Abstract
Alkaloid analgesics have been associated with adverse effects on the central nervous system (CNS). Therefore, it is crucial to characterize the effects of alkaloid analgesics. Plants rich in lycorine, an alkaloid, have shown promise as analgesics. However, the exploration of their CNS side effects, and analgesic effectiveness remains incomplete. The aim of the present study was to investigate the CNS safety profiles of lycorine and its potential analgesic efficacy. Lycorine (3, 10, and 30 mg/kg, intraperitoneal) did not affect motor coordination, and doses of 3 and 10 mg/kg of lycorine did not lead to any impairment in spontaneous locomotor activity. However, the highest dose (30 mg/kg) demonstrated a significant impairment in rearing behavior and an increase in immobility. The safety doses were subsequently used to assess the analgesic efficacy of lycorine in a mouse model of inflammatory pain. Lycorine (1, 3, and 10 mg/kg, intraperitoneal) demonstrated a dose-dependent reduction in pain-like behaviors in formalin-induced mice. In the in vitro study, lycorine regulated immune cells, suggesting its involvement as a cellular mechanism underlying the suppression of pain-like behaviors observed in the formalin model. Overall, our findings delineate the CNS safety range of lycorine in mice and suggest its potential use as an analgesic.
Similar content being viewed by others
Introduction
Alkaloids, organic nitrogen-containing bases derived from plants, are significant potential sources of pharmacological agents1. Plants rich in alkaloids have a long history of use in traditional medicine systems, including traditional Chinese2, African3, and Thai medicine4. These alkaloids exhibit a wide range of biological and pharmacological activities, encompassing antiarrhythmic, antiviral, antibacterial, anti-virulence, anti-neurodegenerative, antidote, anesthetic, antihypertensive, and analgesic properties5,6. Moreover, several alkaloids such as taxol, reserpine, morphine, quinine, codeine, pilocarpine, vinblastine, yohimbine, and vincristine7, have attracted pharmaceutical and commercial interest due to their significant therapeutic potential.
In recent years, there has been an increasing interest in alkaloid compounds as potential analgesics. Several alkaloids with potential analgesic properties have been identified, including aloperine8, brucine9, piperine10, sinomenine11, matrine12, mitragynine13, and tetrahydropalmatine14. These alkaloids have demonstrated efficacy in pain treatment, supported by preclinical, clinical, or combined evidence. However, caution must be exercised when using them as analgesics. Certain opiate alkaloids have been linked to central nervous system (CNS) side effects, such as sedation, sleep disturbance, delirium, psychomotor impairment, excitement, calmness, and hallucinations15. Furthermore, tetrahydropalmatine not only possesses analgesic efficacy but also exhibits hypnotic and sedative effects16. Matrine is known to modulate GABAergic neurotransmission17. Other potential analgesic alkaloids, including caffeine18 and nicotine19 have also been reported to have CNS stimulant effects. Therefore, the discovery of alkaloid analgesics without CNS safety concerns would be highly advantageous for their use as drugs in clinical settings.
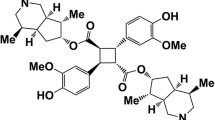
Lycorine (Fig. 1a) is an alkaloid that is commonly found in species belonging to the Amaryllidaceae family. It possesses a wide range of biological and pharmacological activities, such as antiviral, anti-inflammatory, antibacterial, antitumor, and antinociceptive activity20,21. However, it is important to note that certain Amaryllidaceae species containing lycorine are considered as poisonous plants22. These plants have been extensively utilized in Africa for the treatment of CNS diseases, possibly owing to the presence of CNS-active alkaloids, including lycorine23. Alkaloids naturally serve as metabolites that function as a defense system in plants, making them potentially toxic to other species, including humans. Several studies have highlighted the potential CNS side effects and toxicity associated with plants containing lycorine, such as Boophone disticha, Scadoxus puniceus (L.), Clivia miniata (Lindl.) Regel, and Amaryllis belladonna L.22,24. In particular, Boophone disticha, is often abused due to its hallucinogenic effects25. While previous research has reported side effects of lycorine such as nausea and emesis, its specific CNS side effects have not been fully elucidated26. Therefore, a comprehensive study is required to thoroughly assess, validate, and determine the involvement of lycorine in mediating CNS side effects.
Chemical structure of lycorine (a) and profiles of lycorine administration on motor coordination in mice (b). The data are expressed as mean ± SEM (n = 8 mice/group). Differences between groups were analyzed using two-way ANOVA followed by Dunnett’s post hoc test. ***p < 0.001 compared to vehicle-treated mice. VHC, vehicle; CPM, chlorpromazine 5 mg/kg; LCR 3, lycorine 3 mg/kg; LCR 10, lycorine 10 mg/kg; LCR 30, lycorine 30 mg/kg.
The primary objective of this study is to empirically investigate the CNS side effects associated with lycorine. The study design involves administering escalating doses of lycorine to determine dosages that do not induce potential CNS side effects. Furthermore, the study aims to explore the potential analgesic effects of lycorine on pain-like behaviors using a formalin-induced mouse model of inflammatory pain. Additionally, the role of lycorine at the cellular level is examined using in vitro models of inflammation.
Results
Serial doses of lycorine did not impair the motor coordination of mice
Impaired motor coordination is known to be linked to the development of CNS side effects following drug administration27. In the present study, the effect of serial doses of lycorine on mouse motor coordination was investigated using the rotarod test. As shown in Fig. 1b, mice treated with lycorine at doses of 3, 10 and 30 mg/kg administered intraperitoneally did not exhibit a significant difference in rotarod latency compared to the mice treated with the vehicle at all measured time points. In contrast, administration of chlorpromazine led to a significant reduction in rotarod latency compared to the vehicle-treated mice at each time point during the 240 min post-treatment period. These findings suggest that lycorine does not affect motor coordination, as evidenced by the absence of significant changes in rotarod latency across all tested doses.
