Abstract
Supraphysiological doses of anabolic–androgenic steroids (AAS) is popular among recreational weightlifters and bodybuilders due to the performance-enhancing properties but is also associated with adverse cardiovascular effects. The knowledge about how AAS affect the vasculature is limited, although results from previous studies suggest alterations in vasoreactivity and morphology. In the present study we investigate the association between long-term use of AAS and vascular function. Hundred and twenty-three males were included in the study, 56 of them current AAS users and 67 weightlifting controls. Vascular function was evaluated by carotid artery reactivity and flow-mediated dilation. AAS users had significantly reduced carotid artery reactivity (p < 0.001) and flow-mediated dilation (p < 0.001) compared to weightlifting controls. Results from the present study indicate that long-term use of AAS affect the cardiovascular system negatively, measured as reduced carotid artery reactivity and flow-mediated dilation. These findings could partly explain sudden cardiovascular events among young long-term users of AAS.
Similar content being viewed by others
Introduction
Anabolic–androgenic steroids (AAS) consist of the male sex hormone testosterone and its synthetic derivatives1,2. Testosterone was first discovered in 1935, and in 1937 it was first used clinically3. The discovery of the anabolic properties of AAS resulted in AAS use propagating into elite sports in the 1950s4. The use of AAS expanded during the next decade before it was banned4. It was not until the late 1980s that use gained ground among gym members in the general population and is today primarily used to improve body image and build muscles more easily5,6,7. Recent estimates suggest that about 2–3% of the population have used AAS at some point5,6, but higher prevalence is seen among men6 and subpopulations such as recreational weightlifters, bodybuilders, inmates8 and substance use patients9.
High-dose AAS use is associated with both adverse medical and psychological outcomes10, with cardiovascular effects posing a significant health risk10,11,12. There are numerous case reports and observational studies suggesting an association between AAS use and a variety of cardiovascular complications, including myocardial infarction13,14, cardiac dysfunction10,11,12,15,16, unfavorable lipid profile12,17,18, increased coronary plaque burden10 and hypertension19. Prospective studies also show cardiac dysfunction20, adverse changes in blood pressure, lipid metabolism and erythrocytosis21 after administration of AAS for one cycle, median duration of 16 and 13 weeks, respectively. Previous studies have also demonstrated that long-term AAS use is linked to impaired vascular function22,23,24 and arterial stiffening25.
Carotid artery reactivity (CAR%) to cold pressor test (CPT) represents a novel measure for evaluating carotid artery endothelial function. The CPT induces sympathetic activation, that among others results in production of nitric oxide26,27,28 and dilation of healthy arteries29. CAR% tends to decline with advancing age and in the presence of cardiovascular risk factors, exhibiting associations with coronary endothelial function30.
Flow-mediated dilation (FMD) is a widely adopted non-invasive, ultrasound-based modality for the assessment of peripheral artery endothelium-dependent dilation31. It serves as a prognostic indicator for future atherosclerotic development and cardiovascular events31,32. Prior investigations has yielded conflicting results regarding the effect of long-term AAS use on FMD, with one study showing no difference between AAS users and non-users33, while others demonstrating reduced FMD after prolonged AAS use22,23,24. These studies, however, are limited by small sample sizes.
In the present study we aimed to assess vascular function through both CAR% and FMD, hypothesizing that prolonged AAS use detrimentally impact vascular health, resulting in diminished CAR% and FMD.
Methods
Ethical approval
All experiments were approved by the Regional Committees for Medical and Health Research Ethics South East Norway (REC) (2013/601), and the research was carried out in accordance with the Declaration of Helsinki. All participants received oral and written information prior to participation and provided written informed consent. The participants were compensated for their participation with a gift certificate equivalent to 500 NOK (approximately 45 USD).
Study population
This study is part of a longitudinal study focusing on brain, medical and mental health consequences of long-term AAS use, conducted by the Anabolic Androgenic Steroid Research Group at Oslo University Hospital, Norway (https://www.ous-research.no/anabolic-steroids/). The study population aligns with the one detailed in a prior investigation25.
The participants consist of men engaged in heavy resistance strength training, categorized into two groups: AAS users and a weightlifting control (WLC). AAS users have reported a minimum of 1 year of cumulative AAS use, including recent use within the past 12 months.
