Abstract
Phthalates used in the industry penetrate the environment and negatively affect humans and animals. Hair samples seem to be the best matrix for studies on long-term exposure to phthalates, but till now they were used only in investigations on humans. Moreover, the knowledge of the wild terrestrial animal exposure to phthalates is extremely limited. This study aimed to establish of concentration levels of selected phthalate metabolites (i.e. monomethyl phthalate—MMP, monoethyl phthalate—MEP, mono-isobutyl phthalate—MiBP, monobutyl phthalate—MBP, monobenzyl phthalate—MBzP, mono-cyclohexyl phthalate—MCHP, mono(2-ethylhexyl) phthalate—MEHP and mono-n-octyl phthalate—MOP) in wild boar hair samples using liquid chromatography with mass spectrometry (LC–MS) analysis. MEHP was noted in 90.7% of samples with mean 66.17 ± 58.69 pg/mg (median 49.35 pg/mg), MMP in 59.3% with mean 145.1 ± 310.6 pg/mg (median 64.45 pg/mg), MiBP in 37.0% with mean 56.96 ± 119.4 pg/mg (median < limit of detection—LOD), MBP in 35.2% with mean 19.97 ± 34.38 pg/mg (median < LOD) and MBzP in 1.9% with concentration below limit of quantification. MEP, MCHP, and MOP have not been found in wild boar hair samples during this study. The results have shown that wild boars are exposed to phthalates and hair samples may be used as a matrix during studies on levels of phthalate metabolites in wild animals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Phthalates are a large group of synthetic substances, which chemically are the esters of 1,2-benzenedicarboxylic acid1. These substances are synthesized during a reaction between an alcohol and phthalic anhydrite. Due to their properties, such as elasticity and resistance to difficult conditions, phthalates are commonly used in the production of plastics as additives that increase the softness, flexibility, and durability of products2. The history of phthalates in the industry dates back to the 1920s when they began to be added to polyvinyl chloride (PVC)3. In recent years the total production of phthalates is estimated at a level of about 5.5 million tons per year1,2. Phthalates are divided into two groups: high molecular weight phthalates with 7–13 carbon atoms in the carbon chain and low molecular weight phthalates with 3–6 carbon atoms1,2. Phthalates are present in a wide range of everyday objects. High molecular weight phthalates are present among others in household goods, fabrics, toys, food containers, electronic elements, cables, furniture, and car equipment2,3. In turn, low molecular weight phthalates are included in PCV products, paints, inks, cosmetics, and medical devices3,4.
Phthalates can leach out from the objects, in which they are found and enter the environment2. Till now, the presence of phthalates has been observed in various parts of the world in surface water, air, and soil, as well as in food and drinking water, that have been in contact with packaging containing these substances2,5,6. It has also been shown that phthalates may penetrate to human and animal organisms through the digestive tract, respiratory system, and/or the skin2,7. Moreover, in prenatal life, phthalates may enter the body through the placenta8. In living organisms, phthalates are subjected to quick transformation, which involves hydrolysis of the diesters to their respective monoesters, oxidation, and glucuronidation2. Previous studies have shown that the half-life of phthalate diesters in blood plasma and urine is less than 24 h9,10. Therefore, in the biomonitoring not only concentration levels of phthalates but also their metabolites are determined11. The phthalates most frequently used in the industry and their primary metabolites are presented in Table 1.
Phthalates show multidirectional harmful effects on living organisms. As endocrine disruptors, phthalates mimic the actions of natural estrogens and androgens, bind the receptors of these hormones, and therefore cause dysfunction of the endocrine system resulting in disturbances in the proper functioning of many internal organs2. Previous studies have shown the influence of phthalates on the reproductive system. Exposure to these substances in females alters ovarian and uterine functions and may lead to the development of polycystic ovarian syndrome and endometriosis, as well as disorders during pregnancy12. In turn, in males phthalates affect the development and functions of the testis, mainly depressing the functions of Leydig and Sertoli cells12. Therefore, exposure to phthalates may result in reduced semen quality e.g. decreased sperm concentration, smaller sperm motility and an increased percentage of abnormal sperm heads and flagella12. It is also known that phthalates, among others, adversely affect the nervous, cardiovascular, respiratory, gastrointestinal, and immune systems2,3,13. Moreover, previous studies have shown the correlations between the degree of exposure to phthalates and the risk of hypertension and atherosclerosis, diabetes, obesity, autism, allergy, and asthma2,14. Carcinogenic, teratogenic, and genotoxic effects of phthalates are also known, even under the impact of very low concentrations15.
Due to the abovementioned multidirectional harmful influences, monitoring the concentration levels of phthalates and their metabolites in living organisms is an important issue of modern toxicology. Urine is the most preferred matrix for such studies because the excretory system is the main route of elimination of phthalates from the organism16,17. However, the presence of phthalate metabolites has also been documented in other matrices, including saliva, blood serum, semen, breast milk, amniotic fluid, and even cerebrospinal fluid18,19. Among matrices used during the analysis of human exposure to phthalates and their metabolites hair samples deserve special attention, because they seem to be the best matrix for determination of long-term environmental exposure20,21. In the light of previous studies, analysis of hair samples provides a more stable piece of information about phthalates concentration levels, which allows for better determination of chronic level exposure22. Moreover, hair samples can be easily collected, stored, and transported even over long distances. Therefore, hair samples are increasingly used to monitor human exposure to phthalates11,20,22. Till now analysis of hair samples has been used for biomonitoring of phthalates23 and their metabolites11,24 only in humans.
Contrary to humans, the knowledge on wild animal exposure to phthalates is relatively limited and first of all concerns water invertebrates25, fish26, birds27, and marine mammals28. Only single investigations concern terrestrial animals28, and according to the best knowledge of the authors, till now there have been no investigations on the assessment of phthalate or their metabolites conducted on the hair samples collected from wild animals.
