tongue pressure and EMG generated by elevating the posterior tongue for isometric movements of the posterior part of the tongue3. We also reported that the EMG activity is generated by muscle groups associated with posterior tongue movement; namely, the styloglossus, stylohyoideus, and hyoglossus, based on the electrode attachment and anatomical muscle positions27. It has been reported that the styloglossus and hyoglossus muscles are involved in lowering and raising the lateral margins of the tongue11. Furthermore, the styloglossus muscle is involved in pulling not only the root but also the entire length of the tongue28. In this study, Spearman's rank correlation coefficient revealed a significant, yet weak, positive association between tongue pressure and EMG activity. This may be due to the presence of intrinsic muscles of tongue, which activity may have changed as tongue pressure increased. On the other hand, the results of the mixed-effects model analysis suggest that sound pressure level has a significant positive effect on EMG activity, particularly at higher sound pressure levels (70 dB). Future research should consider the roles of the intrinsic and extrinsic muscles of tongue, clarifying their respective involvement in generating pressure during pronunciation across various sound pressure levels.
Impact of syllables on tongue pressure and EMG activity
Syllables had no significant effect on the tongue pressure. Four syllables were used in this study: /ka/, /ko/, /ga/, and /go/, all of which are velar stops. Therefore, the similarities in articulation method among these syllables may have contributed to the lack of significant differences in tongue pressure. However, while syllables did not significantly affect tongue pressure, the syllable /ko/ significantly increased EMG activity. It is hypothesized that while /ka/, /ko/, /ga/, and /go/ are all produced as velar stops with no significant differences in tongue pressure, the balance of activity among the soft palate, intrinsic, extrinsic tongue muscles, and other related structures may change to achieve articulation. Within the scope of this study, the production of /ko/ suggests increased muscle activity of the extrinsic tongue muscles associated with the elevation of the posterior part of the tongue. Investigating the muscle activity related to the extrinsic tongue muscles, in addition to the intrinsic tongue muscles and the soft palate, for other syllables may yield new insights. Currently, the articulation of /ka/ is used to examine the dexterity of the posterior tongue through oral diadochokinesis. However, by incorporating the articulation of /ko/, it may become possible to discuss the function of the extrinsic tongue muscles as well. In the present study, participants were instructed to pronounce each syllable at constant sound pressure levels of 40 dB, 50 dB, 60 dB, and 70 dB. To date, no study has examined the effect of syllable type on sound pressure level under such controlled conditions. Further research with more strictly designed experimental conditions is needed to clarify this point, with the addition of techniques that can visualize the movement of the posterior part of the tongue29.
Limitations
This study had several limitations. First, the participants were restricted to healthy young adults. Considering the external validity of this study, it is necessary to conduct further research with participants representing a wider range of ages and health conditions, including older individuals. Second, only surface EMG was used to evaluate muscle activity. Many muscles are involved in tongue movement, and it is difficult to evaluate the activity of specific muscles using surface EMG alone. The extrinsic tongue muscles, the styloglossus and hyoglossus, have also been reported to penetrate the interior of the tongue and to be involved in the morphological changes of the tongue30. Therefore, the use of wire electrodes and imaging tests will be necessary in future studies to clarify the function of the extrinsic tongue muscles during pronunciation using EMG. Third, we did not fully consider the potential influence of individual variations in pronunciation, regional differences in accent, and the distinction between monolingual and bilingual speakers. These factors may have an impact on the interpretation and generalization of our results. Moreover, the number of explanatory variables and the sample size, which may affect the reliability of the statistical analyses. Future research should take these aspects into account, provide a more detailed description of the participants' linguistic background and characteristics, and ensure a large sample size in relation to the number of explanatory variables. Fourth, the acoustic characteristics of the measurement environment were not described in detail. Although the measurements were conducted in a soundproof shield room, specific details such as reverberation time were not measured. In future studies, it is important to provide a more comprehensive description of the acoustic properties of the measurement environment and to refer to relevant literature on voice disorder measurement standards. This will enhance the transparency and reproducibility of the research and allow for more rigorous comparisons with other studies in the field. Future research should take these aspects into account, provide a more detailed description of the participants’ linguistic background and characteristics, and ensure an adequate sample size in relation to the number of explanatory variables. Additionally, the significant random effects of subjects in both tongue pressure and muscle activity suggest the importance of considering individual differences. This finding highlights the variability in tongue function and muscle activity among individuals, which may be influenced by factors such as anatomical differences, muscle strength, and coordination skills. Future studies should aim to investigate these individual differences in more detail, potentially by incorporating larger and more diverse participant samples, and by using personalized analysis techniques to account for individual variability in speech production.
Conclusion
In conclusion, this study showed that in native Japanese speakers, tongue pressure and muscle activity significantly increase during high sound pressure pronunciation above 70 dB, while the tongue-palate contact pressure may remain constant below conversational levels. Furthermore, tongue pressure was influenced by sound pressure levels, while muscle activity was affected by both sound pressure levels and the syllable /ko/. This suggests that the balance of activity among the soft palate, intrinsic and extrinsic tongue muscles, and other related structures may change to achieve articulation. Future research should aim to provide a more detailed description of participants' linguistic background and characteristics, ensure an adequate sample size, and consider environmental noise to further evaluate tongue function during articulation and clarify these findings.
Data availability
The data of this study are available from the corresponding author upon reasonable request.
References
Minakuchi, S. et al. Oral hypofunction in the older population: Position paper of the Japanese Society of Gerodontology in 2016. Gerodontology 35, 317–324 (2018).