Eligibility criteria for the WLC group were being male engaging in heavy resistance training, with no history of AAS use or other prohibited doping substances. To match the AAS users commitment to heavy strength training, we targeted men with bench press capability of 120 kg (~ 265 pounds) for at least one repetition, with 100 kg (~ 220 pounds) as the minimum inclusion criteria. Recruitment strategies involved outreach through various internet forums, social media platforms, and local gyms in Oslo.
All participants were tested as described by Bjørnebekk et al.34, encompassing urine samples, questionnaire, and interviews to confirm alleged AAS use. Doping analysis confirmed the use of AAS among AAS users and confirmed no use among WLC, as previously described35. Detailed information on blood and urine samples are described previously by Melsom et al.25.
Preparation
Subjects were instructed to fast for > 6 h, refrain from exercise for ≥ 24 h and abstain from caffeine, tobacco, alcohol and vitamin C ≥ 18 h prior to testing, according to guidelines for assessing endothelial function32. All factors are known to alter endothelial function32. Before measurements, subjects rested in a supine position for 10 min in a dark, quiet, temperature-controlled room (20 °C) followed by assessment of blood pressure, measured manually with pressure cuff and stethoscope.
Flow-mediated dilation (FMD)
Endothelial function was assessed by the flow-mediated dilation method (FMD method)32. Participants were examined in the supine position after 20 min of rest. A standard blood pressure cuff was positioned around the right arm, two inches below the ante-cubital fossa, and the brachial artery was imaged 5–9 cm above the ante-cubital fossa. A linear-array multifrequency transducer probe 9 MHz (GE, E-95 scanner) was used to acquire images of the brachial artery. To optimize the stability of recordings, the transducer was placed in a fixed position at the brachial artery and stabilized with a custom-made tripod. When an optimal image was found and the probe placed in a fixed position, the cuff was inflated to 230 mmHg for 5 min. Digitized images of the right brachial artery were continuously captured for 190 s, starting 10 s before the cuff deflation, to document the vasodilator response to reactive hyperemia. Semi-automated readings of the digitized images were then used to determine the FMD %:
FMD % = ((maximum diameter—diameter 10 s before cuff deflation)/diameter 10 s before cuff deflation) × 100. The results were analyzed offline using the Brachial analyzer software (Medical Imaging Applications LLC, US).
Carotid artery reactivity (CAR%)
Participants were in a supine position for assessment of the left carotid artery. Diameter of the left carotid artery was measured continuously for 30 s before and 90 s during cold pressor test (CPT) with the left hand immersed in ice slush (4 °C). A linear array multifrequency transducer probe 9 MHz (GE E-95 scanner) was used to acquire images of the carotid artery. When an optimal image was found, all parameters were set to optimize the image and the probe was held stable. After 30 s of baseline assessment of the carotid artery, the hand was immersed for 90 s with continuous assessment of the carotid artery.
CAR% responses were measured as the relative change from baseline diameter to peak dilation or constriction during 90 s of immersion in ice slush. Baseline diameter was calculated as the mean of the diameter measured every 10th sec the first 30 s. The diameter of the carotid artery was measured every 10th sec during the CPT. CAR% was then calculated as the relative change from baseline diameter to peak dilation or constriction.
Statistics
Statistical analysis was performed using Sigmaplot 14.0 (Systat Software, Inc., GmbH, Erkrath, Germany). Given the non-normal distribution of the data, the Mann–Whitney U Test was used to test for statistical differences between groups. Data are presented as median values (25th-75th percentile), unless otherwise stated. A p-value < 0.05 was considered statistically significant.
Results
We included 123 male participants, 56 with proven use of AAS and 67 WLC. Demographical data and statistics for the two groups are presented in Table 1. Doping analysis confirmed the use of AAS among AAS users and confirmed no use among WLC, as previously described35. Of the 56 AAS users, 49 of them were currently in an AAS cycle. There were no differences between the two groups with respect to age, height, systolic blood pressure, or training hours per week. Body weight was significantly higher in the AAS group, compared to the WLC. AAS users exhibited significantly elevated maximal strength levels in both bench press and ground lift when compared to WLC. Education was significantly lower among AAS users than WLC.
One AAS user and one WLC was excluded from the CAR% analysis due to loss of data, and one WLC was excluded from the FMD analysis due to loss of data.
Carotid artery reactivity (CAR%)
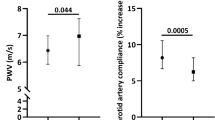
We observed a significantly reduced CAR% in the AAS group compared to the control group [3.58 (2.78–5.80) %, 6.33 (4.48–8.78) % respectively, p < 0.001] (Fig. 1).