The selection of wild boars for this study was not accidental. At present days wild boars more and more often live in the immediate vicinity of human settlements, visit villages and even big cities, and feed near landfills or cultivated fields29,30,31. For this reason, they are exposed to anthropogenic environmental pollutants to a significant extent. Therefore wild boars seemed to be an optimal wild terrestrial mammal species for monitoring the degree of environmental pollution and determining the extent to which anthropogenic substances may affect wild animals. Moreover, it is known that phthalates may disrupt the immune system32, which may be associated with a greater risk of infectious diseases. In turn, wild boars living near cities are often the source of pathogenic microorganisms33, and their high exposure to endocrine disruptors (including phthalates) may result in a higher incidence of infectious diseases in animals and therefore pose a threat to humans and livestock. So, monitoring the exposure of wild boars to endocrine disruptors seems important also for this reason. Simultaneously the collection of samples from wild boars is easier than from other wild animals because the wild boar is a rare species of terrestrial mammal, which is not fully protected and can be legally hunted.
Therefore, the aim of the present study was the evaluation of concentration levels of metabolites of phthalates in the hair samples collected from wild terrestrial mammals. The investigations covered the following substances: monomethyl phthalate (MMP), Monoethyl phthalate (MEP), mono-isobutyl phthalate (MiBP), monobutyl phthalate (MBP), monobenzyl phthalate (MBzP), Mono-cyclohexyl phthalate (MCHP) Mono (2-ethylhexyl) phthalate (MEHP)—mono-n-octyl phthalate (MOP), which were analyzed in the hair samples collected from wild boars living in various regions of Poland. Substances included in the study are primary metabolites of both short and long-alkyl chain phthalates (Table 1), which are commonly used in the industry and, in the light of previous research, most often and significantly pollute the natural environment and affect the living organisms4,6,34,35. Moreover, most of the substances included in this investigation have been previously determined in human hair, which proves that hair samples are an appropriate matrix for the assessment of their levels in the organism11,22.
Results
Substances analyzed in this investigation were observed in the wild boar hair samples at very different concentration levels and with different frequencies (Supplementary materials—Table S1, Table 2). Noteworthy are the significant differences in the concentration levels of substances between samples from particular animals from even one voivodeship (Table S1).
MEHP was noted in the largest number of samples included in the study. It was found in 90.7% of samples and its concentration levels ranged from 14.1 to 312.3 pg/mg (mean 66.17 ± 58.69 pg/mg, median 49.35 pg/mg). The second most common substance noted in the hair samples was MMP. It was present in 59.3% of samples, and its mean concentration levels amounted to 145.1 ± 310.6 pg/mg (median 64.45 pg/mg). Concentration levels of MMP in particular samples ranged from below the limit of quantification—LOQ (14.1 pg/mg) to as many as 1667.9 pg/mg. Other substances were less common in the studied hair samples. MiBP and/or MBP were found in 37.0% and 35.2% of all samples, respectively. The concentration levels of the first of these substances ranged from below LOQ (20.0 pg/mg) to 747 pg/mg (with mean 56.96 ± 119.4 pg/mg and median < LOD) and the second – from below LOQ (15.7 pg/mg) to 171.1 pg/mg (with mean 19.97 ± 34.38 pg/mg and median < LOD). In turn, MBzP was noted sporadically in 1.9% of samples, and its concentration levels did not exceed LOQ (13.4 pg/mg). The presence of other studied substances, namely MEP, MCHP, and MOP, was not found in any of the analyzed samples.
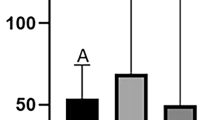
In the case of substances, which were most frequently observed in the hair samples (i.e. MMP, MiBP, MEHP, and MBP) the correlations between their concentration levels and gender of animals (Fig. 1), as well as industrialization and human population density of areas, where animals were hunted were evaluated during the present study (Fig. 2). In males the mean concentration levels (± SD) amounted to 188.9 ± 400.7 pg/mg (median 84.4 pg/mg) for MMP, 29.15 ± 52.93 pg/mg (median < LOD) for MiBP, 17.13 ± 30.06 pg/mg (median < LOD) for MBP and 62.78 ± 63.34 pg/mg (median 47.70 pg/mg) for MEHP. In females mean concentration levels achieved 94.25 ± 143.9 pg/mg (median < LOD), 89.21 ± 161.9 pg/mg (median < LOD), 23.27 ± 39.18 pg/mg (median < LOD), and 70.10 ± 53.81 pg/mg (median 55.40 pg/mg) for MMP, MiBP, MBP, and MEHP, respectively. The observed intragender differences in concentration levels of the above-mentioned substances were not statistically significant in any case (Fig. 1).
Mean concentration levels (± SD) of (A) monomethyl phthalate—MMP, (B) mono-isobutyl phthalate (MiBP), (C) monobutyl phthalate—MBP, and (D) mono(2-ethylhexyl) phthalate in wild boar hair samples of males (M) and females (F). Intragender statistically significant differences (P ≤ 0.05) were not observed. The figure was created using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California USA).
Mean concentration levels (± SD) of (A) monomethyl phthalate—MMP, (B) mono-isobutyl phthalate (MiBP), (C) monobutyl phthalate—MBP, and (D) mono(2-ethylhexyl) phthalate in wild boar hair samples—MEHP in (1) voivodeships with a high degree of industrialization and high human population density (Silesian and Pomeranian) and (2) voivodeships with a low and medium degree of industrialization and low human population density (Kuyavian-Pomeranian, West Pomeranian and Holy Cross). Statistically significant differences (P ≤ 0.05) were not observed. The figure was created using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California USA).
