Abstract
Limited data exist on long-term renal outcomes in patients with hyperglycemic crisis (HC) as initial type 2 diabetes presentation. We evaluated the risk of chronic kidney disease (CKD) development in those with concurrent HC at diagnosis. Utilizing Taiwan’s insurance claims from adults newly diagnosed with type 2 diabetes during 2006–2015, we created HC and matched non-HC cohorts. We assessed incident CKD/diabetic kidney disease (DKD) by 2018’s end, calculating the hazard ratio (HR) with the Cox model. Each cohort comprised 13,242 patients. The combined CKD and DKD incidence was two-fold higher in the HC cohort than in the non-HC cohort (56.47 versus 28.49 per 1000 person-years) with an adjusted HR (aHR) of 2.00 (95% confidence interval [CI] 1.91–2.10]). Risk increased from diabetic ketoacidosis (DKA) (aHR:1.69 [95% CI 1.59–1.79]) to hyperglycemic hyperosmolar state (HHS) (aHR:2.47 [95% CI 2.33–2.63]) and further to combined DKA-HHS (aHR:2.60 [95% CI 2.29–2.95]). Subgroup analysis in individuals aged ≥ 40 years revealed a similar trend with slightly reduced incidences and HRs. Patients with HC as their initial type 2 diabetes presentation face a higher CKD risk than do those without HC. Enhanced medical attention and customized interventions are crucial to reduce this risk.
Similar content being viewed by others
Introduction
Diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state (HHS) represent life-threatening hyperglycemic crises (HC) in patients with diabetes1. Although commonly seen in patients with preexisting diabetes, up to 20% of cases occur in those newly diagnosed2,3,4. Individuals with HC episodes face higher risks of subsequent morbidity and mortality compared with those without such episodes5,6,7. However, limited research explores the risk of chronic complications in patients experiencing HC during diabetes diagnosis, underscoring the need for further investigation.
Previous studies have reported an increased risk of subsequent stroke8,9, cardiovascular events10, long-term mortality11,12, and end-stage renal disease13 in patients with diabetes who have experienced an HC. Nevertheless, these studies overlook the confounding effect of glycemic control on the development of diabetes-related chronic complications. Moreover, type 1 and type 2 diabetes possess distinct pathophysiologies and should not be considered as a single entity; however, only two studies exclusively included patients with type 2 diabetes8,9, and none were conducted in patients with newly diagnosed diabetes. In contrast, an Italian multicenter cohort study demonstrated that patients with DKA upon type 1 diabetes onset were not at an increased risk of diabetic retinopathy or albuminuria14. The reasons behind these conflicting findings and the impact of HC that occurs in patients newly diagnosed with type 2 diabetes remain to be elucidated.
Therefore, in this study, we aimed to examine the risk of developing chronic kidney disease (CKD), one of the major chronic complications of diabetes, in patients experiencing HC upon type 2 diabetes diagnosis. We hypothesized that HC occurring upon type 2 diabetes diagnosis is associated with a higher risk of developing CKD. Our findings could hold significance if this unique initial presentation of type 2 diabetes can be utilized to identify patients at increased risk of developing CKD; moreover, it could facilitate the implementation of preventive interventions to reduce CKD risk in this population.
Methods
Data sources
In this study, we utilized Taiwan’s insurance claims data to identify adults newly diagnosed with type 2 diabetes between 2006 and 2015. We used the Taiwan National Health Insurance Research Database (NHIRD), a comprehensive repository established in 1995 that covers over 99% of the nation’s residents15,16. The NHIRD comprises encrypted records encompassing sociodemographic information, household income, residency, outpatient/inpatient care, and prescribed medications. Diseases in the NHIRD are coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) before 2016 and Tenth Revision (ICD-10-CM). Previous investigations have validated the NHIRD’s accuracy and reliability for population-based studies17,18.