Lycorine impaired spontaneous locomotor activity in mice at a dose of 30 mg/kg
Due to concerns regarding the CNS safety of lycorine as a pharmacological agent, this study aimed to assess its impact on mouse spontaneous locomotor activity. Previous research has established the automated home-cage monitoring as a valuable model for the initial screening of compounds to predict their effects on the CNS28. Therefore, the automated home-cage monitoring was used in this study to investigate the potential CNS side effects associated with the intraperitoneal administration of lycorine (Fig. 2a). Mice were administered various doses of lycorine (3, 10, 30 mg/kg). The movement of mice in the cage was tracked using automated home-cage monitoring and visualized as position distribution (Fig. 2b). As shown in the figure, mice treated with the vehicle and lycorine explored the entire cage, while chlorpromazine-treated mice remained immobile in one corner of the cage. To quantitatively assess the effect of lycorine on mouse locomotive behaviors, the duration of mice engaged in each locomotive behavior is summarized in Fig. 2c–h. The administration of lycorine at doses of 3 and 10 mg/kg did not result in impaired spontaneous locomotor activity. This finding suggests that these doses of lycorine can be considered as the optimum dose to be utilized in future assessments of its therapeutic efficacy. However, 30 mg/kg dose of lycorine resulted in noticeable CNS side effects, as indicated by a significant decrease in rearing behavior and an increase in immobility compared to the vehicle-treated mice. Therefore, the 30 mg/kg dose of lycorine administered intraperitoneally is considered too high for pharmacological testing in mice. Additionally, as anticipated, mice treated with chlorpromazine demonstrated significantly reduced mobility and increased immobility compared to the vehicle-treated mice, confirming the validity of the experimental model.
Profiles of lycorine administration on spontaneous locomotor activity in mice. (a) Experimental set-up of automated home-cage monitoring system. (b) position distribution of mice in the cage and behaviors of spontaneous locomotor activity assessed for 30 min. The behaviors are presented as duration of (c) climbing, (d) locomotion, (e) immobility, (f) rearing, (g) speed, and (h) distance traveled. The data are expressed as mean ± SEM (n = 8 mice/group). The differences between groups were analyzed by ANOVA followed by Dunnett’s post hoc test. **p < 0.01 and ***p < 0.001 compared to the vehicle-treated group. VHC, vehicle; CPM, chlorpromazine 5 mg/kg; LCR 3, lycorine 3 mg/kg; LCR 10, lycorine 10 mg/kg; LCR 30, lycorine 30 mg/kg.
Lycorine at 10 mg/kg dose did not impair general behaviors and well-being of mice
To confirm 10 mg/kg dose of lycorine as the optimum dose for pharmacological testing in mice, the effects of lycorine on general behaviors and the overall well-being of mice were assessed. These behaviors are often used as models to simulate certain aspects of CNS side effects observed in humans. As shown in Fig. 3a–f, mice exhibited increased mobility during the nighttime due to their nocturnal nature. Therefore, locomotor impairments associated with chlorpromazine were more pronounced during the nighttime compared to the daytime. During the nighttime, mice treated with chlorpromazine displayed significantly reduced mobile behaviors (locomotion, climbing, rearing, speed, and distance traveled) and increased immobility compared to the vehicle-treated mice. Conversely, exposure of mice to a 10 mg/kg dose of lycorine did not result in any significant changes in their home cage behaviors compared to the vehicle-treated mice throughout the 24 h assessment period. This finding confirms that the intraperitoneal administration of lycorine at a dose of 10 mg/kg did not disrupt natural behaviors of mice within their familiar environment.
Effects of lycorine administration on home cage behaviors. Treatments were administered at 18.00 h, and home cage behaviors were assessed over 24 h, covering both nighttime and daytime periods. Home cage behaviors are presented as the duration of (a) climbing, (b) locomotion, (c) immobility, (d) rearing, (e) speed, and (f) distance traveled. The data are expressed as mean ± SEM (n = 8 mice/group). The differences between groups were analyzed using ANOVA followed by Dunnett’s post hoc test. *p < 0.05, **p < 0.01, and ***p < 0.001, compared to the vehicle-treated group. VHC, vehicle; CPM, chlorpromazine 5 mg/kg; LCR, lycorine 10 mg/kg.
In addition, the changes in body weight, food consumption, and water intake of the mice were also measured to gain further insights into the effects of lycorine on their overall wellbeing. As indicated in Fig. 4a–c, administration of lycorine at a dose of 10 mg/kg did not significantly alter the body weight, food consumption, or water intake of the mice compared to those treated with the vehicle. Overall, these results confirm the optimum dose of lycorine, 10 mg/kg (intraperitoneal), for further investigations in pharmacological testing in rodents.