In voivodeships with a high and very high degree of industrialization and a higher human population density (Silesian and Pomeranian), the mean concentration levels of MMP amounted to 200.0 ± 381.5 pg/mg (median 78.35 pg/mg), while in the samples collected in voivodeships with medium and low degree of industrialization and a lower human population density (Kuyavian-Pomeranian, West Pomeranian and Holy Cross), these values amounted to 112.7 ± 261.3 pg/mg (median 64.45 pg/mg) (Fig. 2A). Similar situation was noted in the case of MiBP. Its mean concentration amounted to 73.74 ± 172.4 pg/mg (median < LOD) in areas with high and very high degrees of industrialization and a higher human population density and 47.08 ± 74.33 pg/mg (median < LOD) in less urbanized regions (Fig. 2B).
Interestingly in the case of MBP and MEHP, the situation was reversed (Fig. 2C,D). The mean concentration levels of MBP achieved 7.62 ± 12.44 pg/mg (median < LOD) in areas with high and very high degree of industrialization and a higher human population density and 27.24 ± 40.77 pg/mg (median < LOD) in voivodeships with medium and low degree of industrialization and a lower human population density (Fig. 2C). In turn mean concentration levels of MEHP amounted to 49.07 ± 30.82 pg/mg (median 45.05 pg/mg) and 76.23 ± 68.58 pg/mg (median 54.30 pg/mg) in more and less industrialized regions, respectively. However, all mentioned above differences were not statistically significant.
The presence of MBzP was found in too few samples to determine the correlations mentioned above.
Discussion
The results obtained in this study have shown that phthalate metabolites are present in the hair samples collected from wild boars. Simultaneously clear differences have been observed between the frequency of occurrence and concentration levels of particular substances studied. In the light of present studies MEHP and MMP were the most common in the wild boar hair samples, and generally, it is in agreement with previous investigations. Namely, the majority of previous studies describing the environmental pollution with phthalates have found that parent compounds of MEHP and MMP (i.e. DEHP and DMP, respectively) are phthalates, which are the most common in the environment36,37,38. Some studies have shown that DEHP and DMP may even constitute up to 60% to over 90% of all phthalates polluting the surface water and from 85 to 98% of surface sediments39. Because DEHP and DMP are very widespread in the environment living organisms are exposed to these substances to a significant extent2. This is reflected in previous studies that have shown a high frequency of occurrence of MEHP and MMP, i.e. metabolites of DEHP and DMP, in human bodies11,40. On the other hand, previous studies have proven that the degree of human exposure to phthalates and levels of phthalate metabolites in human organisms clearly depend on the area, where observations have been made41,42. These differences are connected with various factors including the degree of industrialization and urbanization, as well as lifestyle including among others people's habits, diet, or the frequency of using cosmetics and personal care products41,43.
It should be pointed out that the analysis of phthalate metabolites in hair samples is a relatively new method because the first studies on this issue were performed in 201324. Nevertheless, the hair seems to be a good matrix for biomonitoring not only phthalates or their metabolites21 but also other endocrine-disrupting chemicals polluting the environment44, especially in studies on long-term exposure. This is due to the fact that the levels of substances accumulating in hair do not fluctuate as quickly as in blood or urine45,46. Moreover, the collection of hair samples is easy, completely non-invasive, and stress-free, which is particularly important in studies on animals. Another advantage of hair analysis for toxic substances in wild animals is the fact that samples can be taken even some time after the animal's death47. Hair samples may be also easily stored and transported. Despite these undeniable advantages, analysis of hair samples is relatively rarely used to determine the concentration levels of phthalate metabolites, and till now such observations have been performed only in humans (Table 3).
It would seem that wild animals are exposed to anthropogenic pollutants to a far lesser degree than humans. Interestingly, comparing these results with observations concerning the levels of phthalate metabolites in human hair (Table 3), this cannot be stated unequivocally. Of course, in some cases, the exposure of humans is extremely higher as in the case of MEP, which in the present study has not been detected in wild boar hair, but in observations on humans, its concentration levels amounted even above 100 pg/mg. On the other hand maximum concentration levels of MMP and MiBP notes in the present study are higher than those observed in some observations performed in humans (Table 3).
Such relatively high concentration levels of phthalate metabolites in the wild boar hair may result from two reasons. Firstly, it may be connected with the lifestyle of wild boars, which more and more often live in close proximity to human sites49. Animals even enter big cities, live in parks, and feed in dumpsters and landfills. In such conditions, wild boar exposure to anthropogenic pollutants, including phthalates may be high. Secondly, relatively high exposure of wild boars may result from a generally high level of environmental contamination with these substances in the places, where samples were taken.
Unfortunately, previous studies on the presence of phthalates and their metabolites in the environment and living organisms in Poland are not numerous. It has been found that phthalates are present in sea sediments, air and indoor dust50,51. Moreover, phthalate metabolites were commonly found in human blood serum and urine52,53. It has also been found that effluents from municipal wastewater treatment plants and leachates from municipal solid waste landfills are a big threat to the natural environment in Poland and the main source of phthalate pollution54,55,56. Among phthalates in effluents from municipal wastewater treatment plants and leaches from solid waste landfills DEHP is the most predominant56, and its concentration levels are often (in 75% of samples) from 1.7 to 56 times higher than the acceptable UE limit of this substance for surface water (1.3 μg/L)54. Such a situation results in a high risk of pollution of surface waters and soil around the waste landfills54,56 with DEHP and other phthalates. In turn, it poses a serious threat to water organisms and mammals feeding on earthworms and grubs living in the soils around the waste landfills or in the areas, where sewage sludge applications in agriculture take place55. It should be underlined that during the present study, MEHP was noted in the highest number of studied samples, which may suggest that effluents from wastewater treatment plants and leachates from solid waste landfills may be an important source of phthalates for wild boars exposure. This is all the more likely that the grubs, earthworms, and other organisms living in the soil are an important component of the wild boar diet.
