Abstract
Mental fatigue during long-term motor imagery (MI) may affect intention recognition in MI applications. However, the current research lacks the monitoring of mental fatigue during MI and the definition of robust biomarkers. The present study aims to reveal the effects of mental fatigue on motor imagery recognition at the brain region level and explore biomarkers of mental fatigue. To achieve this, we recruited 10 healthy participants and asked them to complete a long-term motor imagery task involving both right- and left-handed movements. During the experiment, we recorded 32-channel EEG data and carried out a fatigue questionnaire for each participant. As a result, we found that mental fatigue significantly decreased the subjects’ motor imagery recognition rate during MI. Additionally the theta power of frontal, central, parietal, and occipital clusters significantly increased after the presence of mental fatigue. Furthermore, the phase synchronization between the central cluster and the frontal and occipital lobes was significantly weakened. To summarize, the theta bands of frontal, central, and parieto–occipital clusters may serve as powerful biomarkers for monitoring mental fatigue during motor imagery. Additionally, changes in functional connectivity between the central cluster and the prefrontal and occipital lobes during motor imagery could be investigated as potential biomarkers.
Similar content being viewed by others
Introduction
Motor imagery (MI) is verified as a mental activity in which participants mentally simulate body movements without any actual actions, which means that participants will feel themselves performing the actions1,2. MI has been used in the rehabilitation of various neurological diseases and motor disorders3, and studies have verified the role of MI in motor rehabilitation of stroke patients and cerebral palsy patients4,5. Mental fatigue is a psychobiological state caused by prolonged and demanding cognitive activity6. Mental fatigue can manifest as somnolence, lethargy, or directional attention fatigue7. Prolonged physical or mental work can cause mental fatigue, which can reduce attention levels and even lead to serious accidents8. Suoqing Niu et al. used the Stroop task to induce mental fatigue and confirmed that mental fatigue impairs dart-throwing performance9. Akira Nakashima et al. designed a controlled experiment to verify that continuous repetition of MI as actual exercise training can decrease corticospinal excitability10. Other Studies also have shown that repeated MI can cause mental fatigue, but this phenomenon is usually ignored11,12,13. Franck Di Rienzo et al. confirmed in a controlled experiment that mental fatigue impairs MI vividness and evokes negative emotions in participants during motor tasks with low physical demands14. Mental fatigue is gradually becoming a focus of attention for researchers.
Most current methods for assessing mental fatigue are subjective and/or time-consuming. Fatigue questionnaires such as the National Aeronautics and Space Administration (NASA) Task Load Index (NASATLX)15 and Brief Fatigue Inventory (BFI)16,17, are easily affected by subjective factors. Gold-standard tests such as N-back tests for working memory18 are time-consuming and unmeasurable. Therefore, it is very important to select appropriate indicators or biomarkers of mental fatigue19, spectral electroencephalogram as a potential biomarker has received extensive attention from researchers20. Yvonne Tran et al.21 conducted a meta-analysis based on 21 studies on mental fatigue, and the analysis results supported that the theta band activity in the frontal, central, and posterior sites can serve as a robust biomarker of mental fatigue, and the alpha band changes were considered to be related to individual differences. Some studies have focused on mental fatigue during MI, Upasana Talukdar et al.22 used the kernel partial least squares method to show the changes of mental fatigue during MI. Vianney Rozand et al.23 compared the effects of the MI combined with the motor execution and motor imagery and proposed that regular execution of actual movements can counteract the mental fatigue caused by MI. Upasana Talukdar et al.24 proposed an unsupervised adaptive feature extraction scheme, which adjusted the feature extractor of MI-BCI by tracking the fatigue level of the participants, and found that adapting the common spatial pattern (CSP) according to mental fatigue can improve the MI recognition rate. However, there are still two problems in the current research on mental fatigue during MI. One is that the biomarker of mental fatigue during MI is temporarily unclear. Most of the related studies have been carried out based on mental fatigue generated during simulated driving, simulated flying, and cognitive tasks20,21,25,26,27,28,29, but the results are poorly generalized. The other problem is that the existing studies have only suggested the effects of mental fatigue on MI performance, and some researchers believe that controlling the MI training time in a suitable range can avoid the effects of mental fatigue10,30. It is difficult to set a universal MI training time considering the different time nodes that induce mental fatigue among individuals. Therefore, it is important to investigate the mechanism by which mental fatigue impairs MI performance during prolonged MI training to modulate mental fatigue in the future.