Fletcher, S. G. Time-by-count measurement of diadochokinetic syllable rate. J. Speech Hear. Res. 15, 763–770 (1972).
Izuno, H. et al. Physical fitness and oral function in community-dwelling older people: A pilot study. Gerodontology. 33, 470–479 (2016).
Sawada, N., Takeuchi, N., Ekuni, D. & Morita, M. Oral function, nutritional status and physical status in Japanese independent older adults. Gerodontology 39, 359–365 (2022).
Watanabe, Y. et al. Relationship between frailty and oral function in community-dwelling elderly adults. J. Am. Geriatr. Soc. 65, 66–76 (2017).
Dworkin, J. P. & Aronson, A. E. Tongue strength and alternate motion rates in normal and dysarthric subjects. J. Commun. Disord. 19, 115–132 (1986).
Kent, R. D. et al. Speech deterioration in amyotrophic lateral sclerosis: A case study. J. Speech Hear. Res. 34, 1269–1275 (1991).
Heller, K. S., Levy, J. & Sciubba, J. J. Speech patterns following partial glossectomy for small tumors of the tongue. Head Neck. 13, 340–343 (1991).
International Phonetic Association. The International Phonetic Alphabet and the IPA Chart. http://www.internationalphoneticassociation.org/content/ipa-chart (2018).
Yazawa, K., Whang, J. & Escudero, P. Australian English listeners’ perception of Japanese vowel length reveals underlying phonological knowledge. Front. Psychol. 26(14), 1122471 (2023).
Stone, M. & Lundberg, A. Three-dimensional tongue surface shapes of English consonants and vowels. J. Acoust. Soc. Am. 99, 3728–3737 (1996).
Sanders, I., Mu, L., Amirali, A., Su, H. & Sobotka, S. The human tongue slows down to speak: Muscle fibers of the human tongue. Anat. Rec. (Hoboken) 296, 1615–1627 (2013).
Ikebe, K., Hatta, K., Mihara, Y. & Murakami, K. Key points under discussion on “oral hypofunction”. Rounen Shika Igaku. 34, 451–456 (2020).
Manda, Y. et al. New method of neck surface electromyography for the evaluation of tongue-lifting activity. J. Oral Rehabil. 43, 417–425 (2016).
Ono, T., Hori, K. & Nokubi, T. Pattern of tongue pressure on hard palate during swallowing. Dysphagia. 19, 259–264 (2004).
Hori, K. et al. Newly developed sensor sheet for measuring tongue pressure during swallowing. J. Prosthodont. Res. 53, 28–32 (2008).
Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007).
Shiraishi, K. & Kanda, Y. Measurements of the equivalent continuous sound pressure level and equivalent A-weighted continuous sound pressure level during conversational Japanese speech. Audiol. Japan 3, 199–207 (2010).
Zhang, Z. Mechanics of human voice production and control. J. Acoust. Soc. Am. 140, 2614 (2016).
Titze, I. R. & Sundberg, J. Vocal intensity in speakers and singers. J. Acoust. Soc. Am. 91, 2936–2946 (1992).
Liker, M. & Gibbon, F. E. Tongue palate contact patterns of velar stops in normal adult English speakers. Clin. Linguist. Phon. 22, 137–148 (2008).
Szabo-Portela, A., Granqvist, S., Ternström, S. & Södersten, M. Vocal behavior in environmental noise: Comparisons between work and leisure conditions in women with work-related voice disorders and matched controls. J. Voice 32, 126 (2018).
Sala, E. et al. Vocal loading among day care center teachers. Logoped. Phoniatr. Vocol. 27, 21–28 (2002).
Pekkarinen, E. & Viljanen, V. Acoustic conditions for speech communication in classrooms. Scand. Audiol. 20, 257–263 (1991).
Baseer, M. A. et al. Noise levels encountered in university dental clinics during different specialty treatments. J. Fam. Med. Prim. Care 10, 2987–2992 (2021).
da Cunha, K. F., Dos Santos, R. B. & Klien, C. A. Assessment of noise intensity in a dental teaching clinic. BDJ Open 3, 17010 (2017).
Mori, K. et al. Coordination of surface electromyography activity in the posterior tongue region during mastication of differently textured foods. J. Oral Rehabil. 48, 403–410 (2021).
Sakamoto, Y. Configuration of the extrinsic muscles of the tongue and their spatial interrelationships. Surg. Radiol. Anat. 39, 497–506 (2017).
Yano, J. et al. Effect of visual biofeedback of posterior tongue movement on articulation rehabilitation in dysarthria patients. J. Oral Rehabil. 42, 571–579 (2015).
Hiiemae, K. M. & Palmer, J. B. Tongue movements in feeding and speech. Crit. Rev. Oral Biol. Med. 14, 413–429 (2003).
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research (B) (No. 17H04391) from the Japan Society for the Promotion of Science. We gratefully acknowledge the work of past and present members of our laboratory. We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Contributions
Investigation: Y.M., K.M. Methodology: Y.M., S.M. Validation: Y.M., R.A., and M.M. Writing up: Y.M., N.K., and S.M. Review & editing: All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Manda, Y., Kodama, N., Mori, K. et al. Basic characteristics of tongue pressure and electromyography generated by articulation of a syllable using the posterior part of the tongue. Sci Rep 14, 20756 (2024). https://doi.org/10.1038/s41598-024-71909-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71909-y
- Springer Nature Limited