Lycorine attenuates pain-like behaviors in formalin-injected mice
To determine the efficacy of lycorine on acute nociceptive and inflammatory pain (Fig. 5a–e), mice were administered lycorine at doses of 1, 3, 10 mg/kg one hour before formalin exposure (10 µL of 5% formalin) and further followed by observation of hind paw licking behaviors (Fig. 5a). As shown in Fig. 5, during the first phase (nociceptive phase, 0–5 min), the mice predominantly engaged in hind paw licking, with an average duration of 142.0 ± 7.5 s. In the second phase (inflammatory phase, 10–40 min), mice spent 320.0 ± 22.6 s engaged in hind paw licking, and the peak behavior was observed at 25–30 min post-formalin administration (88.6 ± 12.4 s) (Fig. 5b). Administration of lycorine dose-dependently reduced hind paw licking behaviors in both phase I and phase II of the formalin test. At doses of 3 and 10 mg/kg lycorine caused significant reduction in the area under the curves (Fig. 5c) and duration of licking behaviors in both phase I and phase II (Fig. 5d) compared to the vehicle-treated mice. In contrast, indomethacin significantly reduced hind paw licking behaviors only in phase II. In phase I of the formalin test, the highest dose of lycorine (10 mg/kg) exhibited 39.6 ± 5.6% antinociception. In phase II, the highest dose of lycorine exerted 79.6 ± 8.9% antinociception, which was comparable to the effect of indomethacin at 10 mg/kg (60.2 ± 9.5%) (Fig. 5e). These results indicate that lycorine has the potential to modulate nociceptor and inflammatory responses in the formalin model, with a more potent activity observed during the inflammatory phase.
The effects of lycorine on pain-like behaviors in formalin-injected mice. (a) Representative figure of hind paw licking behaviors, (b) hind paw licking behaviors after lycorine administration at each time point (c) area under the curve (AUC) of the phase I and phase II, (d) total duration of licking in phase I and phase II, and (e) % antinociception in phase I and phase II. Data are presented as mean ± SEM (n = 8 mice/group). *p < 0.05, ***p < 0.001 vs. formalin group (one-way ANOVA followed by Bonferroni post hoc test). FML, formalin-injected mice receiving a vehicle solution; IND, indomethacin 10 mg/kg; LCR 1, lycorine 1 mg/kg; LCR 3, lycorine 3 mg/kg; LCR 10, lycorine 10 mg/kg.
Lycorine reduces proinflammatory mediators in activated macrophages and microglia
Previous research has established the involvement of peripheral and central immune cells in the pathogenesis of the phase II pain response in the formalin model29,30,31. Therefore, we utilized RAW 264.7 macrophage and BV-2 microglial cells to substantiate the in vivo findings and provide insights into the role of lycorine in reducing inflammatory response. Cytokines released by immune cells are key molecular determinants in the pathophysiology of inflammatory pain32. In the present study, the ability of lycorine inhibiting the releases of proinflammatory mediators in activated microglia and macrophages was assessed. First, the non-toxic concentrations of lycorine were determined using cell viability assay (Fig. 6a). The maximum non-toxic concentration of lycorine was found to be 2 μM for both cell lines. Consequently, lycorine concentrations of 0.5, 1, and 2 μM were selected to evaluate its effects on lipopolysaccharide (LPS)-induced activation of immune cells. The activated immune cells such as macrophages and microglia are manifested by increased release of proinflammatory mediators29. As demonstrated in Fig. 6, incubation with LPS significantly increased the release of inflammatory mediators from the cells compared to the untreated cells. However, the non-toxic concentrations of lycorine concentration-dependently attenuated the levels of proinflammatory mediators, including nitric oxide (NO), tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), in LPS-stimulated macrophage and microglial cells (Fig. 6b–d).
The effects of lycorine on the release of proinflammatory mediators in LPS-induced activation of macrophage and BV-2 microglial cells. (a) Viability of the cells after treatment with lycorine. Expression of (b) NO, (c) TNF-α, and (d) IL-6 after treatment with lycorine in activated macrophage and microglia. Data are presented as mean ± SEM (n = 3). The differences between groups in figure (a) were analyzed using one-way ANOVA followed by Dunnett post hoc test, **p < 0.01 ***p < 0.001 vs. VHC group. The differences between groups in figures (b)-(d) were analyzed using one-way ANOVA followed by Bonferroni post hoc test, ###p < 0.001 VHC vs LPS group, and **p < 0.01, ***p < 0.001 vs. LPS group.
Discussion
Compounds such as lycorine, which have the potential to modulate macrophages and microglia, offer promise as potential analgesic agents. However, concerns regarding potential side effects, particularly CNS side effects, have hindered the use of lycorine as a pharmacological agent. In this study, we first elucidated the optimal dose range of lycorine by assessing the effects of multiple serial doses on the behaviors of mice in CNS safety pharmacology models. The results demonstrated that an intraperitoneal dose of 10 mg/kg of lycorine did not induce any adverse effects on motor coordination, spontaneous locomotor activity, home cage behaviors, or the overall well-being of mice. Furthermore, we investigated the potential analgesic effects of lycorine using a formalin-induced mouse model of pain, which revealed a significant improvement in pain-like behaviors upon lycorine administration at 3 and 10 mg/kg doses. Additionally, we explored the role of lycorine at the cellular level using in vitro models of inflammation with activated macrophage and microglial cells, where lycorine demonstrated the ability to suppress the release of proinflammatory mediators such as nitric oxide and pro-inflammatory cytokines. Overall, our results signify the therapeutic relevance of lycorine in the management of pain, while highlighting the importance of precise dose adjustments to minimize its potential CNS side effects.
The growing popularity of using plant-derived compounds in healthcare has raised concerns regarding the limited availability of toxicity data for these products. Natural products are often perceived as safe and are frequently used for self-therapy without professional supervision. As a result, there is an urgent need to expand our understanding of the potential toxicity of natural compounds to ensure their safe and responsible use33. Lycorine, the primary active alkaloid found in numerous Amaryllidaceae plants, has garnered significant attention in scientific research due to its diverse biological functions and favorable pharmacokinetic profiles. It has been extensively investigated for its anticancer properties, acetylcholinesterase inhibitory activity, as well as its potential as an anti-inflammatory, antifungal, antiviral, and anti-malarial agent34.