Design setting and study cohorts
From the NHIRD, we used the ICD-9-CM or ICD-10-CM and Anatomical Therapeutic Chemical Codes (Tables S1 and Table S2) to identify newly diagnosed cases with type 2 diabetes (ICD-9-CM codes: 250. X0 or 250. × 2) during the study period (Fig. 1). We excluded individuals who were diagnosed with diabetes or using glucose-lowering drugs before the study period, younger than 20 years of age, had a history of kidney disease including benign or malignant neoplasms of the kidney, chronic kidney disease of any cause, glomerulonephritis, nephrotic syndrome, urolithiasis, and congenital renal anomalies, or were deceased at baseline. Individuals with concurrent HC at the time of their type 2 diabetes diagnosis (first-time type 2 diabetes diagnosis appeared with at least one of the following ICD-9-CM codes: 250.10, 250.12, 250.20, or 250.22) were categorized into the HC cohort. The clinical diagnostic criteria for HC were based on previous guidelines19,20, and our data were based on diagnostic codes from all medical facilities regardless of inpatient/outpatient care or emergency department visits. Regarding individuals without HC, we selected a control (non-HC) cohort with the same sample size frequency matched by diagnosis year and propensity score. The propensity scores were calculated using multivariable logistic regression for each person at baseline, including sex, age, type of residence, enrollment category, monthly income, comorbidities, and use of angiotensin-converting enzyme inhibitors (ACEis) or angiotensin II receptor blockers (ARBs). Comorbidities were diseases with at least two outpatient claims or one inpatient claim within 1 year of the index date and included hypertension, heart failure, coronary artery disease, ischemic stroke, transient ischemic attack, peripheral arterial disease, hyperlipidemia, obesity, and malignancy. Variables with a between-group standardized mean difference of < 0.1 were considered well-balanced21. Sodium-glucose cotransporter-2 inhibitors were not included due to their unavailability in Taiwan during the enrollment period.
The two cohorts were followed up until the end of 2018. Our outcome of interest was the combined incidence of CKD (ICD-9-CM codes: 585–586; ICD-10-CM code: N184–N186, N189–N19) or diabetic kidney disease (DKD) (ICD-9-CM codes: 250.40 or 250.42; ICD-10-CM: E112), or both. In Taiwan, it is generally accepted that CKD is characterized by a decreased glomerular filtration rate of less than 60 mL/min per 1.73 m2 and/or markers of kidney damage that persist for longer than 3 months. DKD is a clinical diagnosis that refers to those cases with CKD presumed to be caused by diabetes. Clinicians are free to use whichever code is appropriate based on their judgment. Hence, the data of patients with diabetes and CKD may be coded using CKD, DKD, or both. As DKD is within the spectrum of CKD and our aim was to explore the risk of CKD, we used both codes to maximize the probability of capturing our main outcome of interest.
Institutional review board statement
This study was approved by the Institutional Review Board of Mackay Memorial Hospital (approval number: 22MMHIS382e). Our study was performed in accordance with the Declaration of Helsinki and all our methods were carried out under relevant guidelines and regulations. As all personal identifications in the database were encrypted and unidentifiable, the requirement for informed consent from the insured individuals was waived.
Statistical analysis
Baseline characteristics and comorbidities were compared between the HC and non-HC cohorts using Pearson’s χ2 test for categorical variables and Student’s t-test for continuous variables. The HC cohort comprised three sub-cohorts: patients with DKA (ICD-9-CM codes: 250.10 or 250.12), HHS (ICD-9-CM codes: 250.20 or 250.22), and combined DKA and HHS (DKA-HHS). The cumulative incidence rate of combined CKD and DKD between the HC and non-HC cohorts, as well as between the HC sub-cohorts, was estimated and plotted using the Kaplan–Meier method. Inter-group differences were examined using the log-rank test. Cox proportional hazards regression analysis was used to calculate the crude hazard ratio comparing the HC and non-HC cohorts; moreover, the adjusted hazard ratio (aHR), along with its corresponding 95% confidence interval (CI), was used for the combined occurrence of CKD and DKD. The aHR was estimated after adjusting for age, sex, socioeconomic factors, and significant comorbidities. As patients who died before the event occurred will never be coded with CKD and/or DKD, the competing risk of death was managed using the Fine-Grey analysis model to estimate the sub-distribution hazard ratio (sHR)22. To address potential misclassification and pollution bias from claims data, where type 1 diabetes might have been incorrectly recorded as type 2 diabetes, we performed a subgroup analysis to estimate the combined incidence rates of CKD and DKD for individuals aged 40 years and older. We selected this cutoff because the incidence of type 1 diabetes drops significantly after age 40 years and remains relatively low in this age group. This approach helps to minimize the risk of pollution bias in our results23. To validate that the incident events were not the result of undiagnosed preexisting CKD or DKD, a supplementary sensitivity analysis was performed by excluding outcomes that occurred within 6 months after the diagnosis of type 2 diabetes. Finally, we conducted a nested case–control analysis to explore the risk factors for CKD or DKD, including DKA, HHS, acute kidney injury, nonsteroidal anti-inflammatory drug use, and ACEi or ARB use. Medication use was stratified based on the prescription length into three categories: no exposure (0 days), 90 days or less, and more than 90 days. Statistical analyses were performed using the Statistical Package for SAS V. 9.4 (SAS Institute, Cary, North Carolina, USA), and a two-sided P-value of less than 0.05 was considered statistically significant.