Above all, this study sought to explore the physiological mechanisms about how mental fatigue affects MI performance and further investigate a robust biomarker of mental fatigue during long-term MI training. We first conducted a novel MI experiment and recorded EEG signals and subjective BFI scores during the MI experiment. Subsequently, we demonstrated that participants’ state of mental fatigue affected training performance as MI time increased. At the same time, we determined that changes in theta rhythm activity in specific parts of the cerebral cortex during the resting state can effectively reflect mental fatigue caused by motor imagery. Finally, it is difficult to monitor mental fatigue during motor imagery in real time with the use of only resting EEG power spectral density as a biomarker. For this reason, we chose phase synchronization indicators to explore the effect of mental fatigue on functional connectivity during MI.
Method
Participants
Ten healthy participants (four females and six males) were recruited. The participants had no prior experimental experience before participating in the experiment, and all of them signed an informed consent form and were also informed of the experimental procedure and the use of the experimental data. This study was approved by the Ethics Committee of The First Hospital of Qinhuangdao (Protocol Number: 2021A032 Date: May 22, 2021) and conducted in accordance with the Declaration of Helsinki. None of the participants had motor or cognitive impairments, the basic information of the subjects is shown in Table 1.
Procedure
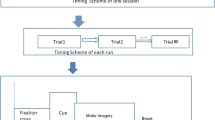
Before and after the formal experiment, each participant is required to undergo a 5 min resting-state experiment while recording EEG data and filling out a BFI questionnaire. Participants had to indicate their level of fatigue by selecting a score on an 11-point Likert scale17. The BFI used in this study is the Chinese version of the scale in the Supplementary Materials. Some questions in BFI ask the level of fatigue within 24 h. To avoid repetition, participants were prompted to answer these questions only once during the pre-test. Before commencing the experiment, participants were instructed to sit in a relaxed and natural position in front of the computer screen. The MI experiment consisted of four blocks, with each block containing around 50 randomly generated left and right-hand MI trials. In a trial, participants performed MI for 4s, and then viewed a fixation cross on a white background for 4s. Participants performed MI by judging the left or right hand from the picture. When the cross appeared on the screen, participants were asked to stop MI but focus on the screen. After each block, participants only need to choose a number to indicate their current level of fatigue, followed by a 3 min rest. The whole experiment would take about 50 min, while EEG data was recorded throughout. The experimental paradigm is shown in Fig. 1a.
EEG recording
We recorded 32-channel EEG signal with the EEG signal acquisition device (JL-EEG32W, Jiangxi Brain Modulation Technology Development Company, China), which includes a matching amplifier, Multichannel data recording software, and an AgCl electrode 32-lead EEG cap. The electrode position is determined according to the international 10–20 standard system as shown in Fig. 1b. Before the experiment, the subjects wore the EEG cap and used EEG paste to reduce the electrode impedance to below 50 kΩ. The amplifier sampling rate was 2000 Hz without filtering and the analog-to-digital conversion bits is 24bit.
Data analysis and statistics
Pre-processing
We used EEGLAB V20.031 to process the raw EEG data. After electrode positioning and removal of unnecessary electrodes, the EEG signals from 31 channels (Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, O2, F7, F8, T7, T8, P7, P8, Fz, Cz, Pz, FC1, FC2, CP1, CP2, FC5, FC6, CP5, CP6, TP9, TP10, FT9, FT10) were high-pass filtered at 1 Hz and low-pass filtered at 80 Hz. After that, a notch filter was used to remove 50 Hz power interference. At this point, there were still artifacts such as eye, heart, and muscle movements in the EEG signals. Therefore, the independent component analysis (ICA) algorithm was used to further process the signals, and the signals were finally downsampled to 500 Hz.