Previous studies on levels of phthalate metabolites in wild animals are relatively scanty25,26,27,28, especially in terrestrial species28. Moreover, the comparison of present results with previous studies is difficult due to the fact that previous observations have been made in completely different parts of the world and on completely different animal species and matrices. Despite the difficulties resulting from various matrices, it can be concluded that phthalate metabolites concentration levels noted in wild boars during this study are higher than values observed in wild animals in previous studies27,28.
The present study also covered dependencies between phthalate metabolites concentration levels in the hair samples and animal gender, as well as urbanization and industrialization of areas, where samples were collected. Some previous studies have reported intragender differences in the levels of various endocrine-disrupting chemicals. But it should be underlined that the results concerning this issue are not clear. Some studies have reported higher concentration levels of endocrine-disrupting chemicals in males, other in females, and still others have shown that exposure levels of such substances are the same for both genders57,58,59. The majority of studies on levels of endocrine-disrupting substances in various genders concern humans. In this case, intragender differences may result from various lifestyles, habits, diet, or more frequent use of cosmetics by women, i.e. factors, which do not matter in wild animals41,43. However, some studies suggest that intragender differences in levels of endocrine-disrupting chemicals may result from differences in hormonal activity, metabolic rate, and/or ratio of body weight to the amount of food consumed60. In the case of phthalate and their metabolites studies conducted on humans have shown that in women levels of these substances are higher than in men61,62. Moreover, intragender differences in correlations between exposure to phthalates and disturbances in metabolism have been reported63,64.
Intragender differences in levels of phthalate metabolites have not been observed in the present study. This fact suggests that differences noted in humans resulted from various lifestyles, habits, and frequency in the use of cosmetics, which has also been suggested by previous studies41,43,62. Moreover, the lack of intragender differences noted in the present work is in agreement with previous investigations in rats, in which no significant differences in toxicokinetics and toxicodynamics of DiBP have not been observed65.
Previous studies have shown that phthalate metabolites levels in human organisms vary significantly depending on the place, where the research was conducted41,62. It would seem that levels of anthropogenic pollutants are higher in areas with a higher degree of urbanization and industrialization. However, results concerning phthalates and their metabolites are inconclusive. The presence of these substances in the environment and human organisms has been observed in various regions, not only highly urbanized ones (Table 3). Moreover, there is evidence that the concentration levels of phthalate metabolites in hair collected from people living in rural areas may be higher than values noted in the hair of people living in cities20. Such a situation is probably connected with the use of phthalates in agriculture, for example as a component of artificial fertilizers and plant protection products66, as well as with the pollution of the rural environment with mentioned effluents from wastewater treatment plants and leachates from solid waste landfills54,55,56. Moreover, previous investigations have described the presence of phthalates in agricultural soils, vegetables, and crop plants67,68. The present results concerning dependents between phthalate metabolites levels in wild boar samples and the degree of urbanization and industrialization are ambiguous. Admittedly, such differences have been shown, but they were not statistically significant. Moreover, mean levels of some phthalate metabolites were higher in less industrialized regions. This fact may confirm previous research that exposure to phthalates may also be significant in agricultural areas20. The exposure of wild boars to phthalates in non-industrially, agricultural areas results from the contamination of the soil and the organisms living in it (earthworms, grubs), which (as already mentioned) are the food of wild boars. The second possible sources of exposure to phthalates for wild boars in the rural areas are vegetables and crop plants, which also may be polluted with phthalates and are often part of the wild boar diet.
A fundamental question arises, whether the levels of phthalate metabolites observed in this study have an adverse effect on the health of wild boars. Unfortunately, till now there are no studies on the phthalate metabolism in the wild boar and correlations between the phthalate concentration levels in the hair, blood, urine, and other tissues of this animal species. Previous studies in humans have shown that correlations between phthalate metabolites levels in hair and urine are often not exact and suggest that analysis of these matrices usually give not the same but complementary information22. Namely, analysis of the hair samples allows for the assessment of long-term exposure, and levels of phthalate metabolites in the urine reflect short-term exposure22,46. Therefore, a clear answer to the above question is very difficult at the current stage of knowledge. It can be only assumed that phthalates may affect the wild boar’s health status. This assumption is supported by the fact that in other mammal species, even small environmental doses of phthalates cause disorders in various internal organs69,70. Moreover, phthalates are only one group of endocrine-disrupting chemicals that pollute the environment. Living organisms are usually exposed to a wide range of such substances, which often have a synergistic effect71. In this case, even low exposure to particular substances may result in negative health effects.
Materials and methods
Reagents
During the present study, the following reagents have been used: phthalate metabolites, MMP, MBP, MCHP and MEHP purchased from Sigma-Aldrich (St. Louis MO, USA), MEP, MiBP, MBZP, and MOP from Toronto Research Chemicals (TRC Inc), methanol and acetonitrile (LC–MS grade) purchased from Fisher Chemical, phenobarbital (internal standard—IS) purchased from Lipomed AG, (Arlesheim Switzerland)), ultrapure water produced by a Direct-Q 3UV water purification system (Merck, Germany).
Sample collection
The method of sample collection has been previously described by Gonkowski et al.72. In short, 54 adult wild boars of both genders (29 male and 25 female) were included in this study. The animals were hunted during legal hunting organized by the Polish Hunting Association. The huntings took place in the Kuyavian-Pomeranian, West Pomeranian, Pomeranian, Silesian, and Holy Cross Voivodeships in the years 2020–2022. Characterization of animals included in this study are presented in supplementary materials (Table S1), and a description of voivodeships, where hunting took place is presented in Table 4.
Hair samples were collected within a maximum of 30 min after the death of animals. Hair (about 2 g) from each animal included in the study was cut closest to the skin from the same place on the abdomen. Immediately after cutting hair was wrapped in aluminum foil and placed in a dark dry place at room temperature. In such conditions, hair samples were stored until further investigations. Due to the fact that hair samples were collected from dead animals hunted during legal hunting permitted by Polish legislation, consent for research from the ethical committee was not required. This is in accordance with the Act for the Protection of Animals for Scientific or Educational Purposes of 15 January 2015 (Official Gazette 2015, No. 266), applicable in the Republic of Poland. The number of samples included in the study was limited by the number of legal hunting organized by the Polish Hunting Association and animals hunted.