Ethics-approval and consent to participate
This study was approved by the Institutional Review Board of Mackay Memorial Hospital (approval number: 22MMHIS382e) and informed consent was waived.
Presentation at a meeting
The current study was presented as an e-poster at the IDF 2022 Congress in Lisbon, Portugal, on December 5–8.
Results
Cohort characteristics
There were 13,242 participants in each cohort (Fig. 1), and their baseline characteristics are shown in Table 1. The mean age of the study participants was approximately 54 years (men: 62%). Compared with the non-HC cohort, the HC cohort had a lower income and higher prevalence of malignancy (6.24% vs. 2.99%, respectively); however, they had a lower prevalence of hypertension (32.7% vs. 37.5%, respectively) and hyperlipidemia (13.2% vs. 20.7%, respectively). The two cohorts did not differ in the proportion of patients who received either ACEis or ARBs.
Combined incidence of CKD and DKD
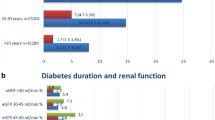
Table 2 depicts the combined incidence of CKD and DKD in the HC cohort, its sub-cohorts, and in the non-HC cohort. The HC cohort comprised mainly patients with DKA (55.1%) and HHS (39.2%), with a few cases of combined DKA-HHS (5.7%). There were 4106 (31%) and 2735 (20.7%) events observed in the HC and non-HC cohorts during median follow-ups of 4.97 years and 7.15 years, respectively. This corresponded to incidence rates of 56.47 and 28.49 per 1000 person-years, respectively. The cumulative incidence of CKD and DKD was significantly greater in the HC cohort than in the non-HC cohort (Fig. 2A). Within the HC cohort, the cumulative incidence was higher in patients with HHS and combined DKA-HHS than in those with DKA (Fig. 2B). The aHR among the HC sub-cohorts increased from 1.69 (95% CI 1.59–1.79) for DKA to 2.47 (95% CI 2.33–2.63) for HHS and 2.60 (95% CI 2.29–2.95) for combined DKA-HHS. In the sub-distribution hazard models, the sHRs were attenuated but remained significantly higher in the main HC cohort and its sub-cohorts.
Group comparisons of the Kaplan–Meier estimated cumulative incidence of combined chronic kidney disease and diabetic kidney disease between hyperglycemic crisis (HC) and non-hyperglycemic crisis (non-HC) cohorts (A), and among hyperglycemic hyperosmolar state (HHS), diabetic ketoacidosis (DKA), combined DKA-HHS sub-cohorts and non-HC cohorts (B), from the National Health Insurance Research Database, Taiwan, 2006–2018.
Subgroup analysis in individuals aged 40 years and older
There were 10,266 (77.5%) and 10,419 (78.7%) individuals aged 40 years and older in the HC and non-HC cohorts, respectively (Table 3). This subgroup comprised 5104 (49.7%) individuals with DKA and 4591 (44.7%) with HHS in the HC cohort. A similar increase in the HR between the HC and non-HC cohorts, compatible with the result in our primary analysis, was observed in this age group. The aHR among the HC sub-cohorts also showed a stepwise escalation from 1.62 (95% CI 1.51–1.72) for DKA to 2.33 (95% CI 2.19–2.49) for HHS and 2.59 (95% CI 2.25–2.98) for combined DKA-HHS.