Carotid artery reactivity. The figure shows the relative change in carotid artery reactivity (CAR%) from baseline to peak dilation during 90 s immersion of the hand to ice slush. The CAR% was significantly reduced in the AAS group compared to the control group [3.58 (2.78–5.80) %, 6.33 (4.48–8.78) % respectively, p < 0.001].
Flow-mediated dilation (FMD)
The FMD response was significantly lower in the AAS group compared to the control group [3.99 (2.85–6.81) %, 6.72 (4.63–9.48) % respectively, p < 0.001] (Fig. 2). No significant difference was observed in absolute change in flow after cuff release between the AAS group and the WLCs [522.50 (410.50–637.25) mL/min, 474.00 (377.25–578.00) mL/min respectively, p = 0.152] (Fig. 3). AAS users demonstrated a significantly greater baseline diameter compared to the WLCs [5.11 (4.51–5.50) mm, 4.54 (4.09–4.97) mm respectively, p < 0.001]. Additionally, AAS users exhibited a significantly smaller absolute change in diameter compared to the WLCs [0.21 (0.14–0.33) mm, 0.30 (0.23–0.40) mm respectively, p < 0.001].
Absolute change in flow. The figure represents the absolute change in flow (mL/min) from baseline (10 s before deflation) to peak flow after deflation. There was no difference in absolute change in flow (mL/min) between the AAS group and the control group [522.50 (410.50–637.25) mL/min, 474.00 (377.25–578.00) mL/min respectively, p = 0.152].
Discussion
The main findings in this study were that long-term AAS users showed impaired vascular function compared to WLC, evaluated by CAR% and FMD.
To our knowledge, this is the first study to evaluate CAR% among men with a history of long-term AAS use. The finding of reduced CAR% among AAS users indicate diminished vascular function. Reduction in CAR% heightens the susceptibility to acute myocardial infarction, due to the shift from laminar to turbulent blood flow, thereby increasing the likelihood of clot formation36. Dilation of the coronary arteries is an important response in situations with increased oxygen demand, such as physical activity or other situations where sympathetic activity increases. To meet the increased oxygen demand, blood flow increases, leading to arterial dilation. In 1988 Nabel et al. explored how healthy and atherosclerotic coronary arteries respond to an exogenous stimulus that excites the sympathetic activity, and found that healthy coronary arteries dilate in response to this exogenous stimulus37. The vascular responses to CPT involve intricate mechanisms not fully elucidated. However, three key contributors to coronary vasodilation are identified28: increased flow induces shear stress, resulting in flow-mediated dilation contingent on an intact endothelium; epinephrine release activates β2-adrenergic receptors on coronary smooth muscle cells, promoting vasodilation; and norepinephrine is suggested to stimulate nitric oxide synthesis via α2-adrenergic receptors on coronary artery endothelial cells28. The carotid arteries comprise central vessels with similar properties as the coronary arteries and are recognized surrogate measures of coronary artery function30. Thus, we speculate that AAS use negatively affects the balance between the dilation and constriction during the CPT. The reduced dilation of the carotid and coronary arteries under increased blood flow has profound consequences for blood circulation. When arteries fail to dilate adequately in response to increased blood flow, there is a risk that blood flow velocity accelerates and reaches the critical speed where laminar flow is replaced by turbulent flow, as seen in stenotic arteries. The difference is that the turbulent flow is created due to reduced ability of the artery to dilate and thus the whole artery is too narrow when the blood flow velocity increases. The result is increased risk for turbulent flow and subsequently clot formation, potentially resulting in cardiovascular events like myocardial infarction. This could offer insight into sudden cardiovascular events among young users of AAS, despite the absence of morphological changes in the artery wall13,14.
The diminished CAR% observed in AAS users may also be indicative of accelerated arterial ageing process. Previous studies demonstrate that CAR% is reduced in older individuals compared to younger ones30,38. Individuals at risk for CVD also exhibit reduced CAR% in response to a cold pressor test compared to those without risk factors29,30. CAR% is considered a marker of physiological arterial ageing, as its response diminishes with age38, and reduced CAR% is pathological when found in the younger population. In this context, we might argue that AAS use appears to expedite the ageing process and increase the risk for CVD.