Extraction of phthalate metabolites
Samples were prepared according to the method described previously by Tzatzarakis et al.44. At first hair samples were cut into small fragments with a length of several millimeters. Then external contaminations were removed from the hair by double rinsing of the samples with ultrapure water and double rinsing with methanol. After rinsing the samples were dried at 50 °C. The extraction of phthalate metabolites was made up according to the method described by Tzatzarakis et al.44 and Katsikantami et al.11. 100 mg of each sample were put into glass screw tubes with 2 × 2 ml of methanol and 25 ng of IS. To avoid any contamination, only glass tubes, which were washed with methanol (LC–MS grade) and dried at 80 °C were used in the extraction procedure, while the hair samples were stored in aluminum foil. Blank samples were analyzed with each batch. Extraction was carried out in an ultrasonic water bath for 2 × 2 h with periodic mixing with a vortex system. The extracts were evaporated to dryness under nitrogen steam at 35 °C and reconstituted by adding 100 μl of methanol. The obtained solution was transferred into 2 ml vials with inserts for liquid chromatography–mass spectrometry (LC–MS) analysis. 10 μl ml of solution was injected into the system.
Instrumentation
An LC–MS system (2010 EV, Shimadzu) was used for the detection and quantification of phthalate metabolites after the separation of the substances on a Supelco Discovery column C18 (250 mm, 4.6 mm, 5 μm, Sigma-Aldrich, St. Louis, MO, USA), which was conducted at 30 °C. The analysis was made up with a flow rate of 0.6 ml/min using 5 mM ammonium acetate (solvent A) and acetonitrile 0.1% formic acid (solvent B). To monitor the analytes, an atmospheric pressure chemical ionization (APCI) and a quadrupole mass filter in negative selected ion monitoring (SIM) mode were used. Retention times and selected ions m/z for each substance were as follows: IS: 14.08 min, 231.05 m/z, MMP: 9.15 min, 179.00, 225.05 m/z, MEP: 12.71 min, 193.00, 239.05 m/z, MiBP: 17 min, 221.10, 267.10 m/z, MBP: 17.15 min, 221.10, 267.1 m/z, MBzP: 17.48 min, 255.10, 301.05 m/z, MCHP: 18.4 min, 247.10, 293.1 m/z, MEHP: 22.21 min, 277.10, 323.1 m/z and MOP: 22.76 min, 277.10, 323.1 m/z (Table 3). The interface, CDL, and heat block temperatures were set at 400 °C, 200 °C, and 200 °C, respectively. The detector voltage was set at 1.5 kV, the drying gas pressure was set at 0.02 MPa and the nebulizing gas flow at 2.5 L/min.
Method validation
The performance of the analytical method was examined. To this aim, standard solutions of phthalate metabolites were made and their linearity was found to be from 0.9896 for MMP to 0.9949 for MEP. Spiked sample analysis was made for concentrations of 0, 10, 25, 50, 100, and 250 pg/mg with linearity from 0.9837 for MEHP to 0.9986 for MEP (Table 5).
The limit of detection (LOD) and limit of quantification (LOQ) were evaluated using the signal to noise ratio, (signal to noise ratio > 3 and > 10, respectively). Three repeats of spiked samples (n = 3) were used for the evaluation of the recovery and inter-day precision (%RSD), and the accuracy of the method. The mean values of precision, accuracy and recovery of the applied method are depicted in Table 5.
Statistical analysis
The statistical analysis was made using GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, California, USA) and the nonparametric Mann–Whitney test was used. Data are presented as mean ± standard deviation (SD) and median and the differences were considered as statistically significant at P < 0.05. In the statistical analysis values below LOQ and LOD were taken into account as LOQ/2 and LOD/2, respectively.
Conclusions
Results obtained during the present study clearly indicate that wild boars in Poland are exposed to phthalates, especially to DEHM and DMP, whose metabolites MEHP and MMP have been found in the majority of hair samples included in the study. There were no intragender differences in phthalate metabolites levels in wild boars. Phthalate metabolites were found in samples collected both in regions with a high degree of industrialization and urbanization and in areas of an agricultural nature. The concentration levels of some phthalate metabolites in wild boars are relatively high, which is connected with the penetration of phthalates to soil, surface water, and plants. It can be assumed that concentration levels of phthalate metabolites may affect wild boar health status. However, due to the fact that exact metabolism of phthalates in wild boars is unknown and the knowledge on the correlation between levels of phthalate metabolites in hair and urine or serum is very limited, the precise explanation of phthalates impact on the wild boar health status requires further studies. It should be underlined that this is the first use of analysis of hair samples in studies concerning phthalate metabolites levels in wild animals, and results confirm that such analysis in animals, similarly to humans, is suitable for biomonitoring of long-term exposure to phthalates.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
References
Wang, Y., Zhu, H. & Kannan, K. A review of biomonitoring of phthalate exposures. Toxics 7, 21. https://doi.org/10.3390/toxics7020021 (2019).
Chang, W. H., Herianto, S., Lee, C. C., Hung, H. & Chen, H. L. The effects of phthalate ester exposure on human health: A review. Sci. Total Environ. 786, 147371. https://doi.org/10.1016/j.scitotenv.2021.147371 (2021).
Benjamin, S. et al. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard. Mater. 340, 360–383. https://doi.org/10.1016/j.jhazmat.2017.06.036 (2017).
Zhang, Y. J., Guo, J. L., Xue, J. C., Bai, C. L. & Guo, Y. Phthalate metabolites: Characterization, toxicities, global distribution, and exposure assessment. Environ. Pollut. 291, 118106. https://doi.org/10.1016/j.envpol.2021.118106 (2021).