Sensitivity analysis
The sensitivity analysis demonstrated that the patterns of CKD or DKD development 6 months after the diagnosis of type 2 diabetes were consistent with the findings of the primary analysis (Table S3).
Nested case–control analyses
The nested case–control analysis showed that the risk of developing CKD or DKD was significantly higher for patients with a history of hyperlipidemia (adjusted odds ratio [aOR] 1.22; 95% CI 1.15–1.30), acute kidney injury (aOR 1.33; 95% CI 1.18–1.50), DKA (aOR 1.56; 95% CI 1.47–1.66), and HHS (aOR 1.75; 95% CI 1.64–1.86). Compared with patients who did not receive ACEis or ARBs, those who had received treatment with ACEis or ARBs also had a higher risk, with an aOR of 1.93 (95% CI 1.75–2.13) for those treated for 90 days or less and an aOR of 1.69 (95% CI 1.57–1.82) for those who were treated for more than 90 days (Table S4).
Discussion
This population-based cohort study revealed a higher risk of incident CKD and/or DKD in patients with HC as their initial presentation of type 2 diabetes than in patients who present type 2 diabetes without HC. This association remained consistent across all HC sub-cohorts and stayed significant in the subgroup analysis for those aged 40 years and older. The risk was higher in patients with HHS and in those with both DKA and HHS than in those with DKA. This association remained robust after excluding cases that appeared within 6 months of diabetes diagnosis. Our nested case–control analysis corroborates that, compared with patients with type 2 diabetes who did not develop CKD or DKD, those who did were more likely to have experienced HC upon diabetes diagnosis.
The surprisingly high proportion of patients having DKA instead of HHS on the initial presentation of their type 2 diabetes in our cohort may raise concerns of pollution bias by the presence of patients with type 1 diabetes. Although there is currently no data reporting the proportion of DKA versus HHS in patients with HC as the initial presentation of type 2 diabetes, we believe that our findings are valid because previous studies have also reported a high percentage of newly diagnosed type 2 diabetes among patients with DKA. In an early study of 141 episodes of DKA in a tertiary referral center in Taiwan, 32 (22.7%) episodes were caused by newly diagnosed diabetes24. Twenty-five of the newly diagnosed patients were followed for at least 12 months, and 11 (44%) of them were not using insulin and exhibited metabolic features of type 2 diabetes. A recent study that retrospectively reviewed the medical records of consecutive patients with index DKA in four general hospitals in Qatar showed that 442 (48%) of them had type 2 diabetes25. Of the 324 patients with DKA and newly diagnosed diabetes, 176 (54.3%) had type 2 diabetes, and 93 (52.8%) were Asian. We speculate that the excessive DKA cases observed in our study and previous studies may be attributed to ‘ketosis-prone diabetes (KPD)’26. This syndrome is characterized by the acute onset of severe hyperglycemia with ketoacidosis, necessitating hospital admission and treatment. However, it often undergoes spontaneous remission, with patients maintaining long-term insulin independence several weeks after discharge27. Initially identified in individuals of African descent and African Americans28, KPD is now recognized as a significant clinical entity in Asian populations27. Patients with KPD are typically young or middle-aged and predominantly male29, consistent with the clinical characteristics of our study population. Another reason we believe that we secured the cases of type 2 diabetes in our study is due to the unique characteristics of the NHIRD. In Taiwan, type 1 diabetes is classified as a catastrophic illness by the National Health Insurance Administration. When a physician diagnoses a patient with such a condition, the patient can apply for a catastrophic illness certificate by submitting the necessary documentation. Upon issuance, this certification is recorded on the patient’s National Health Insurance Card. During the validity period of this certificate, patients are exempt from co-payment of outpatient or inpatient care related to the certified illness30. However, this exemption is contingent upon the physician using the correct ICD codes, as is the case with type 1 diabetes. An incorrect ICD coding that misclassifies type 1 diabetes as type 2 diabetes would prevent patients with type 1 diabetes from receiving exemptions for medical expenses, a scenario which is unlikely to occur in real-world practice or in the NHIRD. Moreover, the result of our subgroup analysis for those aged 40 years and older, which comprised nearly 78% of all patients, did not differ from our main findings. As the incidence of type 2 diabetes increases with age in Taiwan, and the incidence of type 1 diabetes over 40 years of age is remarkably low (0.02 per 100,000 population)23, the possibility that our findings are biased due to the presence of patients with type 1 diabetes is negligibly low.