This study also identified a significantly lower FMD response in AAS users compared to the WLC group. The baseline diameter was significantly larger in the AAS group. Notably, FMD represents a relative alteration in diameter, meaning the size of the artery impacts the observed relative change32. This means that a small artery will have a bigger relative change than a bigger artery, when the absolute change in diameter is equal32. If both the baseline diameter and the absolute change in diameter would be bigger in the AAS group compared to the WLC, the reduced FMD could be explained by the bigger baseline diameter in the AAS group. However, in this study, the absolute change in diameter was significantly smaller in the AAS group compared to the WLC, with no difference in absolute change in flow. This suggests comparable flow stimulus for the FMD were applied across both groups, supporting the notion that attenuated FMD response among AAS users reflects endothelial dysfunction. FMD can be measured in different conduit arteries and are mediated through increased shear stress on the arterial wall due to increased blood flow32,39. The FMD method in large conduit arteries were first introduced in 1992 when Celemajer et al. described reduced arterial dilation in response to increased blood flow in children and adults at higher risk of developing CVD39. Reduced FMD is considered a warning sign for unhealthy vasculature and a predictor for future cardiovascular events40 and support our hypothesis that long-term use of AAS causes vascular impairment, posing a risk factor for CVD.
Sader et al. reported significantly higher carotid intima media thickness (cIMT) among AAS-users compared to controls33, which is in line with results previously published by our group25. This reinforces the notion that AAS negatively impact the vascular system. However, Sader et al. did not find significant differences in FMD between AAS using bodybuilders, non-using bodybuilders, and controls33, conflicting with results from the present study. The small sample size in the study of Sader et al. might explain the disparities. Moreover, AAS use is associated with increased sympathetic tone41,42,43 and reduced baroreflex sensitivity43. The sympathetic tone is also important in regulating the vascular tone in central arteries44,45. The endothelium and the smooth muscle cells express adreno-receptors, which respond to regional and systemic sympathetic stimuli44,45. While activation of β-receptors induce vasodilation, activation of α-receptors induce constriction44. In healthy individuals sympathetic stimulation leads to dilation, whilst in individuals with coronary disease sympathetic activation leads to vasoconstriction46. It is suggested that AAS use can increase vascular responsiveness to catecholamines47. Thus, an alternative explanation for the reduced FMD found in the present study could be that the AAS user group has an initial dilation of brachial artery, resulting in a relatively lower dilation compared to the WLC. Moreover, Santos et al. found lower baroreflex sensitivity among AAS users compared to non-users and discuss that this could explain the increased sympathetic tone43. Furthermore, they discuss reduced baroreflex sensitivity as a potential consequence of vascular damage, e.g. increased arterial stiffness43,48. Considering existing literature on medical consequences of AAS, reduced FMD most likely reflects endothelial dysfunction. Further, our results from the present study regarding FMD align with those of Ebenbichler et al. and Severo et al.22,24, reporting reduced FMD among AAS users compared to non-users both in phases of AAS use and non-use22, suggesting that reduced FMD reflects endothelial dysfunction rather than a lack of flow stimulus and sympathetically induced vasodilation of the resting diameter.
Limitations
Some limitations should be considered when interpreting the present findings. The cross-sectional study design precludes establishing causality. Also, AAS is legally prohibited in Norway, leading to secrecy around personal use and making it challenging to recruit users. Despite this, the study boasts a relatively large population compared to prior investigations. The diversity in AAS substances used, administration methods, polypharmacy, and duration of use may exert varying effects on the vascular system. Moreover, ethical constraints prevent placebo-controlled studies where specific AAS compounds in high dose are administered over longer time. Consequently, we must study individuals taking AAS at their own initiative. We also cannot rule out the influence of genetic effects or the possibility that AAS use is associated with other lifestyle factors such as diet and drug use that are difficult to control for and might influence our results. Furthermore, our sample mainly comprising men with Scandinavian ethnicity, meaning that the findings may not be generalizable to other ethnicities or female AAS users. While AAS users and WLC align on training hours per week and share a common interest in heavy resistance training, discrepancies in exercise regimens that could differentially affect the cardiovascular system cannot be excluded.
Conclusion
Long-term use of AAS is associated with impaired vascular function, as evaluated by CAR % and FMD. The findings align with our previous study, where morphological changes in the vasculature were observed25. The results suggest that long-term use of AAS negatively affect the cardiovascular system, evident in reduced CAR % and FMD, a pattern also seen in other groups at increased risk for CVD. Therefore, AAS should be considered a significant threat to cardiovascular health, potentially contributing to severe cardiovascular events, such as myocardial infarction and sudden cardiovascular death, observed among young male users of AAS.
