Abstract
Reaumuria soongorica is an important biological barrier for ecological protection in the Gobi Desert in northwestern China, where soil nitrogen availability is low. N deposition has recently increased significantly in Gobi Desert, and the responses of R. soongorica to N enrichment may become a problem for ecological restoration and protection. However, little is known about the effects of N addition on the biomass, non-structural carbohydrates (NSC), and carbon:nitrogen:phosphorus (C:N:P) stoichiometry of R. soongorica in this region. Here, we examined changes in biomass, NSC and C:N:P ratios of different organs of R. soongorica seedlings in four N addition treatments: 0 (N0), 4.6 (N1), 9.2 (N2), and 13.8 (N3) g m−2 year−1. N addition up to 9.2 g m−2 year−1 significantly increased the biomass of different organs, simultaneously increasing the belowground: aboveground ratio of R. soongorica seedlings. Root NSC concentrations significantly increased under all N addition treatments, but leaf and stem NSC concentrations only increased under the N1 and N2 addition treatments. Nitrogen addition enhanced the soluble sugar concentrations (SSC) of leaves and roots, and reduced starch concentrations (SC) of all organs. Stem and root N concentrations significantly increased under the N2 and N3 treatments, and leaf N concentrations only increased under the N3 treatment, but N addition had no significant effect on plant C and P concentrations. Leaf and stem C:N ratios decreased significantly under the N2 and N3 treatments, but root C:N decreased significantly in all N addition treatments. The N3 treatment significantly increased the N:P ratio of all organs. N addition significantly enhanced available N (AN), available P (AP) and total phosphorus (TP) in rhizosphere soil. Our results suggest that N addition alters the biomass, NSC, N concentrations, C:N and N:P ratios of all plant organs, but roots responded more strongly than stems or leaves to N addition, potentially allowing the plants to absorb more water from the arid soil in this region ensuring the survival of R. soongorica seedlings. Rhizosphere soil AP, AN and TP concentrations were important factors affecting the NSC concentrations and stoichiometric characteristics of R. soongorica.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Since the industrial revolution, atmospheric nitrogen (N) deposition has increased rapidly due to the widespread use of N-containing synthetic fertilizers and increased fossil fuel combustion, motivating increased research on the influence of N deposition on terrestrial ecosystems1,2. It has been shown that N deposition can affect plant photosynthesis and nutrient transport3, and accordingly the form and distribution of carbohydrates, the main product of photosynthesis, in plant organs should be affected by N deposition4. N deposition has also been shown to affect the stoichiometry (carbon, nitrogen, and phosphorus) of plants in terrestrial ecosystems and thus alter plant physiological activity and growth rates5.
Non-structural carbohydrates (NSC) include soluble sugars and starch. These NSCs provide the main energy supply for plant growth, development and reproduction, and perform important functions in metabolism, transporting water and assimilation products, regulating osmotic potential, and acting as a nutrient reservoir6,7. Understanding plant NSC concentrations and their variation among organs is thus the ideal entry point to explore the response and adaptation of plant growth and physiology to environmental stressors. Under environmental stress, plants will alter C allocation among different organs. For example, drought either significantly increased or maintained total NSC concentrations in aboveground plant organs, but reduced total NSC concentrations in sapling roots8. N and P fertilization greatly reduced leaf, stem, and root NSC concentrations in Moringa oleifera seedlings, possibly because of the dilution effects of increased biomass following fertilization9. Thus NSCs and their components (soluble sugar and starch) display different responses to different environmental factors among different organs10,11. Understanding fluctuations in NSC concentrations under different types of environmental stress has become an important tool for exploring plant adaptation strategies to diverse stressors.
Carbon (C), nitrogen (N) and phosphorus (P) are structural elements and the major nutrients maintaining biogeochemical cycling and energy flow within ecosystems12. Many studies have found that N addition significantly increased the C, N and P contents of plant organs13,14,15. Plant C:N and C:P ratios usually increased16 or decreased17 under N addition, but plant N:P ratios may increase15, decrease18 or remain the same17 depending on the species18 and soil nutrient conditions19 of the study. However, most studies have focused on aboveground stoichiometry rather than whole-plant stoichiometry20,21, and the responses of different plant organs to N addition remain largely unexplored in the Gobi Desert region.