Our findings align with those of previous Taiwan NHIRD studies demonstrating the detrimental effects of HC in patients with diabetes8,9,10,11,12,13. The present research adds value to the previous literature in several aspects. First, unlike previous studies that examined diabetes as a single entity10,11,12,13, we focused exclusively on type 2 diabetes and examined the individual effects of different types of HC. Given the distinct pathophysiologies underlying DKA and HHS, as well as those underlying type 1 and type 2 diabetes, our study design may ensure a more robust association between HC and CKD. The higher risk of CKD and/or DKD observed in patients with HHS or those with combined DKA-HHS than in patients with isolated DKA also aligns with previous studies reporting worse in-hospital outcomes in patients with combined DKA-HHS than in those with isolated DKA or HHS31. Second, HC typically signifies uncontrolled diabetes32, which is a well-recognized risk factor for diabetic complications33. Studies not adjusting for patients’ glycemic control may confound the impact of HC on long-term outcomes. We minimized such confounding by focusing on patients newly diagnosed with diabetes, where subsequent glycemic control acts as a mediator or effect modifier that requires no adjustment. Third, this pioneering study investigates the impact of HC on patients newly diagnosed with type 2 diabetes without prior kidney disease, offering potential insights for future clinical practice. Given their increased risk of CKD, patients experiencing HC upon type 2 diabetes diagnosis should receive proactive early preventive measures to mitigate such risk.
Several mechanisms may explain our findings. A longitudinal study on type 2 diabetes in Taiwan highlighted that individuals with lower income levels were more likely to have hospitalization-diagnosed diabetes, although it did not report how many of these patients were diagnosed via HC34. Patients with type 2 diabetes who are not diagnosed until hospitalization may be less likely to receive early detection screenings or may lack sufficient awareness of diabetes-related symptoms to seek appropriate healthcare34. Although the two study groups in our study did not differ in most baseline characteristics after propensity-score matching, individuals in the HC group exhibited significantly lower income levels than those in the non-HC group. Such difference may suggest that our HC group, compared with the non-HC group, included more individuals facing healthcare inequality, potentially resulting in worse renal outcomes. Furthermore, common risk factors for CKD, such as hypertension and hyperlipidemia, often remain undiagnosed among underprivileged individuals35. The paradoxically higher risk of CKD despite a lower prevalence of hypertension and hyperlipidemia in the HC group than in the non-HC group may be a result of more undiagnosed rather than healthier cases in the former group. As our propensity score considered only established comorbidities, the true risk difference of CKD at baseline between the two groups may be unbalanced. This could explain the remaining two-fold higher risk of CKD in the HC group, even after adjusting for income level, comorbidities, and other covariates.
From a biological perspective, our finding may be a consequence of initial priming by hyperglycemia, as the onset of diabetes followed by subsequent insults from acute kidney injury (AKI) during HC ultimately leads to persistent nephron damage. Hyperglycemia-induced epigenetic change can lead to progressive and irreversible renal injury, a phenomenon known as the “metabolic memory of DKD”36. Following exposure to hyperglycemia, vascular endothelial cells continue to increase oxidative stress and elicit inflammation even after normalization of blood glucose levels37. Owing to the slow progression of type 2 diabetes, the exact duration between disease onset and diagnosis is difficult to ascertain. Previous studies have suggested that the interval between the onset and diagnosis of type 2 diabetes is at least 5 years38,39. Moreover, low income is significantly associated with delayed diagnosis and inadequate diabetes care and management34. We speculate that patients experiencing HC upon diabetes diagnosis had a longer duration from diabetes onset to diagnosis compared with those who had diabetes diagnosed without HC. Such a latent period aggravates metabolic memory and leads to an increased risk of CKD. Moreover, AKI is a known risk factor for CKD40,41,42 and is common in patients with DKA due to volume depletion7,43. Despite this, there is a paucity of data reporting the incidence of AKI during HHS. A higher rate of AKI in patients with HHS is expected as patients with HHS are more likely to be dehydrated than patients with DKA. The occurrence of AKI during HC may exacerbate renal damage and increase the risk of CKD. A recent study revealed a higher incidence of AKI in patients with HHS and combined DKA-HHS than in those with DKA44, which may also explain the greater risk of CKD in patients with HHS and combined DKA-HHS than in those with DKA in our study. Nevertheless, we cannot exclude the possibility that our findings result from surveillance bias between the two cohorts. As patients experiencing HC upon diabetes diagnosis have more severe disease compared with those without HC at diagnosis, clinicians should provide more vigilant follow-ups for earlier detection of CKD.