Data availability
The raw data cannot be shared publicly due to General Data Protection Regulation (GDPR), and is available from the corresponding author (h.m.tungesvik@medisin.uio.no) on reasonable request.
References
Graham, M. R., Davies, B., Grace, F. M., Kicman, A. & Baker, J. S. Anabolic steroid use: Patterns of use and detection of doping. Sports Med. 38, 505–525. https://doi.org/10.2165/00007256-200838060-00005 (2008).
Yesalis, C. E. & Bahrke, M. S. Anabolic-androgenic steroids and related substances. Curr. Sports Med. Rep. 1, 246–252. https://doi.org/10.1249/00149619-200208000-00009 (2002).
Handelsman, D. J. Mechanisms of action of testosterone–unraveling a Gordian knot. N. Engl. J. Med. 369, 1058–1059. https://doi.org/10.1056/NEJMe1305307 (2013).
Wood, R. I. & Stanton, S. J. Testosterone and sport: Current perspectives. Horm. Behav. 61, 147–155. https://doi.org/10.1016/j.yhbeh.2011.09.010 (2012).
Sagoe, D. & Pallesen, S. Androgen abuse epidemiology. Curr. Opin. Endocrinol. Diabetes Obes. 25, 185–194. https://doi.org/10.1097/MED.0000000000000403 (2018).
Sagoe, D., Molde, H., Andreassen, C. S., Torsheim, T. & Pallesen, S. The global epidemiology of anabolic-androgenic steroid use: A meta-analysis and meta-regression analysis. Ann. Epidemiol. 24, 383–398. https://doi.org/10.1016/j.annepidem.2014.01.009 (2014).
Brennan, R., Wells, J. S. G. & Van Hout, M. C. The injecting use of image and performance-enhancing drugs (IPED) in the general population: A systematic review. Health Soc. Care Community 25, 1459–1531. https://doi.org/10.1111/hsc.12326 (2017).
Havnes, I. A., Bukten, A., Rognli, E. B. & Muller, A. E. Use of anabolic-androgenic steroids and other substances prior to and during imprisonment—Results from the Norwegian Offender Mental Health and Addiction (NorMA) study. Drug Alcohol. Depend. 217, 108255. https://doi.org/10.1016/j.drugalcdep.2020.108255 (2020).
Havnes, I. A., Jorstad, M. L., McVeigh, J., Van Hout, M. C. & Bjornebekk, A. The anabolic androgenic steroid treatment gap: A national study of substance use disorder treatment. Subst. Abuse 14, 1178221820904150. https://doi.org/10.1177/1178221820904150 (2020).
Baggish, A. L. et al. Cardiovascular toxicity of illicit anabolic-androgenic steroid use. Circulation 135, 1991–2002. https://doi.org/10.1161/circulationaha.116.026945 (2017).
Abdullah, R. et al. Severe biventricular cardiomyopathy in both current and former long-term users of anabolic-androgenic steroids. Eur. J. Prev. Cardiol. https://doi.org/10.1093/eurjpc/zwad362 (2023).
Fyksen, T. S. et al. Cardiovascular phenotype of long-term anabolic-androgenic steroid abusers compared with strength-trained athletes. Scand. J. Med. Sci. Sports 32, 1170–1181. https://doi.org/10.1111/sms.14172 (2022).
Halvorsen, S., Thorsby, P. M. & Haug, E. Acute myocardial infarction in a young man who had been using androgenic anabolic steroids. Tidsskr. Nor. Laegeforen. 124, 170–172 (2004).
Christou, G. A., Christou, K. A., Nikas, D. N. & Goudevenos, J. A. Acute myocardial infarction in a young bodybuilder taking anabolic androgenic steroids: A case report and critical review of the literature. Eur. J. Prev. Cardiol. 23, 1785–1796. https://doi.org/10.1177/2047487316651341 (2016).
Rasmussen, J. J. et al. Cardiac systolic dysfunction in past illicit users of anabolic androgenic steroids. Am. Heart J. 203, 49–56. https://doi.org/10.1016/j.ahj.2018.06.010 (2018).