Data analysis
Motor imagery classification accuracy rate during MI training
In order to observe the impact of fatigue on motor imagination ability during motor imagery and confirm the necessity of studying mental fatigue during MI. We first calculated the motor imagery classification accuracy rate during long-term MI. Considering that CSP32 can effectively utilize the spatial correlation of EEG signals, and reduce data dimensions and computational complexity, in this study, we used it to reduce the dimensionality of EEG signals and obtained CSP features that can be represented in two dimensions. After that, we used support vector machines (SVM)33 to classify the EEG data. The SVM algorithm finds a hyperplane in the sample space to separate different samples while maximizing the distance between the margin points of the two-point sets. To ensure the reliability of the results, we used tenfold cross-validation to calculate the recognition rate of the motion imagery. The data is randomly divided into 10 parts and nine of them are used as training data and one as testing data in turn for calculating the accuracy rate. The average of the results was obtained as the motor imagery classification accuracy rate.
Power spectral density
A meta-analysis of 21 studies on mental fatigue revealed that changes in theta band activity in the frontal, central, and parietal sites could serve as biomarkers of mental fatigue, while the article also suggested that the changes in the alpha band were not consistent across subjects21. This meta-analysis was based on studies that included only simulated driving, simulated flying, real driving, cognitive tasks, and visual stimuli. We found it difficult to extend the results to MI training. Therefore, we used the resting-state EEG data from the pre-and post-test phases to calculate the power spectral density as shown in Eq. (1). The study calculated the proportions of theta and alpha bands within 1–80 Hz (delta (1–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), and gamma (30–80 Hz) bands) in EEG signal from frontal, central, and parieto-occipital clusters. The channels for the three clusters are shown in Fig. 234.
Phase synchronization
Functional and effective connectivity in the brain represents statistical dependence and directed information flow between cortical areas. In particular functional connectivity represents the underlying interconnections between brain regions in some way35. We speculated that mental fatigue induced by long-term MI training may impair the phase synchronization of the brain further affecting MI performance. The phase difference can indicate the degree of synchronization or coherence between two EEG signals, which reflects the functional connectivity between the brain regions that generate these signals36,37. It has been suggested that functional connectivity in the right hemisphere was increased in high compared to low aptitude MI-BCI users during motor imagery38. Phase locking value (PLV) is an important method to indicate synchronization of EEG signals39, as a common indicator of functional connectivity, it has the advantage of being unaffected by artifacts on the signal amplitude. We used the feature to observe the changes in brain functional connectivity due to mental fatigue during the MI and selected the EEG signals with the main MI frequency of 8–13 Hz as the research object. The formula for calculating the PLV of the alpha band (8–13 Hz) during a trial is as follows:
where N is the number of time points in a trail, \(\psi_{q}^{n}\) represents the phase of channel q at the time point n, and j is the imaginary unit.
Statistical analysis
All data in this study was collected in the lab for research purposes. Participants were allowed to withdraw from the experiment at any time and request their experimental data be removed. Data normality was tested using the Shapiro–Wilk normality test. Wilcoxon test was used for nonparametric data for paired comparisons and one-way t-test was used for data with normal distribution. One-way repeated measures analysis of variance (ANOVAs) was conducted to compare participants’ BFI scores and MI recognition rate after completing each block of MI training in the study (BFI score: F = 56.72, p < 0.001, \(\eta_{p}^{2}\) = 0.8631; MI recognition rate: F = 8.301, p < 0.001, \(\eta_{p}^{2}\) = 0.4798). Firstly, the data were tested for normality and variance homogeneity using the Shapiro–Wilk test and Levene’s test. Subsequently, Mauchly’s test of sphericity was utilized to verify the equality of covariances across conditions. For data not meeting these criteria, Greenhouse–Geisser correction was applied. Finally, Bonferroni correction was conducted for pairwise comparisons to get the trends in the data.