Regarding the pharmacokinetic parameters, lycorine exhibited a considerably high oral bioavailability of 40% in beagle dogs26. In mice, intraperitoneal administration of lycorine at a dose of 10 mg/kg resulted in approximately 76.28% bioavailability, with a maximum drug concentration (Cmax) of 4.73 ± 0.52 μg/mL and a time to maximum drug concentration (Tmax) of 10 min35. In beagle dogs, subcutaneous administration of lycorine at a dose of 2 mg/kg did not show potential toxicity to the liver and kidneys, as assessed using biochemical and hematological analyses26. However, future pharmacokinetics and toxicity studies in animals as well as in humans are required to ascertain the drug-likeness of lycorine.
Despite the numerous pharmacological activities and favorable pharmacokinetic profiles of lycorine, previous studies have reported the possible induction of CNS side effects with its administration23,26. Therefore, in order to address this concern, we assessed the effects of lycorine on motor coordination, spontaneous locomotor activity, home cage behaviors, and the overall well-being of mice. Impairment of these behaviors has been previously linked to potential CNS side effects27,36. Moreover, these assessments align with the European Medicines Agency (EMA) guidelines in ICH S7A Safety pharmacology studies, which includes spontaneous locomotor activity, motor coordination and general behaviors of mice as measures of CNS side effects and are considered core battery studies36. In the present study, we compared the effects of lycorine with chlorpromazine, a standard drug known for its potent CNS depressant effects. Lycorine doses of 3, 10, and 30 mg/kg administered intraperitoneally showed no effects on motor coordination, except for the 30 mg/kg dose which impaired rearing behaviors and increased immobility in the mice. Lower doses of lycorine (≤ 10 mg/kg) did not affect general behaviors and wellbeing of mice, as confirmed by the evaluation of home cage behaviors over 24 h. These results emphasize the importance of using appropriate doses of lycorine to minimize potential CNS effects.
Several alkaloids derived from plants with potential analgesic effects have been reported, including morphine37, berberine38, piperine39, quinine40, and mescaline41. The effects of these alkaloids on spontaneous locomotor activities have been characterized by both decreased and increased spontaneous locomotor activity after administration. For instance, morphine administered subcutaneously at a dose of 20 mg/kg impaired spontaneous locomotor activity in rats, while a dose of 5 mg/kg increased the activity42. Similarly, in mice, orally administered berberine at a dose of 500 mg/kg decreased spontaneous locomotor activity due to its effects on modulating the serotonin system43. Piperine, when orally administered, also impaired spontaneous locomotor activity39. Quinine has been reported to decrease locomotion and induce a hypnotic effect in mice44, with its modulatory effects on the CNS, including the dopaminergic and serotonin systems45,46. Belladonna alkaloids, known for potential analgesic properties, also demonstrated impairment in rearing behaviors47. Furthermore, mescaline, when administered intraperitoneally at a dose of 50 mg/kg, impaired rearing activities during the 0–30 min post-drug administration48. In the present study, the administration of lycorine at 30 mg/kg dose impaired rearing behaviors, consistent with the effects observed with others alkaloids in previous studies47,48.
Although lycorine has been extensively studied for its potential in treating various diseases, its effects in the formalin model have not been fully evaluated. The efficacy of lycorine in the formalin model was assessed along with its CNS safety evaluation. The formalin model is a widely used chemically induced pain model, where the administration of formalin directly sensitizes nociceptors and triggers the release of inflammatory mediators. In the formalin test, there are two distinct phases: phase I, which is neurogenic, and phase II, which is inflammatory49. In phase II of the formalin model, non-neuronal cells release mediators, including cytokines, bradykinin, and pronociceptive lipids, which sensitize nociceptors and projection neurons in the CNS50. As shown in the present study, lycorine exhibited the ability to alleviate pain-like behaviors in both phases of the formalin model, indicating its potential for modulating nociceptors and non-neuronal immune cells.
In an attempt to characterize the role of lycorine at cellular level, activated macrophage and microglial cells were used. The activation of macrophages and microglia is associated with the pathogenesis of inflammatory pain and contributes to development and maintenance of chronic inflammatory pain29,51. The findings from our study demonstrate that lycorine regulates non-neuronal cells by reducing inflammatory cytokines and nitrite oxide. Similar results were observed with other potential analgesic alkaloids in the literature, demonstrating their potential ability to modulate immune cells. Numerous studies have shown the ability of morphine to modulate immune cells via regulating the production of proinflammatory cytokines and the polarization stage of the cells52. Furthermore, piperine has been shown to suppress proinflammatory mediators such as prostaglandin E2 (PGE2) and NO via the nuclear factor-kappa B pathway in activated macrophages53. Piperine has also been reported to reduce the release of proinflammatory cytokines, including TNF-α, IL-6, IL-1β, and PGE2, in activated microglia via the nuclear factor-kappa B and Nrf2 pathways54. In macrophages, berberine has been found to promote the stimulation of M2 macrophages and suppress M1 macrophages (pro-inflammatory phenotype)55. In a mouse model of diabetic neuropathy, berberine reduced the activation of microglia characterized by the suppression of TNF-α, IL-6, IL-1β, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2)56. Therefore, it is plausible to suggest that the observed ability of lycorine to reduce pain responses in phase II of formalin model is achieved through the modulation of immune responses.
Furthermore, our findings are consistent with a previous study that demonstrated the inhibitory effects of lycorine on LPS-induced nitric oxide, IL-6, TNF-α, and PGE2 release in LPS-induced RAW 264.7 macrophage cells by suppressing p38 and STATs pathways57. It is noteworthy that our study is the first to report the anti-inflammatory effects of lycorine in central immune cells. However, to fully understand the mechanisms underlying the anti-nociceptive and anti-inflammatory effects of lycorine in the formalin model, further investigations are warranted. Furthermore, the limitation of the present study is that only male mice were included, excluding female mice. Using both sexes of mice in pain studies facilitates the robustness and representativeness of the result58.