Baggish, A. L. et al. Long-term anabolic-androgenic steroid use is associated with left ventricular dysfunction. Circ. Heart Fail. 3, 472–476. https://doi.org/10.1161/CIRCHEARTFAILURE.109.931063 (2010).
Thiblin, I. & Petersson, A. Pharmacoepidemiology of anabolic androgenic steroids: A review. Fundam. Clin. Pharmacol. 19, 27–44. https://doi.org/10.1111/j.1472-8206.2004.00298.x (2005).
Hurley, B. F. et al. High-density-lipoprotein cholesterol in bodybuilders v powerlifters Negative effects of androgen use. JAMA 252, 507–513 (1984).
Rasmussen, J. J. et al. Increased blood pressure and aortic stiffness among abusers of anabolic androgenic steroids: Potential effect of suppressed natriuretic peptides in plasma?. J. Hypertens. https://doi.org/10.1097/hjh.0000000000001546 (2017).
Smit, D. L., Voogel, A. J., den Heijer, M. & de Ronde, W. Anabolic androgenic steroids induce reversible left ventricular hypertrophy and cardiac dysfunction. Echocardiography results of the HAARLEM study. Front. Reprod. Health 3, 732318. https://doi.org/10.3389/frph.2021.732318 (2021).
Smit, D. L. et al. Prospective study on blood pressure, lipid metabolism and erythrocytosis during and after androgen abuse. Andrologia 54, e14372. https://doi.org/10.1111/and.14372 (2022).
Ebenbichler, C. F. et al. Flow-mediated, endothelium-dependent vasodilatation is impaired in male body builders taking anabolic-androgenic steroids. Atherosclerosis 158, 483–490 (2001).
de Souza, F. R. et al. Retrograde and oscillatory shear rate in young anabolic androgenic steroid users. Scand. J. Med. Sci. Sports 29, 422–429. https://doi.org/10.1111/sms.13332 (2019).
Severo, C. B. et al. Increased atherothrombotic markers and endothelial dysfunction in steroid users. Eur J Prev Cardiol 20, 195–201. https://doi.org/10.1177/2047487312437062 (2013).
Melsom, H. S. et al. Reduced arterial elasticity after anabolic-androgenic steroid use in young adult males and mice. Sci. Rep. 12, 9707. https://doi.org/10.1038/s41598-022-14065-5 (2022).
Tousoulis, D., Davies, G., Tentolouris, C., Crake, T. & Toutouzas, P. Inhibition of nitric oxide synthesis during the cold pressor test in patients with coronary artery disease. Am. J. Cardiol. 79, 1676–1679. https://doi.org/10.1016/s0002-9149(97)00222-1 (1997).
van Mil, A. et al. Similarity between carotid and coronary artery responses to sympathetic stimulation and the role of alpha1-receptors in humans. J. Appl. Physiol. 1985(125), 409–418. https://doi.org/10.1152/japplphysiol.00386.2017 (2018).
Pouwels, S. et al. Utility of the cold pressor test to predict future cardiovascular events. Expert Rev. Cardiovasc. Ther. 17, 305–318. https://doi.org/10.1080/14779072.2019.1598262 (2019).
Rubenfire, M., Rajagopalan, S. & Mosca, L. Carotid artery vasoreactivity in response to sympathetic stress correlates with coronary disease risk and is independent of wall thickness. J. Am. Coll. Cardiol. 36, 2192–2197. https://doi.org/10.1016/s0735-1097(00)01021-4 (2000).
van Mil, A. C. et al. Correlation of carotid artery reactivity with cardiovascular risk factors and coronary artery vasodilator responses in asymptomatic, healthy volunteers. J. Hypertens. 35, 1026–1034. https://doi.org/10.1097/hjh.0000000000001274 (2017).
Thijssen, D. H. J. et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 40, 2534–2547. https://doi.org/10.1093/eurheartj/ehz350 (2019).
Thijssen, D. H. et al. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am. J. Physiol. Heart Circ. Physiol. 300, H2-12. https://doi.org/10.1152/ajpheart.00471.2010 (2011).
Sader, M. A., Griffiths, K. A., McCredie, R. J., Handelsman, D. J. & Celermajer, D. S. Androgenic anabolic steroids and arterial structure and function in male bodybuilders. J. Am. Coll. Cardiol. 37, 224–230. https://doi.org/10.1016/s0735-1097(00)01083-4 (2001).