For frequency band changes a three-way repeated measures ANOVA was used (3[brain region (frontal, central, parieto-occipital)] \(\times\) 2[band (theta, alpha)] \(\times\) 2[time (pre, post)]), similarly to before normality and variance homogeneity were tested, followed by testing for equality of data covariances to determine whether a correction should be applied. Finally, post-hoc pairwise comparisons using the Bonferroni correction were used to explore significant main effects further. Statistical analyses were performed using the IBM SPSS Statistics program (version 26) and fatigue drawing with Prism 9.0 software. In this study, p values < 0.05 were considered statistically significant.
Results
Changes in BFI scores and MI recognition with mental fatigue during MI
To observe changes in participants’ subjective fatigue perception during MI, we recorded changes in participants’ BFI scores and trends in average BFI scores during MI training (Table 2), higher BFI scores indicate greater subjective fatigue in participants. It can be seen in Table 2 that each participant’s BFI score increased after the end of motor imagery. Figure 3 showed that, except for the second block of MI training, the average BFI value increased significantly after each block of motor imagery compared with the previous block (p < 0.05). All p values are shown in Fig. 3.
Additionally, we considered that the scale evaluation is not objective enough and is easily affected by the subject’s physical condition and individual cognitive differences. We also calculated the recognition rate of each subject during the MI in Fig. 4a. It was observed that during the MI, the majority of participants achieved their maximum MI recognition rate in either block 2 or block 3. Furthermore, to show the changing trend of motor imagery recognition rate with MI, we have plotted Fig. 4b. As in Fig. 3, all p values were shown in the figure. The figure shows that participants’ average motor imagery recognition rate increased with the number of training times in the first three blocks, peaking at the third block of MI training. However, at the end of the last block group, instead of improving, the motor imagery recognition rate showed a significant decrease.
Changes in theta band activity as biomarkers of mental fatigue are feasible
To investigate the feasibility of EEG power spectra densities reflecting changes in mental fatigue, we calculated the power spectral densities of resting EEG signals in the frontal, central, and parieto–occipital sites (Fig. S1), and performed Three-way repeated measures ANOVA on the pre-and post-test data (brain region: F = 34.54, p < 0.001, \(\eta_{p}^{2}\) = 0.7933; band: F = 38.52, p < 0.001, \(\eta_{p}^{2}\) = 0.8105; time: F = 5.688, p = 0.041, \(\eta_{p}^{2}\) = 0.3873; brain region * band: F = 40.87, p < 0.001, \(\eta_{p}^{2}\) = 0.8195, Fig. 5). Our findings suggested that changes in theta waves in the frontal, central, and parieto–occipital sites were more suitable as biomarkers of mental fatigue than alpha waves. The study also indicated that mental fatigue resulting from long-term recurrent MI may mainly affect wave activity in the frontal, central, and parietal sites, which can be used as a reference for future research on the modulation of mental fatigue.
Three-way repeated measures ANOVA results of EEG signal power spectrum changes in alpha and theta bands in frontal and central sites at resting state. (a) Changes in theta band of EEG signals in the frontal site (p < 0.001, \(\eta_{p}^{2}\) = 0.7452). (b) Changes in theta band of EEG signals in the central site (p < 0.001, \(\eta_{p}^{2}\) = 0.8029). (c) Changes in theta band of EEG signals in the parieto–occipital site (p < 0.001, \(\eta_{p}^{2}\) = 0.5977). (d) Changes in alpha band of EEG signals in the frontal site (p > 0.05, \(\eta_{p}^{2}\) = 0.1659). (e) Changes in alpha band of EEG signals in the central site (p > 0.05, \(\eta_{p}^{2}\) < 0.01). (f) Changes in alpha band of EEG signals in the parieto–occipital site (p > 0.05, \(\eta_{p}^{2}\) < 0.01).
Changes in phase synchronization reflect the generation of mental fatigue
To investigate whether changes in the brain’s functional connections during motor imagery can reflect the emergence of mental fatigue. We calculated PLV values for whole-brain pathways in the alpha band during MI in blocks 1 and 4 (Fig. 6a, b). From Fig. 6c, it can be seen that there was a decrease in whole-brain phase synchronization in participants after long-term MI training. We selected a nonparametric test to statistically compare whole-brain functional connectivity using the two-tailed Wilcoxon signed-rank test (Fig. 6d). Significant mental fatigue was found to significantly reduce phase synchrony between the central cluster and the frontal and occipital clusters.