Conclusion
In conclusion, our study provides valuable insights into the profile of lycorine in terms of CNS side effects, along with its potential analgesic efficacy in a mouse model of formalin-induced pain. Further studies are warranted to assess the efficacy of lycorine on CNS systems at mechanistic and molecular levels. Furthermore, other aspects of safety pharmacology for lycorine, such as respiratory and cardiovascular safety are also required. Additionally, evaluating the efficacy of lycorine in other animal models of pain would be beneficial. Furthermore, a through characterization of the safety profile of lycorine will contribute to its potential clinical use in the treatment of inflammatory pain and other pain conditions.
Methods
Animals
Male mice of the ICR strain were obtained from Nomura Siam International Co. Ltd., Bangkok, Thailand. The mice were housed in cages with 4–5 mice per cage under conditions of a 12:12 h light–dark cycle, 50–60% humidity, and a temperature of 22 ± 2 °C. Mice were provided with free access to food and water. All protocol and procedures were approved by Institutional Animal Care and Use Committee (IACUC) of Faculty of Pharmaceutical Sciences, Chulalongkorn University (No. 22-33-020).
A total of 144 mice were used, with the sample size estimated using power analysis to ensure sufficient statistical power. Before the experiment, mice were randomly assigned to experimental groups with the blinding procedure. The administration of lycorine for each dose, positive control and vehicle occurred randomly within each cage to minimize bias and confounding variables. During the experiment, two researchers were involved in the process: one researcher was informed and aware of the group allocation, directly administering compounds to the experimental groups. Additionally, another researcher performed subsequent experiments while remaining unaware of the group allocation and the administration of compounds. Throughout the experiment, no animals were excluded from the analysis. After the experiment, mice underwent euthanasia via inhalation of carbon dioxide, followed by cervical dislocation. All the tests were in compliance with the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments). All methods were performed in accordance with the relevant guidelines and regulations.
Drug administration
Lycorine was isolated from the leaves of Crinum latifolium L. as described in a previous study from our group members59, and its purity was determined to be > 95% by UHPLC analysis. Lycorine was initially dissolved in dimethylsulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO, USA), and then adjusted to the required concentrations using normal saline, while maintaining a final concentration of 5% DMSO in each dose. Each mouse received a constant volume of diluted lycorine at a dose of 10 ml/kg. To assess the potential CNS side effects of lycorine, mice were administered with escalating doses of lycorine (3, 10 and 30 mg/kg, intraperitoneal). The selection of the escalation dose was in accordance with the dosages used in previous studies60,61,62,63,64,65. For the evaluation of therapeutic efficacy, a mouse model of formalin-induced pain was used, and the mice were given doses of lycorine at 1, 3, and 10 mg/kg intraperitoneally. Positive controls, chlorpromazine and indomethacin, were dissolved in normal saline and administered at doses of 5 and 10 mg/kg, respectively, via intraperitoneal injection.
Assessment of motor coordination by rotarod test
The effects of lycorine on motor coordination were evaluated using a rotarod apparatus (Ugo-Basile, Varese, Italy) set at a constant speed of 17 rpm. Prior to the experiment, mice were trained to remain on the rotating rod for 180 s over three consecutive days. Only mice that were able to remain on the rotating rod for 180 s during training were included in the experiment. On the fourth day, mice administered escalating doses of lycorine (3, 10, and 30 mg/kg) or chlorpromazine (5 mg/kg) through intraperitoneal injection. Subsequently, the mice were placed on the rotarod at various time intervals (0, 15, 30, 60, 90, 120, and 240 min) after treatment. The duration each mouse remained on the rotating rod, known as the rotarod latency, was recorded.
Assessment of spontaneous locomotor activity by LABORAS
The spontaneous locomotor activity of mice was assessed using the LABORAS automated behavioral analysis (Metris, Hoofddorp, Netherlands). The LABORAS system consists of mouse home cages measuring 22 × 16 × 14 cm, a control unit, and a computer equipped with the LABORAS software. One hour after treatment, each mouse was placed individually into the cage for a period of 30 min. The behaviors of the mice inside the LABORAS home cages were recorded and analyzed using the LABORAS software.
Assessment of general behaviors and wellbeing of mice
The LABORAS automated behavioral analysis system was utilized to assess general behaviors of mice, including long-term locomotor behavior, changes in body weight, water intake, and food consumption. Mice were administered with the 10 mg/kg dose of lycorine and monitored continuously for a 24 h period, encompassing both day and nighttime. After 24 h, alterations in body weight, as well as the quantities of food and water consumed, were measured and recorded.
Formalin-induced pain-like behaviors
The formalin test was used to assess the antinociceptive and anti-inflammatory efficacy of lycorine, as detailed in a previous study66. One hour after administrating lycorine and indomethacin, each mouse received a subcutaneous (intraplantar) injection of 5% formalin (10 μL) into the left hind paw. The duration of hind paw licking, which serves as a measure of pain-like behaviors, was recorded using a camera and analyzed with the Behavioral Observation Research Interactive Software (BORIS)67. The percentage inhibition of paw licking behavior in the treatment groups compared with the formalin group was defined as %antinociception and determined using the following equation:
Cell culture
The RAW 264.7 cells (ATCC, Manassas, VA) and BV-2 cells (AcceGen Biotechnology, Fairfield, NJ) were cultured in DMEM media (Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (PAN-Biotech, Aidenbach, Germany) and 1% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO, USA). The cells were maintained at a temperature of 37 °C, 5% CO2 and 95% humidity.