Bjornebekk, A. et al. Structural brain imaging of long-term anabolic-androgenic steroid users and Nonusing weightlifters. Biol. Psychiatry 82, 294–302. https://doi.org/10.1016/j.biopsych.2016.06.017 (2017).
Bjornebekk, A. et al. Long-term anabolic-androgenic steroid use is associated with deviant brain aging. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 6, 579–589. https://doi.org/10.1016/j.bpsc.2021.01.001 (2021).
Grady, P. A. Pathophysiology of extracranial cerebral arterial stenosis—A critical review. Stroke 15, 224–236. https://doi.org/10.1161/01.str.15.2.224 (1984).
Nabel, E. G., Ganz, P., Gordon, J. B., Alexander, R. W. & Selwyn, A. P. Dilation of normal and constriction of atherosclerotic coronary arteries caused by the cold pressor test. Circulation 77, 43–52 (1988).
Monahan, K. D., Feehan, R. P., Sinoway, L. I. & Gao, Z. Contribution of sympathetic activation to coronary vasodilatation during the cold pressor test in healthy men: Effect of ageing. J. Physiol. 591, 2937–2947. https://doi.org/10.1113/jphysiol.2013.251298 (2013).
Celermajer, D. S. et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340, 1111–1115. https://doi.org/10.1016/0140-6736(92)93147-f (1992).
Inaba, Y., Chen, J. A. & Bergmann, S. R. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: A meta-analysis. Int. J. Cardiovasc. Imaging 26, 631–640. https://doi.org/10.1007/s10554-010-9616-1 (2010).
Barbosa Neto, O. et al. Long-term anabolic steroids in male bodybuilders induce cardiovascular structural and autonomic abnormalities. Clin. Auton. Res. Off. J. Clin. Auton. Res. Soc. https://doi.org/10.1007/s10286-017-0470-2 (2017).
Alves, M. J. et al. Abnormal neurovascular control in anabolic androgenic steroids users. Med. Sci. Sports Exerc. 42, 865–871. https://doi.org/10.1249/MSS.0b013e3181c07b74 (2010).
Santos, M. R. D. et al. Resting spontaneous baroreflex sensitivity and cardiac autonomic control in anabolic androgenic steroid users. Clinics 73, e226. https://doi.org/10.6061/clinics/2018/e226 (2018).
Guimaraes, S. & Moura, D. Vascular adrenoceptors: An update. Pharmacol. Rev. 53, 319–356 (2001).
Peace, A., Van Mil, A., Jones, H. & Thijssen, D. H. J. Similarities and differences between carotid artery and coronary artery function. Curr. Cardiol. Rev. 14, 254–263. https://doi.org/10.2174/1573403x14666180910125638 (2018).
Zeiher, A. M., Drexler, H., Wollschlaeger, H., Saurbier, B. & Just, H. Coronary vasomotion in response to sympathetic stimulation in humans: Importance of the functional integrity of the endothelium. J. Am. Coll. Cardiol. 14, 1181–1190. https://doi.org/10.1016/0735-1097(89)90414-2 (1989).
Green, D. J., Cable, N. T., Rankin, J. M., Fox, C. & Taylor, R. R. Anabolic steroids and vascular responses. Lancet 342, 863. https://doi.org/10.1016/0140-6736(93)92720-e (1993).
Rowe, J. W. Clinical consequences of age-related impairments in vascular compliance. Am. J. Cardiol. 60, 68G-71G. https://doi.org/10.1016/0002-9149(87)90594-7 (1987).
Acknowledgements
The authors would like to thank CP, PhD Lisa Evju Hauger for contributing on the recruitment of study participants. We would also thank all the participants who took part in this study. We also thank the Norwegian Research Council (NRC, grant number 271555/F20), and The Medical Student Research Program (MSR) at the University of Oslo.
Author information
Authors and Affiliations
Contributions
HMT recruited participants, collected, analyzed, and evaluated the data. HMT wrote the initial manuscript. AB was the project manager of the study, coordinated recruitment and collection of biometrical data. JH contributed with method training and data interpretation. JH and AB contributed with manuscript writing. JH and HMT contributed to experimental and conceptual planning. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tungesvik, H.M., Bjørnebekk, A. & Hisdal, J. Impaired vascular function among young users of anabolic–androgenic steroids. Sci Rep 14, 19201 (2024). https://doi.org/10.1038/s41598-024-70110-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70110-5
- Springer Nature Limited