Kashyap, D. & Agarwal, T. Concentration and factors affecting the distribution of phthalates in the air and dust: A global scenario. Sci. Total Environ. 635, 817–827. https://doi.org/10.1016/j.scitotenv.2018.04.158 (2018).
Cui, D., Ricardo, M. & Quinete, N. A novel report on phthalates levels in Biscayne Bay surface waters and drinking water from South Florida. Mar. Pollut. Bull. 180, 113802. https://doi.org/10.1016/j.marpolbul.2022.113802 (2022).
Heudorf, U., Mersch-Sundermann, V. & Angerer, J. Phthalates: Toxicology and exposure. Int. J. Hyg. Environ. Health 210, 623–634. https://doi.org/10.1016/j.ijheh.2007.07.011 (2007).
Warner, G. R., Dettogni, R. S., Bagchi, I. C., Flaws, J. A. & Graceli, J. B. Placental outcomes of phthalate exposure. Reprod. Toxicol. 103, 1–17. https://doi.org/10.1016/j.reprotox.2021.05.001 (2021).
Latini, G. Monitoring phthalate exposure in humans. Clin. Chim. Acta 361, 20–29. https://doi.org/10.1016/j.cccn.2005.05.003 (2005).
Frederiksen, H., Skakkebaek, N. E. & Andersson, A. M. Metabolism of phthalates in humans. Mol. Nutr. Food Res. 51, 899–911. https://doi.org/10.1002/mnfr.200600243 (2007).
Katsikantami, I. et al. Biomonitoring of bisphenols A and S and phthalate metabolites in hair from pregnant women in Crete. Sci. Total Environ. 712, 135651. https://doi.org/10.1016/j.scitotenv.2019.135651 (2020).
Hlisníková, H., Petrovičová, I., Kolena, B., Šidlovská, M. & Sirotkin, A. Effects and mechanisms of phthalates’ action on reproductive processes and reproductive health: A literature review. Int. J. Environ. Res. Public Health 17, 6811. https://doi.org/10.3390/ijerph17186811 (2020).
Kimber, I. & Dearman, R. J. An assessment of the ability of phthalates to influence immune and allergic responses. Toxicology 271, 73–82. https://doi.org/10.1016/j.tox.2010.03.020 (2010).
Mariana, M. & Cairrao, E. The relationship between phthalates and diabetes: A review. Metabolites 13, 746. https://doi.org/10.3390/metabo13060746 (2023).
Caldwell, J. C. DEHP: Genotoxicity and potential carcinogenic mechanisms—A review. Mutat. Res. 751, 82–157 (2012).
Genuis, S. J., Beesoon, S., Lobo, R. A. & Birkholz, D. Human elimination of phthalate compounds: Blood, urine, and sweat (BUS) study. Sci. World J. 2012, 615068. https://doi.org/10.1100/2012/615068 (2012).
Domínguez-Romero, E. & Scheringer, M. A review of phthalate pharmacokinetics in human and rat: What factors drive phthalate distribution and partitioning?. Drug Metab. Rev. 51, 314–329. https://doi.org/10.1080/03602532.2019.1620762 (2019).
Kato, K. et al. Quantitative detection of nine phthalate metabolites in human serum using reversed-phase high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. J. Anal. Toxic. 27, 284–289 (2003).
Agin, A. et al. Environmental exposure to phthalates and dementia with Lewy bodies: Contribution of metabolomics. J. Neurol. Neurosurg. Psychiatry 91, 968–974. https://doi.org/10.1136/jnnp-2020-322815 (2020).
He, M. J., Lu, J. F., Ma, J. Y., Wang, H. & Du, X. F. Organophosphate esters and phthalate esters in human hair from rural and urban areas, Chongqing, China: Concentrations, composition profiles and sources in comparison to street dust. Environ. Pollut. 237, 143–153. https://doi.org/10.1016/j.envpol.2018.02.040 (2018).
Hsu, J. F., Chang, W. C., Ho, W. Y. & Liao, P. C. Exploration of long-term exposure markers for phthalate esters in human hair using liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 1200, 339610. https://doi.org/10.1016/j.aca.2022.339610 (2022).
Fäys, F. et al. Biomonitoring of fast-elimination endocrine disruptors. Results from a 6-month follow up on human volunteers with repeated urine and hair collection. Sci. Total Environ. 778, 146330. https://doi.org/10.1016/j.scitotenv.2021.146330 (2021).
Cleys, P. et al. Hair as an alternative matrix to assess exposure of premature neonates to phthalate and alternative plasticizers in the neonatal intensive care unit. Environ. Res. 236, 116712. https://doi.org/10.1016/j.envres.2023.116712 (2023).
Chang, Y.-J., Lin, K.-L. & Chang, Y.-Z. Determination of Di-(2-ethylhexyl)phthalate (DEHP) metabolites in human hair using liquid chromatography–tandem mass spectrometry. Clin. Chim. Acta 420, 155–159. https://doi.org/10.1016/j.cca.2012.10.009 (2013).
Raguso, C. et al. Detection of microplastics and phthalic acid esters in sea urchins from Sardinia (Western Mediterranean Sea). Mar. Pollut. Bull. 185, 114328. https://doi.org/10.1016/j.marpolbul.2022.114328 (2022).
Martins, K. et al. Tissue phthalate levels correlate with changes in immune gene expression in a population of juvenile wild salmon. Arch. Environ. Contam. Toxicol. 71, 35–47. https://doi.org/10.1007/s00244-016-0283-7 (2016).
Allen, S. F. et al. Phthalate diversity in eggs and associations with oxidative stress in the European herring gull (Larus argentatus). Mar. Pollut. Bull. 169, 112564. https://doi.org/10.1016/j.marpolbul.2021.112564 (2021).