Changes in phase synchronization between whole-brain channels during block 1 and block 4 MI periods. (a) Whole-brain functional connectivity matrix during MI in block 1. (b) Whole-brain functional connectivity matrix during MI in block 4. (c) Changes in the functional connectivity matrix between block 1 and block 4. (d) Phase synchronization between the central cluster and the frontal and occipital lobes was significantly reduced, non-white areas indicate significant reduction (p < 0.05), the signed-rank statistic and effect size obtained from the statistical analyses are provided in Table S1.
Discussion
Most studies on mental fatigue focus on fatigue while driving vehicles, such as cars and airplanes. Therefore, we conducted a long-term and high-repetitive MI experiment to explore the characteristics of mental fatigue during MI. Our findings suggested that the changes in the theta band activity in the frontal, central, and parieto–occipital sites of the brain in resting state EEG signals can serve as robust biomarkers of mental fatigue during motor imagery. At the same time, we found that changes in phase synchronization between the central cluster and other cortices during MI may also reflect the onset of mental fatigue. This discussion will cover these findings and phenomena in the study.
Muhammad Awais et al. conducted a driving simulator based study to observe the significant changes that occur in the EEG power spectrum during monotonous driving29. The finding suggested that the alpha and theta band powers in the occipital and parietal regions increased significantly when the participants were fatigued. This study provided a theoretical basis for fatigue driving monitoring and prevention of traffic accidents. Teng Cao et al. used an objective and real-time method based on electroencephalography (EEG) spectral analysis to evaluate the fatigue in SSVEP-based BCIs40. Xiaoli Fan et al.41 designed a long-term visual search task and found that the alpha activity of the frontal, central, posterior temporal, parietal, and occipital lobes increased significantly, and a dip occurred in the beta activity in the pre-frontal, inferior frontal, posterior temporal, and occipital lobes. Our results showed that the theta band power in the frontal, central, and parieto–occipital sites increased significantly, while the alpha band power did not show any regular changes. In previous studies, it was shown that theta band activity increases when individuals feel fatigued42. Theta waves are thought to be related to neural activity such as sleep, working memory, and cognitive performance. Several studies have shown that an increase in the theta frequency band is related to increased levels of mental fatigue43,44. In MI training when participants felt fatigued they became sleepy and difficult to maintain attention and vigilance level. Increased activity in the alpha band was associated with increased mental effort to maintain attention42. This study provided some potential indicators for monitoring and evaluating mental fatigue during MI, which can be used in the future to monitor the changes in mental fatigue during MI rehabilitation training and adjust the training difficulty based on this indicator.
In some studies, researchers have proposed that fatigue caused by exercise can affect MI ability. Thiago Ferreira Dias Kanthack et al.45 designed a comparative experiment that included continuous and intermittent exercise. By testing the MI ability of the participants after exercise, they verified that continuous exercise had an impact on MI. Akira Nakashima et al.30 used a visual analog scale and pinch force to assess subjective mental fatigue and muscle fatigue and confirmed that MI training caused mental fatigue by continuously repeating MI, which reduced the training performance. However, the authors did not reveal how to improve the experimental effect in this study. We designed a fatigue-inducing experiment that included 200 MI trials, and we found that participants’ fatigue was maintained at a low level during the first 100 MI training trials after that mental fatigue increased significantly. We therefore suggested that the MI training effect was optimal between 100 and 150 trials, and the duration should be controlled within 30 min. Moderate levels of fatigue may cause participants to exert more effort during motor imagery. This finding may provide a reference for subsequent MI-related training and psychological paradigm design.