This study had some limitations. First, relying solely on claims data may have led to the misclassification of diseases. However, the accuracy and validity of the NHIRD claims data have been demonstrated previously15,17,18, minimizing the impact of misclassification on our results. Second, we could not adjust for covariates unavailable in the NHIRD, including laboratory tests, blood pressure, waist circumference, body mass index, and lifestyle. The potential impact of this missing data remains unassessed. Furthermore, the inherent limitations of the NHIRD also restricted us from determining the severity of DKA and the stage of CKD, which may also have affected our results. Third, in large-sample studies, even minor differences can achieve statistical significance, warranting cautious interpretation. Because we established our study cohorts using a propensity score-matched design and conducted the analysis using stratification, we believe that the observed risk differences between the two cohorts were not a result of overpowering. Finally, the generalizability of our findings may be limited to populations with similar characteristics.
Conclusions
Patients who experience HC upon type 2 diabetes diagnosis have a higher risk of developing CKD compared with those without HC at diagnosis. As type 2 diabetes and end-stage renal disease are highly prevalent in Taiwan, proactive preventive measures are imperative to mitigate risks in this vulnerable population. These interventions should include early introduction of ACEis or ARBs and sodium-glucose cotransporter-2 inhibitors, stringent control of diabetes and the reduction of other risk factors, and educational programs for continuous diabetes self-care management. Furthermore, healthcare authorities should reinforce government-subsidized diabetes screening programs, especially for the underprivileged, to facilitate early recognition of undiagnosed diabetes and prevent HC incidents.
Data availability
No original data were generated or collected as part of this study. The NHIRD used in this study is available from the Taiwan National Health Insurance Administration, Ministry of Health and Welfare.
References
Gosmanov, A. R. et al. Hyperglycemic Crises: Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar State (Endotext, 2021).
Misra, S. et al. Temporal trends in emergency admissions for diabetic ketoacidosis in people with diabetes in England before and during the COVID-19 pandemic: A population-based study. Lancet Diabetes Endocrinol. 9, 671–680 (2021).
Wachtel, T. J., Tetu-Mouradjian, L. M., Goldman, D. L., Ellis, S. E. & O’Sullivan, P. S. Hyperosmolarity and acidosis in diabetes mellitus: A three-year experience in Rhode Island. J. Gen. Intern. Med. 6, 495–502 (1991).
Fourtner, S. H., Weinzimer, S. A. & Levitt Katz, L. E. Hyperglycemic hyperosmolar non-ketotic syndrome in children with type 2 diabetes*. Pediatr. Diabetes 6, 129–135 (2005).
Desai, R. et al. Temporal trends in the prevalence of diabetes decompensation (diabetic ketoacidosis and hyperosmolar hyperglycemic state) among adult patients hospitalized with diabetes mellitus: A nationwide analysis stratified by age, gender, and race. Cureus 11, e4353 (2019).
Lawrence, S. E., Cummings, E. A., Gaboury, I. & Daneman, D. Population-based study of incidence and risk factors for cerebral edema in pediatric diabetic ketoacidosis. J. Pediatr. 146, 688–692 (2005).
Orban, J. C., Maizière, E. M., Ghaddab, A., Van Obberghen, E. & Ichai, C. Incidence and characteristics of acute kidney injury in severe diabetic ketoacidosis. PLOS One 9, e110925 (2014).