Under natural conditions, Reaumuria soongorica can reproduce through seeds. The success of the plant’s natural renewal mainly depends on seed germination and seedling survival. In the Gobi Desert region, nitrogen is a key factor restricting vegetation growth, distribution, and the restoration of damaged ecosystems. The germination of R. soongorica seeds and the survival of seedlings are thus likely affected by nitrogen availability. N deposition may be beneficial for R. soongorica growth, but recent studies on R. soongorica have focused on growth22,23, photosynthetic physiology24,25, and genetics26 in recent years, and little is known regarding the impacts of N on the biomass, C:N:P stoichiometry, and NSC distribution among organs of R. soongorica seedlings in the Gobi Desert in northwestern China. To evaluate the effects of nitrogen on C:N:P stoichiometry and NSC content in different organs of R. soongorica, we conducted an N addition experiment. We hypothesized that (1) N addition would significantly enhance the biomass of different organs, but aboveground (leaf and stem) biomass would respond more strongly than belowground (root) biomass, resulting in a lower belowground: aboveground biomass ratio; (2) N addition would significantly enhance the NSC content of different organs, and (3) N addition would significantly enhance N concentrations in plant organs and soil, resulting in lower plant C:N ratios and higher N:P ratios. Our study is important for revealing the responses and adaptation mechanisms of desert plants to N deposition.
Results
Biomass across organs
With increasing N addition, total, leaf, stem, and root biomass all increased significantly and reached their maximum values in the N2 treatment (P < 0.05). Compared with the N0 treatment, total, leaf, stem and root biomass increased by 60.49%, 42.85%, 48.86% and 97.5%, respectively, in the N2 addition treatment. The ratio of belowground to aboveground biomass also significantly increased in the N2 and N3 treatments (P < 0.05) (Table 1). The relative growth rates (RGR) of leaves, stems and roots were all highest in the N2 treatment; stem and root RGRs differed significantly from the control in the N1–N3 addition treatments (Fig. 1), while leaf RGR was significantly enhanced in the N2 and N3 treatments compared to the control (P < 0.05). Among the different organs, the RGR of roots was highest under N addition, demonstrating that root biomass responded more rapidly than did leaf and stem biomass.
NSC concentrations in different organs
The soluble sugar concentrations (SSC), starch concentrations (SC) and NSC concentrations of different organs altered with increasing N addition. With increasing N addition, leaf, stem, and root SSC all increased significantly in the N1 and N2 addition treatments, but decreased significantly (by 26.95%, 37%, and 15.68% in leaves, stems, and roots) in the N3 treatment compared to the N2 treatment (Fig. 2a) (P < 0.05). Leaf, stem, and root SC decreased significantly in the N2 and N3 addition treatments compared to the control (Fig. 2b) (P < 0.05). Compared with N0, N addition (N1–N3) significantly enhanced the NSC concentrations of roots, which were highest in the N2 treatment, but leaf and stem NSC concentrations only increased significantly in the N1 and N2 treatments (Fig. 2c) (P < 0.05).
Nutrient concentrations and stoichiometry across organs
N addition had no effect on P concentrations across all organs (Fig. 3a,c). Compared with N0, the N concentrations of stems and roots significantly increased in the N2 and N3 treatments; leaf N concentration was significantly enhanced in the N3 treatment (P < 0.05). There were no significant differences in N concentrations between the N0 and N1 treatments (Fig. 3b).
Leaf and stem C:N ratios decreased significantly in the N2 and N3 treatments compared to the control, but root C:N decreased significantly with increasing N addition (P < 0.05) (Fig. 3d). Compared with N0, the C:P ratio of different organs all reduced slightly with N addition, but displayed no significant differences among N treatments (P > 0.05) (Fig. 3e). The higher N addition treatments (N2–N3) significantly enhanced the stem N:P ratio, but leaf and root N:P ratios only increased in the N3 addition treatment (Fig. 3f) (P < 0.05).
Rhizosphere soil nutrient concentrations
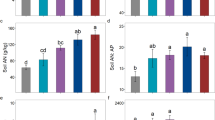
Compared with N0, N addition significantly increased all rhizosphere soil nutrient concentrations [except total carbon (TC) and total nitrogen (TN) in the N1 treatment] relative to the control (P < 0.05). Rhizosphere soil total phosphorus (TP) did not differ significantly among the N1–N3 treatments (P > 0.05) (Fig. 4a). Available N (AN) was significantly higher in the N2 and N3 treatments than in the N1 addition treatment, but available P (AP) in the N3 addition treatment was significantly higher than in the N1 and N2 addition treatments (P < 0.05) (Fig. 4b).
Relationship between measured soil factors, plant stoichiometric characteristics, and NSC
Rhizosphere soil TP was significantly positively correlated with the SSC of different organs, but for roots, SSC was also correlated with the total nitrogen (TN), AN, and AP of rhizosphere soil. Except for TC, other measured soil factors were significantly negatively correlated with SC and were positively correlated with the biomass of different organs. A significant positive correlation was only found between root NSCs and rhizosphere soil TP, AN, AP (Fig. 5). The relationships between rhizosphere soil nutrients and plant stoichiometric characteristics differed among plant organs, but were significantly positively correlated with plant N, and negatively correlated with C:N. Rhizosphere soil nutrients were more strongly related to root stoichiometric characteristics than leaf and stem stoichiometry.