Our study strongly supports the conclusion that excessive MI training can impair MI ability. We also selected the BFI score as a subjective indicator and the MI recognition rate as an objective indicator to ensure the accuracy of the results. Although some studies have pointed out that mental fatigue can reduce MI ability, they did not reveal the mechanism from a physiological perspective. We found that with the occurrence of mental fatigue, the phase synchronization in the alpha band (8–13 Hz) between the central cluster and other brain regions decreased significantly. In recent years, more and more studies have suggested that mental fatigue is associated with the reorganization of functional connectivity between brain regions46. Yang Shuo et al. induced mental fatigue through a sustained cognitive task and found that the dynamic structure and functional properties of the brain functional network in the brain-fatigue state were changed, and the efficiency of information transmission was reduced47. An increase in brain waves at 8–13 Hz is thought to be associated with maintaining concentration42, and when participants feel fatigued they have difficulties in maintaining attention and concentration. Based on this we suggest that mental fatigue altered the phase synchronization between participants’ brain regions affecting MI performance. It is hypothesized that if the alpha band phase synchronization between the central cluster and other brain regions could be improved by neuromodulation techniques, it might be possible to modulate mental fatigue during MI to improve the quality of the EEG signal.
In the present study, we found the pattern of mental fatigue during repeated MI training and provided reliable biomarkers for monitoring mental fatigue. However, this study had several limitations. One limitation of our study is that we only collected data from 10 participants in this study. Although we obtained regular results, we still need to consider the impact of the small sample size on the results when explaining the phenomena. In addition, we chose the BFI scale to collect scores from the participants, and the results were inevitably affected by the participants’ subjective feelings. Another limitation is that our study considered the fatigue caused by sitting in the EEG room for a long time, so we allowed participants to choose a natural and comfortable position to start the experiment. However, this could not completely exclude the effect of sitting for a long time on the experiment. In the future, we will optimize the experimental design to exclude the interference of this factor. It is important to note that the effects of MI on mental fatigue have been studied in other research, and the methods of this study are not completely innovative.
Conclusion
In summary, we found that the increase of theta band activity in the frontal, central, and parieto–occipital regions of the brain could better reflect the increase of mental fatigue than the alpha band activity. We also observed that the MI training effect was better in the range of 100–150 trials, which may guide the clinical application of the MI paradigm. We propose that phase synchrony between central and occipital regions decreases significantly after participants experience mental fatigue. This feature can be studied as a potential biomarker and provide a theoretical basis for future regulation of neurological fatigue.
Data availability
Currently, the data used in this study is not publicly available, they are part of an ongoing study. The data supporting this study’s findings are available on request from the corresponding author.
References
Decety, J. Do imagined and executed actions share the same neural substrate?. Cogn. Brain Res. 3, 87–93 (1996).
Decety, J. & Ingvar, D. H. Brain structures participating in mental simulation of motor behavior: A neuropsychological interpretation. Acta Psychol. 73, 13–34 (1990).
Jackson, P. L., Lafleur, M. F., Malouin, F., Richards, C. & Doyon, J. Potential role of mental practice using motor imagery in neurologic rehabilitation. Arch. Phys. Med. Rehabil. 82, 1133–1141 (2001).
Page, S. J., Levine, P., Sisto, S. A. & Johnston, M. V. Mental practice combined with physical practice for upper-limb motor deficit in subacute stroke. Phys. Ther. 81, 1455–1462 (2001).
Xie, J. et al. Rehabilitation of motor function in children with cerebral palsy based on motor imagery. Cogn. Neurodyn. 15, 939–948 (2021).
Martin, K., Meeusen, R., Thompson, K. G., Keegan, R. & Rattray, B. Mental fatigue impairs endurance performance: A physiological explanation. Sports Med. 48, 2041–2051 (2018).
Marcora, S. M., Staiano, W. & Manning, V. Mental fatigue impairs physical performance in humans. J. Appl. Physiol. 106, 857–864 (2009).
Landrigan, C. P. et al. Effect of reducing interns’ work hours on serious medical errors in intensive care units. N. Engl. J. Med. 351, 1838–1848 (2004).
Niu, S. et al. The effects of mental fatigue on fine motor performance in humans and its neural network connectivity mechanism: A dart throwing study. Cereb. Cortex https://doi.org/10.1093/cercor/bhae085 (2024).