Cell treatments
Prior to evaluating the effects of lycorine on LPS-stimulated RAW 264.7 and BV-2 cells, a cytotoxicity assay was performed using the MTT assay to determine the non-toxic concentrations of lycorine in both cell types. The cells were treated with a range of lycorine concentrations (0, 0.5, 1, 2, 4, 8 µM) for 24 h, followed by the MTT assay to assess cell viability. The non-toxic concentrations of lycorine in RAW 264.7 cells and BV-2 cells were determined and subsequently tested in the experiments with LPS stimulation. After a one-hour treatment period, the cells were exposed to LPS for 22 h, and the culture media was collected for subsequent nitic oxide (NO) and enzyme-linked immunosorbent assay (ELISA) tests.
MTT assay
Cell viability of the treated cells was evaluated using the MTT assay. After a 24 h treatment with lycorine, the cells were incubated with MTT solution at a concentration of 0.5 mg/mL for 3 h. Following incubation, the formazan crystals formed by viable cells were dissolved using DMSO. The absorbance of the dissolved formazan crystals was then measured at a wavelength of 570 nm using a microplate reader (CLARIOstar, BMG Labtech, Ortenberg, Germany).
Griess assay
To determine the nitrite levels in the culture media after treating the cells with lycorine under LPS-stimulated conditions, the Griess assay (Sigma-Aldrich, St. Louis, MO, USA) was performed. Initially, a 100 µL sample of the culture media was added to each well of a 96-well plate. Next, 100 µL of Griess reagent was added to each well. The plate was then incubated for 10 min to allow for the reaction to occur. After the incubation period, the absorbance of the samples was measured at a wavelength of 520 nm using a microplate reader. The absorbance readings provided a quantification of the nitrite concentration, which serves as an indicator of nitric oxide (NO) production by the cells.
ELISA assay
The levels of inflammatory cytokines including tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) in the treated cells were determined using ELISA test. The ELISA test was performed according to the manufacturer's instructions with slight modifications (BioLegend, San Diego, CA, USA).
Statistical analysis
The results are expressed as mean ± standard deviation (SD) for the in vitro data and as mean ± standard error of the mean (SEM) for the in vivo data. Statistical analysis was conducted using analysis of variance (ANOVA), followed by Dunnett’s or Bonferroni post hoc tests to determine differences between groups. GraphPad Prism 9.4 was used for the statistical analysis. A p value of less than 0.05 was considered statistically significant.
Data availability
All the data of this study are available upon request from the corresponding author.
References
Cordell, G. A., Quinn-Beattie, M. L. & Farnsworth, N. R. The potential of alkaloids in drug discovery. Phytother. Res. 15, 183–205 (2001).
Jiang, W. et al. Analgesic alkaloids derived from traditional Chinese medicine in pain management. Front. Pharmacol. 13, 851508 (2022).
Wansi, J. D., Devkota, K. P., Tshikalange, E. & Kuete, V. 14 - Alkaloids from the Medicinal Plants of Africa. In Medicinal plant research in africa (ed. Kuete, V.) 557–605 (Elsevier, Amsterdam, 2013). https://doi.org/10.1016/B978-0-12-405927-6.00014-X.
Mahidol, C., Sahakitpichan, P. & Ruchirawat, S. Bioactive natural products from Thai plants. Pure Appl. Chem. Pure Appl. Chem. 66, 2353–2356 (1994).
Qiu, S. et al. Natural alkaloids: Basic aspects, biological roles, and future perspectives. Chin. J. Nat. Med. 12, 401–406 (2014).
Cushnie, T. P. T., Cushnie, B. & Lamb, A. J. Alkaloids: an overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 44, 377–386 (2014).
Johnson, G. Encyclopedia of analytical science 2nd edn. (Elsevier Academic Press, Amsterdam, 2005).
Yang, Y. et al. Effects of aloperine on acute and inflammatory pain models in mice. Scand. J. Pain 8, 28–34 (2015).
Yu, G. et al. Brucine alleviates neuropathic pain in mice via reducing the current of the sodium channel. J. Ethnopharmacol. 233, 56–63 (2019).
Bang, J. S. et al. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1beta-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res. Ther. 11, R49 (2009).
Jiang, W. et al. Analgesic mechanism of sinomenine against chronic pain. Pain Res. Manag. 2020, 1876862 (2020).
Haiyan, W. et al. Antinociceptive effects of matrine on neuropathic pain induced by chronic constriction injury. Pharm. Biol. 51, 844–850 (2013).
Foss, J. D. et al. Mitragynine, bioactive alkaloid of kratom, reduces chemotherapy-induced neuropathic pain in rats through α-adrenoceptor mechanism. Drug Alcohol Depend. 209, 107946 (2020).
Du, Q., Meng, X. & Wang, S. A comprehensive review on the chemical properties, plant sources, pharmacological activities, pharmacokinetic and toxicological characteristics of tetrahydropalmatine. Front. Pharmacol. 13, 890078 (2022).
Vella-Brincat, J. & Macleod, A. D. Adverse effects of opioids on the central nervous systems of palliative care patients. J. Pain Palliat. Care Pharmacother. 21, 15–25 (2007).
Pérez, E. G. & Cassels, B. K. Alkaloids from the genus Duguetia. Alkaloids. Chem. Biol. 68, 83–156 (2010).
Xiang, J. & Jiang, Y. Antiepileptic potential of matrine via regulation the levels of gamma-aminobutyric acid and glutamic acid in the brain. Int. J. Mol. Sci. 14, 23751–23761 (2013).
Baratloo, A. et al. The role of caffeine in pain management: A brief literature review. Anesthesiol. pain Med. 6, e33193 (2016).