Correlation analysis between soil factors and ecological stoichiometry and NSCs of R. soongorica. RSAP = rhizosphere soil available P, TC = rhizosphere soil total carbon, RSTP = rhizosphere soil total phosphorus, RSTN = rhizosphere soil total nitrogen, RSAN = rhizosphere soil available N, L-SSC = leaf soluble sugar concentration, L-SC = leaf starch concentration, L-NSC = leaf non-structural carbohydrates, L-C = leaf total C, L-N = leaf total N, L-P = leaf total P, L-C:N = leaf C:N ratio, L-C:P = leaf C:P ratio, L-N:P = leaf N:P ratio, LB = leaf biomass, S-SSC = stem soluble sugar concentration, S-SC = stem starch concentration, S-NSC = stem non-structural carbohydrates, S-C = stem total C, S-N = stem total N, S-P = stem total P, S-C:N = stem C:N ratio, S-C:P = stem C:P ratio, S-N:P = stem N:P ratio, SB = stem biomass, R-SSC = root soluble sugar concentration, R-SC = root starch concentration, R-NSC = root non-structural carbohydrates, R-C = root total C, R-N = root total N, R-P = root total P, R-C:N = root C:N ratio, R-C:P = root C:P ratio, R-N:P = root N:P ratio, RB = root biomass.
RDA analyses showed that measured rhizosphere soil nutrients affected the stoichiometric characteristics and NSCs of R. soongorica (Fig. 6). The first axis explained 67.01% of the total variation and the second axis accounted for 13.25% of the total variation, and therefore 79.26% of the variation in plant stoichiometric characteristics and NSC traits were explained by soil factors. Among the five soil factors, rhizosphere soil AP was the most significant variable that affected the stoichiometric characteristics and NSCs of R. soongorica (P = 0.002), explaining 55.1% of the total variance. Rhizosphere soil AN and TP were the next most important variables, explaining 51.2% and 39.7% (P = 0.002) of the total variance, respectively. Rhizosphere soil AP, AN and TP were negatively correlated with the first axis, with correlation coefficients of 0.892, 0.887, 0.802, respectively (Fig. 6). Therefore, rhizosphere soil AP, AN and TP concentrations were important factors affecting the plant stoichiometric characteristics and NSCs of R. soongorica.
Redundancy analysis (RDA) between soil factors and ecological stoichiometry and NSCs of R. soongorica. RSAP = rhizosphere soil available P, TC = rhizosphere soil total carbon, RSTP = rhizosphere soil total phosphorus, RSTN = rhizosphere soil total nitrogen, RSAN = rhizosphere soil available N, L-SSC = leaf soluble sugar concentration, L-SC = leaf starch concentration, L-NSC = leaf non-structural carbohydrates, L-C = leaf total C, L-N = leaf total N, L-P = leaf total P, L-C:N = leaf C:N ratio, L-C:P = leaf C:P ratio, L-N:P = leaf N:P ratio, LB = leaf biomass, S-SSC = stem soluble sugar concentration, S-SC = stem starch concentration, S-NSC = stem non-structural carbohydrates, S-C = stem total C, S-N = stem total N, S-P = stem total P, S-C:N = stem C:N ratio, S-C:P = stem C:P ratio, S-N:P = stem N:P ratio, SB = stem biomass, R-SSC = root soluble sugar concentration, R-SC = root starch concentration, R-NSC = root non-structural carbohydrates, R-C = root total C, R-N = root total N, R-P = root total P, R-C:N = root C:N ratio, R-C:P = root C:P ratio, R-N:P = root N:P ratio, RB = root biomass.
Discussion
Effects of N addition on plant biomass
Plant growth is closely related to nutrients, especially in nutrient-deficient soil. Soil nitrogen content is low in the arid and semi-arid regions of northwestern China, and is one of the main factors limiting plant productivity in the region27. Contrary to our first hypothesis, we found that N addition up to 9.2 g m−2 year−1 significantly increased the biomass and RGR of different organs, but biomass and RGR significantly decreased at highest level of N addition (13.8 g m−2 year−1). This result is consistent with the results of Jin et al.,13 and previous studies have found that the study area is N-limited28, explaining why plant growth was enhanced at 0–9.2 g m−2 year−1 N addition. This finding indicates that appropriate N addition is beneficial to the growth of R. soongorica in the Gobi desert region, and the N saturation value is between 9.2 and 13.7 g m−2 year−1. Once N addition exceeds the maximum N requirements for plant growth, plants will be less sensitive to N addition29.