Nakashima, A. et al. Corticospinal excitability during motor imagery is diminished by continuous repetition-induced fatigue. Neural Regener. Res. 16, 1031–1036 (2021).
Rozand, V., Lebon, F., Papaxanthis, C. & Lepers, R. Does a mental-training session induce neuromuscular fatigue?. Med. Sci. Sports Exerc. https://doi.org/10.1249/MSS.0000000000000327 (2014).
Graham, J. D., Sonne, M. W. L. & Bray, S. R. It wears me out just imagining it! Mental imagery leads to muscle fatigue and diminished performance of isometric exercise. Biol. Psychol. 103, 1–6 (2014).
Foong, R. et al. Assessment of the efficacy of EEG-based MI-BCI with visual feedback and EEG correlates of mental fatigue for upper-limb stroke rehabilitation. IEEE Trans. Biomed. Eng. 67, 786–795 (2020).
Di Rienzo, F., Rozand, V., Le Noac’h, M. & Guillot, A. A quantitative investigation of mental fatigue elicited during motor imagery practice: Selective effects on maximal force performance and imagery ability. Brain Sci. https://doi.org/10.3390/brainsci13070996 (2023).
Hart, S. G. & Staveland, L. E. In Advances in Psychology (eds Hancock, P. A. & Meshkati, N.) 139–183 (Elsevier, 1988).
Ahlberg, K., Ekman, T., Gaston-Johansson, F. & Mock, V. Assessment and management of cancer-related fatigue in adults. Lancet 362, 640–650 (2003).
Mendoza, T. R. et al. The rapid assessment of fatigue severity in cancer patients: Use of the brief fatigue inventory. Cancer 85, 1186–1196 (1999).
Owen, A. M., McMillan, K. M., Laird, A. R. & Bullmore, E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 25, 46–59 (2005).
Borghini, G., Astolfi, L., Vecchiato, G., Mattia, D. & Babiloni, F. Measuring neurophysiological signals in aircraft pilots and car drivers for the assessment of mental workload, fatigue and drowsiness. Neurosci. Biobehav. Rev. 44, 58–75 (2014).
Craig, A., Tran, Y., Wijesuriya, N. & Nguyen, H. Regional brain wave activity changes associated with fatigue. Psychophysiology 49, 574–582 (2012).
Tran, Y., Craig, A., Craig, R., Chai, R. & Nguyen, H. The influence of mental fatigue on brain activity: Evidence from a systematic review with meta-analyses. Psychophysiology 57, e13554 (2020).
Talukdar, U., Hazarika, S. M. & Gan, J. Q. Motor imagery and mental fatigue: Inter-relationship and EEG based estimation. J. Comput. Neurosci. 46, 55–76 (2019).
Rozand, V., Lebon, F., Stapley, P. J., Papaxanthis, C. & Lepers, R. A prolonged motor imagery session alter imagined and actual movement durations: Potential implications for neurorehabilitation. Behav. Brain Res. SreeTestContent1 297, 67–75 (2016).
Talukdar, U., Hazarika, S. M. & Gan, J. Q. Adaptive feature extraction in EEG-based motor imagery BCI: Tracking mental fatigue. J. Neural Eng. 17, 016020 (2020).
Wascher, E. et al. Frontal theta activity reflects distinct aspects of mental fatigue. Biol. Psychol. https://doi.org/10.1016/j.biopsycho.2013.11.010 (2013).
Trejo, L., Kubitz, K., Rosipal, R., Kochavi, R. & Montgomery, L. EEG-based estimation and classification of mental fatigue. Psychology 6, 572–589 (2015).
Dasari, D., Shou, G. & Ding, L. Investigation of independent components based EEG metrics for mental fatigue in simulated ATC task. In 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER) 1331–1334 (IEEE, 2013).
Gharagozlou, F. et al. Detecting driver mental fatigue based on EEG alpha power changes during simulated driving. Iran. J. Public Health 44, 1693–1700 (2015).
Awais, M., Badruddin, N. & Drieberg, M. IEEE Region 10 Symposium 244–247 (IEEE, 2014).