Ditre, J. W., Heckman, B. W., Zale, E. L., Kosiba, J. D. & Maisto, S. A. Acute analgesic effects of nicotine and tobacco in humans: a meta-analysis. Pain 157, 1373–1381 (2016).
Çitoğlu, G. S., Acikara, O. B., Yilmaz, B. S. & Ozbek, H. Evaluation of analgesic, anti-inflammatory and hepatoprotective effects of lycorine from Sternbergia fisheriana (Herbert) Rupr. Fitoterapia 83, 81–87 (2012).
Roy, M. et al. Lycorine: A prospective natural lead for anticancer drug discovery. Biomed. Pharmacother. 107, 615–624 (2018).
Nair, J. J. & van Staden, J. Pharmacological and toxicological insights to the South African Amaryllidaceae. Food Chem. Toxicol. Int J. Publ. Br. Ind. Biol. Res. Assoc. 62, 262–275 (2013).
Stafford, G. I., Pedersen, M. E., van Staden, J. & Jäger, A. K. Review on plants with CNS-effects used in traditional South African medicine against mental diseases. J. Ethnopharmacol. 119, 513–537 (2008).
Nair, J. J. & Van Staden, J. Traditional usage, phytochemistry and pharmacology of the South African medicinal plant Boophone disticha (L.f.) Herb. (Amaryllidaceae). J. Ethnopharmacol. 151, 12–26 (2014).
Gadaga, L. L., Tagwireyi, D., Dzangare, J. & Nhachi, C. F. B. Acute oral toxicity and neurobehavioural toxicological effects of hydroethanolic extract of Boophone disticha in rats. Hum. Exp. Toxicol. 30, 972–980 (2011).
Kretzing, S. et al. Dose-dependent emetic effects of the Amaryllidaceous alkaloid lycorine in beagle dogs. Toxicon 57, 117–124 (2011).
Castagné, V. et al. Central nervous system (CNS) safety pharmacology studies. In BT - drug discovery and evaluation: Safety and pharmacokinetic assays (eds Vogel, H. G. et al.) 17–72 (Springer, Berlin, 2013). https://doi.org/10.1007/978-3-642-25240-2_3.
Lynch, J. J., Castagné, V., Moser, P. C. & Mittelstadt, S. W. Comparison of methods for the assessment of locomotor activity in rodent safety pharmacology studies. J. Pharmacol. Toxicol. Methods 64, 74–80 (2011).
Chen, O., Luo, X. & Ji, R.-R. Macrophages and microglia in inflammation and neuroinflammation underlying different pain states. Med. Rev. 3, 381–407 (2023).
Li, K., Lin, T., Cao, Y., Light, A. R. & Fu, K. Y. Peripheral formalin injury induces 2 stages of microglial activation in the spinal cord. J. Pain 11, 1056–1065 (2010).
Santos, J. M. M., Tatsuo, M. A. K. F., Turchetti-Maia, R. M. M., Lisboa, M. C. G. & De Francischi, J. N. Leukocyte recruitment to peritoneal cavity of rats following formalin injection: Role of Tachykinin receptors. J. Pharmacol. Sci. 94, 384–392 (2004).
Ji, R. R., Chamessian, A. & Zhang, Y. Q. Pain regulation by non-neuronal cells and inflammation. Science 354, 572–577 (2016).
Zhao, Y. L. et al. Genotoxicity and safety pharmacology studies of indole alkaloids extract from leaves of Alstonia scholaris (L.) R. Br. Nat. Products Bioprospect. 10, 119–129 (2020).
Xiao, H. et al. Lycorine and organ protection: Review of its potential effects and molecular mechanisms. Phytomedicine 104, 154266 (2022).
Ren, L., Zhao, H. & Chen, Z. Study on pharmacokinetic and tissue distribution of lycorine in mice plasma and tissues by liquid chromatography–mass spectrometry. Talanta 119, 401–406 (2014).
Gauvin, D. V., Zimmermann, Z. J., Dalton, J. A., Baird, T. J. & Kallman, M.-J. CNS safety screening under ICH S7A guidelines requires observations of multiple behavioral units to assess motor function. Int. J. Toxicol. 38, 339–356 (2019).
Ferreira, S. H. & Nakamura, M. II. Prostaglandin hyperalgesia: The peripheral analgesic activity of morphine, enkephalins and opioid antagonists. Prostaglandins 18, 191–200 (1979).
Del Gaudio, M. P., Kraus, S. I., Melzer, T. M., Bustos, P. S. & Ortega, M. G. Oral treatment with Berberine reduces peripheral nociception: Possible interaction with different nociceptive pathways activated by different allogeneic substances. J. Ethnopharmacol. 321, 117504 (2024).
Boonrueng, P. et al. Combination of curcumin and piperine synergistically improves pain-like behaviors in mouse models of pain with no potential CNS side effects. Chin. Med. 17, 119 (2022).
Amabeoku, G., Ewesuedo, R. & Abuh, F. Influence of some dopaminergic agents on antinociception produced by quinine in mice. Prog. Neuro-Psychopharmacology Biol. Psychiatry 16, 351–360 (1992).
Ferri, S., Santagostino, A. & Braga, P. C. Development of tolerance to the antinociceptive effect of mescaline intraventricularly administered to rabbits. Psychopharmacology (Berl). 47, 261–265 (1976).
Oka, T. & Hosoya, E. Effects of humoral modulators and naloxone on morphine-induced changes in the spontaneous locomotor activity of the rat. Psychopharmacology (Berl). 47, 243–248 (1976).
Peng, W.-H. et al. Anxiolytic effect of berberine on exploratory activity of the mouse in two experimental anxiety models: Interaction with drugs acting at 5-HT receptors. Life Sci. 75, 2451–2462 (2004).