Wang, J. Y. et al. Increased risk of ischemic stroke after hyperosmolar hyperglycemic state: A population-based follow-up study. PLOS One 9, e94155 (2014).
Chen, Y. L., Weng, S. F., Yang, C. Y., Wang, J. J. & Tien, K. J. Long-term risk of stroke in type 2 diabetes patients with diabetic ketoacidosis: A population-based, propensity score-matched, longitudinal follow-up study. Diabetes Metab. 43, 223–228 (2017).
Chang, L. H. et al. Association between hyperglycaemic crisis and long-term major adverse cardiovascular events: a nationwide population-based, propensity score-matched, cohort study. BMJ Open. 6, e012233 (2016).
Kao, Y. et al. Subsequent mortality after hyperglycemic crisis episode in the non-elderly: A national population-based cohort study. Endocrine 51, 72–82 (2016).
Huang, C. C. et al. Long-term mortality risk after hyperglycemic crisis episodes in geriatric patients with diabetes: A national population-based cohort study. Diabetes Care 38, 746–751 (2015).
Kao, Y. et al. Association of hyperglycemic crisis with an increased risk of end-stage renal disease: A nationwide population-based cohort study. Diabetes Res. Clin. Pract. 138, 106–112 (2018).
Salardi, S. et al. Ketoacidosis at diagnosis in childhood-onset diabetes and the risk of retinopathy 20years later. J. Diabetes Complicat. 30, 55–60 (2016).
Lin, L. Y., Warren-Gash, C., Smeeth, L. & Chen, P. C. Data resource profile: The National Health Insurance Research Database (NHIRD). Epidemiol. Health 40, e2018062 (2018).
Wang, T. H., Tsai, Y. T. & Lee, P. C. Health big data in Taiwan: A national health insurance research database. J. Formos. Med. Assoc. 122, 296–298 (2023).
Lin, C. C., Lai, M. S., Syu, C. Y., Chang, S. C. & Tseng, F. Y. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J. Formos. Med. Assoc. 104, 157–163 (2005).
Hsieh, M. T., Hsieh, C. Y., Tsai, T. T., Wang, Y. C. & Sung, S. F. Performance of ICD-10-CM diagnosis codes for identifying acute ischemic stroke in a national health insurance claims database. Clin. Epidemiol. 12, 1007–1013 (2020).
Kitabchi, A. E., Umpierrez, G. E., Murphy, M. B. & Kreisberg, R. A. Hyperglycemic crises in adult patients with diabetes: A consensus statement from the American Diabetes Association. Diabetes Care 29, 2739–2748 (2006).
Kitabchi, A. E., Umpierrez, G. E., Miles, J. M. & Fisher, J. N. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 32, 1335–1343 (2009).
Nguyen, T. L. et al. Double-adjustment in propensity score matching analysis: Choosing a threshold for considering residual imbalance. BMC Med. Res. Methodol. 17, 78 (2017).
Fine, J. P. & Gray, R. J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 94, 496–509 (1999).
Sheen, Y. J. et al. Trends in prevalence and incidence of diabetes mellitus from 2005 to 2014 in Taiwan. J. Formos. Med. Assoc. 118(suppl 2), S66–S73 (2019).
Yan, S. H., Sheu, W. H., Song, Y. M. & Tseng, L. N. The occurrence of diabetic ketoacidosis in adults. Intern. Med. 39, 10–14 (2000).
Ata, F. et al. Differential evolution of diabetic ketoacidosis in adults with pre-existent versus newly diagnosed type 1 and type 2 diabetes mellitus. BMC Endocr. Disord. 23, 193 (2023).
Gaba, R., Mehta, P. & Balasubramanyam, A. Evaluation and management of ketosis-prone diabetes. Expert. Rev. Endocrinol. Metab. 14, 43–48 (2019).
Lebovitz, H. E. & Banerji, M. A. Ketosis-prone diabetes (flatbush diabetes): An emerging worldwide clinically important entity. Curr. Diabetes Rep. 18, 120 (2018).