Routti, H. et al. Concentrations and endocrine disruptive potential of phthalates in marine mammals from the Norwegian Arctic. Environ. Int. 152, 106458. https://doi.org/10.1016/j.envint.2021.106458 (2021).
Csókás, A. et al. Space use of wild boar (Sus Scrofa) in Budapest: Are they resident or transient city dwellers?. Biol. Futur. 71, 39–51. https://doi.org/10.1007/s42977-020-00010-y (2020).
Castillo-Contreras, R. et al. Urban wild boars prefer fragmented areas with food resources near natural corridors. Sci. Total Environ. 615, 282–288. https://doi.org/10.1016/j.scitotenv.2017.09.277 (2018).
Castillo-Contreras, R. et al. Wild boar in the city: Phenotypic responses to urbanisation. Sci. Total Environ. 773, 145593. https://doi.org/10.1016/j.scitotenv.2021.145593 (2021).
Zhang, Y., Lyu, L., Tao, Y., Ju, H. & Chen, J. Health risks of phthalates: A review of immunotoxicity. Environ. Pollut. 313, 120173. https://doi.org/10.1016/j.envpol.2022.120173 (2022).
Jansen, A. et al. Leptospirosis in urban wild boars, Berlin, Germany. Emerg. Infect. Dis. 13, 739–742. https://doi.org/10.3201/eid1305.061302 (2007).
Przybylińska, P. A. & Wyszkowski, M. Environmental contamination with phthalates and its impact on living organisms. Ecol. Chem. Eng. S 23, 347–356. https://doi.org/10.1515/eces-2016-0024 (2016).
Schmidt, N., Castro-Jiménez, J., Oursel, B. & Sempéré, R. Phthalates and organophosphate esters in surface water, sediments and zooplankton of the NW Mediterranean Sea: Exploring links with microplastic abundance and accumulation in the marine food web. Environ. Pollut. 272, 115970. https://doi.org/10.1016/j.envpol.2020.115970 (2021).
Gao, D. W. & Wen, Z. D. Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Sci. Total Environ. 541, 986–1001. https://doi.org/10.1016/j.scitotenv.2015.09.148 (2016).
Abtahi, M. et al. Health risk of phthalates in water environment: Occurrence in water resources, bottled water, and tap water, and burden of disease from exposure through drinking water in Tehran, Iran. Environ. Res. 173, 469–479. https://doi.org/10.1016/j.envres.2019.03.071 (2019).
Vasseghian, Y., Alimohamadi, M., Dragoi, E. N. & Sonne, C. A global meta-analysis of phthalate esters in drinking water sources and associated health risks. Sci. Total Environ. 903, 166846. https://doi.org/10.1016/j.scitotenv.2023.166846 (2023).
Li, X., Yin, P. & Zhao, L. Phthalate esters in water and surface sediments of the Pearl River Estuary: Distribution, ecological, and human health risks. Environ. Sci. Pollut. Res. Int. 23, 19341–19349. https://doi.org/10.1007/s11356-016-7143-x (2016).
Li, Z. et al. Phthalate metabolites in paired human serum and whole blood. Sci. Total Environ. 824, 153792. https://doi.org/10.1016/j.scitotenv.2022.153792 (2022).
Bai, P. Y. et al. The association of socio-demographic status, lifestyle factors and dietary patterns with total urinary phthalates in Australian men. PLoS One 10, e0122140. https://doi.org/10.1371/journal.pone.0122140 (2015).
Xiang, S., Dong, J., Li, X. & Li, C. Urine phthalate levels and liver function in US adolescents: Analyses of NHANES 2007–2016. Front. Public Health 10, 843971. https://doi.org/10.3389/fpubh.2022.843971 (2022).
Hallberg, I. et al. Associations between lifestyle factors and levels of per- and polyfluoroalkyl substances (PFASs), phthalates and parabens in follicular fluid in women undergoing fertility treatment. J. Expo. Sci. Environ. Epidemiol. 33, 699–709. https://doi.org/10.1038/s41370-023-00579-1 (2023).
Tzatzarakis, M. N. et al. Biomonitoring of bisphenol A in hair of Greek population. Chemosphere 118, 336–341. https://doi.org/10.1016/j.chemosphere.2014.10.044 (2015).
Alves, A. et al. Human biomonitoring of emerging pollutants through non-invasive matrices: State of the art and future potential. Anal. Bioanal. Chem. 406, 4063–4088. https://doi.org/10.1007/s00216-014-7748-1 (2014).
Zhang, S. et al. Human hair as a noninvasive matrix to assess exposure to micro-organic contaminants: State of the art review. Sci. Total Environ. 892, 164341. https://doi.org/10.1016/j.scitotenv.2023.164341 (2023).
Iatrou, E. I. et al. Monitoring of environmental persistent organic pollutants in hair samples collected from wild terrestrial mammals of Primorsky Krai, Russia. Environ. Sci. Pollut. Res. Int. 26, 7640–7650. https://doi.org/10.1007/s11356-019-04171-9 (2019).
Li, N., Ying, G. G., Hong, H., Tsang, E. P. K. & Deng, W. J. Plasticizer contamination in the urine and hair of preschool children, airborne particles in kindergartens, and drinking water in Hong Kong. Environ. Pollut. 271, 116394. https://doi.org/10.1016/j.envpol.2020.116394 (2021).
Basak, S. M. et al. Public perceptions and attitudes toward urban wildlife encounters—A decade of change. Sci. Total Environ. 834, 155603. https://doi.org/10.1016/j.scitotenv.2022.155603 (2022).
Lubecki, L. & Kowalewska, G. Plastic-derived contaminants in sediments from the coastal zone of the southern Baltic Sea. Mar. Pollut. Bull. 146, 255–262. https://doi.org/10.1016/j.marpolbul.2019.06.030 (2019).
Szewczyńska, M., Dobrzyńska, E. & Pośniak, M. Determination of phthalates in particulate matter and gaseous phase emitted in indoor air of offices. Environ. Sci. Pollut. Res. Int. 28, 59319–59327. https://doi.org/10.1007/s11356-020-10195-3 (2021).