Nassiri-Asl, M., Zamansoltani, F. & Torabinejad, B. Antiepileptic effects of quinine in the pentylenetetrazole model of seizure. Seizure 18, 129–132 (2009).
Zou, L. et al. The effects of quinine on neurophysiological properties of dopaminergic neurons. Neurotox. Res. 34, 62–73 (2018).
Islahudin, F. et al. The antimalarial drug quinine interferes with serotonin biosynthesis and action. Sci. Rep. 4, 3618 (2014).
Pan, S. Y. et al. Belladonna alkaloids-induced behavioral changes and amnesia on open-field and step-through in 18-, 28-, and 38-day-old mice. Zhongguo Yao Li Xue Bao 19, 112–116 (1998).
Chen, Y., Liu, J., Yao, Y., Yan, H. & Su, R. Rearing behaviour in the mouse behavioural pattern monitor distinguishes the effects of psychedelics from those of lisuride and TBG. Front. Pharmacol. 14, 1021729 (2023).
Tjølsen, A., Berge, O.-G., Hunskaar, S., Rosland, J. H. & Hole, K. The formalin test: An evaluation of the method. Pain 51, 5–17 (1992).
Chichorro, J. G., Lorenzetti, B. B. & Zampronio, A. R. Involvement of bradykinin, cytokines, sympathetic amines and prostaglandins in formalin-induced orofacial nociception in rats. Br. J. Pharmacol. 141, 1175–1184 (2004).
Ji, R.-R., Xu, Z.-Z. & Gao, Y.-J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 13, 533–548 (2014).
Wen, S. et al. Opioids regulate the immune system: Focusing on macrophages and their organelles. Front. Pharmacol. 12, 814241 (2021).
Ying, X. et al. Piperine inhibits LPS induced expression of inflammatory mediators in RAW 264.7 cells. Cell. Immunol. 285, 49–54 (2013).
Wang-Sheng, C. et al. Piperine attenuates lipopolysaccharide (LPS)-induced inflammatory responses in BV2 microglia. Int. Immunopharmacol. 42, 44–48 (2017).
Xiong, K. et al. Berberine promotes M2 macrophage polarisation through the IL-4-STAT6 signalling pathway in ulcerative colitis treatment. Heliyon 9, e14176 (2023).
Liu, M., Gao, L. & Zhang, N. Berberine reduces neuroglia activation and inflammation in streptozotocin-induced diabetic mice. Int. J. Immunopathol. Pharmacol. 33, 2058738419866379 (2019).
Kang, J. et al. Lycorine inhibits lipopolysaccharide-induced iNOS and COX-2 up-regulation in RAW264. 7 cells through suppressing P38 and STATs activation and increases the survival rate of mice after LPS challenge. Int. Immunopharmacol. 12, 249–256 (2012).
Smith, J. C. A review of strain and sex differences in response to pain and analgesia in mice. Comp. Med. 69, 490–500 (2019).
Thongphichai, W. et al. Standardization of the ethanolic extract of Crinum latifolium leaves by two bioactive markers with antiproliferative activity against TGF-β-promoted prostate stromal cells (WPMY-1). BMC Complement. Med. Ther. 22, 139 (2022).
Liu, J., Li, Y., Tang, L. J., Zhang, G. P. & Hu, W. X. Treatment of lycorine on SCID mice model with human APL cells. Biomed. Pharmacother. 61, 229–234 (2007).
Wang, C. et al. Lycorine induces apoptosis of bladder cancer T24 cells by inhibiting phospho-Akt and activating the intrinsic apoptotic cascade. Biochem. Biophys. Res. Commun. 483, 197–202 (2017).
Hu, M. et al. Lycorine is a novel inhibitor of the growth and metastasis of hormone-refractory prostate cancer. Oncotarget 6, 15348–15361 (2015).
Chen, H. et al. Antiviral activity of lycorine against Zika virus in vivo and in vitro. Virology 546, 88–97 (2020).
Shi, S. et al. Lycorine hydrochloride inhibits melanoma cell proliferation, migration and invasion via down-regulating p21(Cip1/WAF1). Am. J. Cancer Res. 11, 1391–1409 (2021).
Yuan, X.-H. et al. Lycorine inhibits tumor growth of human osteosarcoma cells by blocking Wnt/β-catenin, ERK1/2/MAPK and PI3K/AKT signaling pathway. Am. J. Transl. Res. 12, 5381–5398 (2020).
Dasuni Wasana, P. W. et al. Curcumin and metformin synergistically modulate peripheral and central immune mechanisms of pain. Sci. Rep. 12, 9713 (2022).
Friard, O. & Gamba, M. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330 (2016).
Acknowledgements
This research was supported by the Second Century Fund (C2F), Chulalongkorn University (H.H.) and funded by Herb Guardian Co., Ltd.
Author information
Authors and Affiliations
Contributions
H.H.: Conceptualization, Data curation, Formal analysis, Investigation, Visualization, Writing—original draft. P.W.D.W.: Data curation, Formal analysis, Investigation, Writing—original draft. W.T.: Data curation, Formal analysis, Investigation. S.S.: Resources, Supervision, Writing—review & editing. P.T.: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
S.S. and P.T. are the co-founders of Herb Guardian Co., Ltd. H.H. reports a relationship with Herb Guardian Co., Ltd. that includes funding grants. The remaining authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hasriadi, H., Wasana, P.W.D., Thongphichai, W. et al. Exploring the safety of lycorine in the central nervous system and its impact on pain-like behaviors in mice. Sci Rep 14, 16856 (2024). https://doi.org/10.1038/s41598-024-64410-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64410-z
- Springer Nature Limited
Keywords
This article is cited by
-
Uncover the anticancer potential of lycorine
Chinese Medicine (2024)