Banerji, M. A. et al. GAD antibody negative NIDDM in adult black subjects with diabetic ketoacidosis and increased frequency of human leukocyte antigen DR3 and DR4. Flatbush diabetes. Diabetes 43, 741–745 (1994).
Wang, X. & Tan, H. Male predominance in ketosis-prone diabetes mellitus. Biomed. Rep. 3, 439–442 (2015).
National Health Insurance Administration, Ministry of Health and Welfare, Taiwan: Policies and Services: Care for Special Groups (Accessed 15 June 2024). https://www.nhi.gov.tw/en/cp-90-d4e0a-18-2.html
Pasquel, F. J. et al. Clinical outcomes in patients with isolated or combined diabetic ketoacidosis and hyperosmolar hyperglycemic state: A retrospective, hospital-based cohort study. Diabetes Care 43, 349–357 (2020).
Chou, W. et al. Clinical characteristics of hyperglycemic crises in patients without a history of diabetes. J. Diabetes Investig. 5, 657–662 (2014).
An, J. et al. Prevalence and incidence of microvascular and macrovascular complications over 15 years among patients with incident type 2 diabetes. BMJ Open Diabetes Res. Care 9, e001847 (2021).
Hsu, C. C. et al. Poverty increases type 2 diabetes incidence and inequality of care despite universal health coverage. Diabetes Care 35, 2286–2292 (2012).
Ayanian, J. Z., Zaslavsky, A. M., Weissman, J. S., Schneider, E. C. & Ginsburg, J. A. Undiagnosed hypertension and hypercholesterolemia among uninsured and insured adults in the Third National Health and Nutrition Examination Survey. Am. J. Public Health 93, 2051–2054 (2003).
Kato, M. & Natarajan, R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat. Rev. Nephrol. 15, 327–345 (2019).
El-Osta, A. et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J. Exp. Med. 205, 2409–2417 (2008).
Harris, M. I., Klein, R., Welborn, T. A. & Knuiman, M. W. Onset of NIDDM occurs at least 4–7 yr before clinical diagnosis. Diabetes Care 15, 815–819 (1992).
Porta, M. et al. Estimating the delay between onset and diagnosis of type 2 diabetes from the time course of retinopathy prevalence. Diabetes Care 37, 1668–1674 (2014).
Thakar, C. V., Christianson, A., Himmelfarb, J. & Leonard, A. C. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin. J. Am. Soc. Nephrol. 6, 2567–2572 (2011).
Coca, S. G., Singanamala, S. & Parikh, C. R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 81, 442–448 (2012).
Hsu, R. K. & Hsu, C. Y. The role of acute kidney injury in chronic kidney disease. Semin. Nephrol. 36, 283–292 (2016).
Myers, S. R. et al. Frequency and risk factors of acute kidney injury during diabetic ketoacidosis in children and association with neurocognitive outcomes. JAMA Netw. Open 3, e2025481 (2020).
Schmitt, J., Rahman, A. F. & Ashraf, A. Concurrent diabetic ketoacidosis with hyperosmolality and/or severe hyperglycemia in youth with type 2 diabetes. Endocrinol. Diabetes Metab. 3, e00160 (2020).
Acknowledgements
We are grateful to the Health Data Science Center of China Medical University Hospital for providing administrative, technical, and funding support.
Funding
This work was supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial Center (Grant Number: MOHW110-TDU-B-212-124004), the Ministry of Science and Technology (grant number: MOST 110-2321-B-039-003), and China Medical University Hospital (Grant Numbers: DMR-111-228 and CMU110-MF-63). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conception and design: CT, FC. Acquisition, analysis, and interpretation of data: all authors. Drafting of the manuscript: CT. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: CH. Obtained funding: FC, PC. All authors had full access to all the study data and take responsibility for its integrity and the accuracy of data analysis. All authors have read and approved the final version of the manuscript submitted for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, CT., Muo, CH., Sung, FC. et al. Risk of chronic kidney disease in patients with a hyperglycemic crisis as the initial presentation of type 2 diabetes. Sci Rep 14, 16746 (2024). https://doi.org/10.1038/s41598-024-67678-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67678-3
- Springer Nature Limited