Nakashima, A. et al. Continuous repetition motor imagery training and physical practice training exert the growth of fatigue and its effect on performance. Brain Sci. https://doi.org/10.3390/brainsci12081087 (2022).
Delorme, A. & Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21 (2004).
Wang, F. et al. Improved brain-computer interface signal recognition algorithm based on few-channel motor imagery. Front. Hum. Neurosci. https://doi.org/10.3389/fnhum.2022.880304 (2022).
Shi, M. et al. EEG signal classification based on SVM with improved squirrel search algorithm. Biomed. Eng. Biomed. Tech. 66, 137–152 (2021).
Perrier, J. et al. Driving performance and EEG fluctuations during on-the-road driving following sleep deprivation. Biol. Psychol. 121, 1–11 (2016).
Cao, J. et al. Brain functional and effective connectivity based on electroencephalography recordings: A review. Hum. Brain Mapp. 43, 860–879 (2022).
van Mierlo, P. et al. Functional brain connectivity from EEG in epilepsy: Seizure prediction and epileptogenic focus localization. Prog. Neurobiol. 121, 19–35 (2014).
Kang, J.-S., Park, U., Gonuguntla, V., Veluvolu, K. C. & Lee, M. Human implicit intent recognition based on the phase synchrony of EEG signals. Pattern Recognit. Lett. 66, 144–152 (2015).
Leeuwis, N., Yoon, S. & Alimardani, M. Functional connectivity analysis in motor-imagery brain computer interfaces. Front. Hum. Neurosci. https://doi.org/10.3389/fnhum.2021.732946 (2021).
Lachaux, J. P., Rodriguez, E., Martinerie, J. & Varela, F. J. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 8, 194–208 (1999).
Cao, T., Wan, F., Wong, C. M., da Cruz, J. N. & Hu, Y. Objective evaluation of fatigue by EEG spectral analysis in steady-state visual evoked potential-based brain-computer interfaces. Biomed. Eng. Online 13, 28 (2014).
Fan, X., Zhou, Q., Liu, Z. & Xie, F. Electroencephalogram assessment of mental fatigue in visual search. Biomed. Mater. Eng. 26, S1455–S1463 (2015).
Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Brain Res. Rev 29, 169–195 (1999).
Azadi Moghadam, M. & Maleki, A. Fatigue factors and fatigue indices in SSVEP-based brain-computer interfaces: A systematic review and meta-analysis. Front. Hum. Neurosci. 17, 1248474 (2023).
Wascher, E. et al. Frontal theta activity reflects distinct aspects of mental fatigue. Biol. Psychol. 96, 57–65 (2014).
Kanthack, T. F. D., Guillot, A., Clémençon, M., Debarnot, U. & Di Rienzo, F. Effect of physical fatigue elicited by continuous and intermittent exercise on motor imagery ability. Res. Q. Exerc. Sport 91, 525–538 (2020).
Qi, P. et al. Neural mechanisms of mental fatigue revisited: New insights from the brain connectome. Engineering 5, 276–286 (2019).
Yang, S., Ai, N., Wang, L., Yin, N. & Xu, G. Researchon brain functional network during mental fatigue. Trans. Beijing Inst. Technol. 37, 67–71 (2017).
Acknowledgements
The authors would like to thank all researchers at the Key Laboratory of Measuring Technology and Instrument, School of Electrical Engineering, Yanshan University, Hebei Province for help in collecting study data.
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 62371416, 62103354]; the Natural Science Foundation of Hebei Province [grant numbers F2022203002, F2022203079, F2022203081]; and the Hebei innovation capability improvement plan project [grant number 22567619H].
Author information
Authors and Affiliations
Contributions
P.X, X.C, J.W supervised this research and contributed to editing this manuscript; T.L was involved in design, data collection, analysis, and writing; D.Z, and Y.W were involved in data collection and editing the manuscript. Y.Z, S.C were involved in data collection.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, T., Zhang, D., Wang, Y. et al. Research on mental fatigue during long-term motor imagery: a pilot study. Sci Rep 14, 18454 (2024). https://doi.org/10.1038/s41598-024-69013-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69013-2
- Springer Nature Limited