Jurewicz, J. et al. Human urinary phthalate metabolites level and main semen parameters, sperm chromatin structure, sperm aneuploidy and reproductive hormones. Reprod. Toxicol. 42, 232–241. https://doi.org/10.1016/j.reprotox.2013.10.001 (2013).
Specht, I. O. et al. Associations between serum phthalates and biomarkers of reproductive function in 589 adult men. Environ. Int. 66, 146–156. https://doi.org/10.1016/j.envint.2014.02.002 (2014).
Wowkonowicz, P. & Kijeńska, M. Phthalate release in leachate from municipal landfills of central Poland. PLoS One 12, e0174986. https://doi.org/10.1371/journal.pone.0174986 (2017).
Wowkonowicz, P., Kijeńska, M. & Koda, E. Potential environmental risk assessment of di-2-ethylhexyl phthalate emissions from a municipal solid waste landfill leachate. PeerJ. 9, e12163. https://doi.org/10.7717/peerj.12163 (2021).
Kotowska, U., Kapelewska, J. & Sawczuk, R. Occurrence, removal, and environmental risk of phthalates in wastewaters, landfill leachates, and groundwater in Poland. Environ. Pollut. 267, 115643. https://doi.org/10.1016/j.envpol.2020.115643 (2020).
Takeuchi, T. & Tsutsumi, O. Serum bisphenol a concentrations showed gender differences, possibly linked to androgen levels. Biochem. Biophys. Res. Commun. 291, 76–78. https://doi.org/10.1006/bbrc.2002.6407 (2002).
Karzi, V. et al. Biomonitoring of bisphenol A, triclosan and perfluorooctanoic acid in hair samples of children and adults. J. Appl. Toxicol. 38, 1144–1152. https://doi.org/10.1002/jat.3627 (2018).
Mahalingaiah, S. et al. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ. Health Perspect. 116, 173–178. https://doi.org/10.1289/ehp.10605 (2008).
Makowska, K. et al. Biomonitoring parabens in dogs using fur sample analysis—Preliminary studies. Sci. Total. Environ. 807, 150757. https://doi.org/10.1016/j.scitotenv.2021.150757 (2022).
Huang, P. C. et al. Age and gender differences in urinary levels of eleven phthalate metabolites in general Taiwanese population after a DEHP episode. PLoS One 10, e0133782. https://doi.org/10.1371/journal.pone.0133782 (2015).
Stuchlík Fišerová, P. et al. Personal care product use and lifestyle affect phthalate and DINCH metabolite levels in teenagers and young adults. Environ. Res. 213, 113675. https://doi.org/10.1016/j.envres.2022.113675 (2022).
Dong, R. et al. Gender- and age-specific relationships between phthalate exposures and obesity in Shanghai adults. Arch. Environ. Contam. Toxicol. 73, 431–441. https://doi.org/10.1007/s00244-017-0441-6 (2017).
Weng, T. I. et al. Effects of gender on the association of urinary phthalate metabolites with thyroid hormones in children: A prospective cohort study in Taiwan. Int. J. Environ. Res. Public Health 14, 123. https://doi.org/10.3390/ijerph14020123 (2017).
Jeong, S. H., Jang, J. H., Cho, H. Y. & Lee, Y. B. Toxicokinetic studies of di-isobutyl phthalate focusing on the exploration of gender differences in rats. Chemosphere 286, 131706. https://doi.org/10.1016/j.chemosphere.2021 (2022).
Kumari, A. & Kaur, R. A review on morpho-physiological traits of plants under phthalates stress and insights into their uptake and translocation. Plant Growth Regul. 91, 327–347 (2020).
Sun, J., Wu, X. & Gan, J. Uptake and metabolism of phthalate esters by edible plants. Environ. Sci. Technol. 49, 8471–8478 (2015).
Sun, J. et al. Phthalate esters and organochlorine pesticides in agricultural soils and vegetables from fast-growing regions: A case study from eastern China. Environ. Sci. Pollut. Res. 25, 34–42 (2018).
Adegoke, E. O. et al. Environmentally relevant doses of endocrine disrupting chemicals affect male fertility by interfering with sertoli cell glucose metabolism in mice. Chemosphere 337, 139277. https://doi.org/10.1016/j.chemosphere.2023.139277 (2023).
Ducroq, S., Grange-Messent, V. & Mhaouty-Kodja, S. Exposure to low doses of phthalates in male rodents: Effects on reproductive and cognitive behaviors. Neuroendocrinology 113, 1215–1231. https://doi.org/10.1159/000534836 (2023).
Hamid, N., Junaid, M. & Pei, D. S. Combined toxicity of endocrine-disrupting chemicals: A review. Ecotoxicol. Environ. Saf. 215, 112136. https://doi.org/10.1016/j.ecoenv.2021.112136 (2021).
Gonkowski, S., Tzatzarakis, M., Vakonaki, E., Meschini, E. & Rytel, L. Exposure assessment to bisphenol A (BPA) and its analogues bisphenol S (BPS) and bisphenol F (BPF) in wild boars by hair analysis. Sci. Total Environ. 905, 167076. https://doi.org/10.1016/j.scitotenv.2023.167076 (2023).
Author information
Authors and Affiliations
Contributions
SG: Writing—original draft, formal analysis, supervision, conceptualization, planning the investigation, MT: conceptualization, investigation, validation, writing—review & editing, EM and EV: analysis of the hair samples. LK: statistical analysis, investigation, LR: conceptualization, sample collection, writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gonkowski, S., Tzatzarakis, M., Vakonaki, E. et al. Concentration levels of phthalate metabolites in wild boar hair samples. Sci Rep 14, 17228 (2024). https://doi.org/10.1038/s41598-024-68131-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68131-1
- Springer Nature Limited