A previous study has shown that plants can increase their allocation of photosynthetic products to belowground organs under lower 0.15 mM N conditions30, but biomass allocation patterns are species-specific4,31 and also vary with the amount of N addition. We found that the RGR of roots under N addition (up to 9.2 g m−2 year−1) was significantly higher than that of the shoot, thus resulting in a higher belowground : aboveground ratio. This result was inconsistent with the findings of Ai et al. who found that the rate of biomass increase of Bothriochloa ischaemum was more rapid aboveground than belowground4, also failing to support our first hypothesis. This discrepancy may be because R. soongorica allocated additional N from treatments to root biomass production over leaf and stem biomass production to better access water in the arid Gobi Desert region.
Effects of N addition on NSCs
NSCs are the products of photosynthesis and provide energy for plant growth and metabolism, playing an important role in plant responses to environmental changes32. Some studies have reported that N addition increased NSC accumulation31, but other studies found that N addition had no effect on or even decreased NSC concentrations4,9. In this study, we found that NSC concentrations of different organs of R. soongorica significantly increased with mild to moderate N addition (0–9.2 g N m−2 year−1), but slightly decreased under the highest N addition treatment (13.8 g N m−2 year−1). This result failed to support our second hypothesis and was consistent with the results of other studies that found that low N addition contributes to the accumulation of soluble carbohydrates, thereby increasing their concentrations, but high N addition can significantly reduce concentrations33. Meanwhile, some studies have reported that the accumulation of higher NSC concentrations can balance the osmotic pressure of cells34 and be used to cope with environmental stress. Thus the high NSC concentrations in different organs under the moderate N addition treatment (9.2 g m−2 year−1) in our study indicate that adding proper amount of N will benefit for NSC accumulation and potentially improve the resistance of R. soongorica to water deficits in the Gobi Desert region, aiding the successful settlement of R. soongorica in this region.
We found that roots increased NSC concentrations more than leaves and stems under N addition, which is consistent with previous studies4,31. While we found that root NSC concentrations were higher than leaf and stem NSCs in our study, Ai et al4 found that aboveground NSC concentrations were higher than belowground NSC concentrations; this difference may be related to characteristics of C allocation and transport35. In order to facilitate water absorption and survive in the Gobi Desert, R. soongorica may have allocated more carbon to the roots than to leaves and stems36. Some studies have found that N addition significantly affected soil P concentrations, and soil P concentrations had significant effects on belowground starch and NSC concentrations, so N addition can accordingly have strong positive effects on belowground biomass NSC concentrations4. We found that N addition significantly increased rhizosphere soil AP, AN and TP concentrations, but the three major soil factors exerted different influences on the NSC concentrations of different organs of R. soongorica. The effects of soil nutrients on root NSC concentrations were significantly positive, but we observed no significant positive effects on leaf and stem NSC concentrations (Fig. 5), confirmed the conclusions of prior studies.
Effect of N addition on plant stoichiometry
Stoichiometry reflected the utilization ability of C, N and P for plants, which are susceptible to environmental changes37. Many studies have concluded that N addition will result in higher soil N availability, and thus enhanced N concentrations and lower C:N ratios in many species38,39. Some studies also found that N addition significantly increased the C and N contents of plants and thus had no effect on C:N ratios4,14, but the stoichiometry of different organs can respond differently to environmental changes40. In this study, we found that the N2–N3 addition treatments significantly increased leaf and stem N concentrations and reduced leaf and stem C:N ratios, and all three N addition treatments significantly increased root N concentrations and reduced root C:N ratios in R. soongorica. This may be because available N in rhizosphere soil was significantly enhanced by N addition, but treatments had only minimal effects on total carbon. N addition also had greater effects on roots than on other organs, consistent with our third hypothesis and the results of Xiao et al.31, who reported that N addition increased N concentrations in roots compared to shoots. A plant’s N:P ratio has been interpreted as an indicator of N and/or P limitation41,42. It is widely accepted that N:P ratios < 10 indicate N limitation. R. soongorica displayed very low N:P ratios due to relatively high P concentrations in the higher N addition treatment, indicating that these plants remained N-limited despite the massive N doses that were applied. This result is consistent with the research of Wang et al.43, who found that the N:P ratio of R. soongorica was lower in the arid desert region, but is inconsistent with the results of Niu et al.44, who found that the N:P ratios of six shrubs (including R. soongorica) in the desert of the Alxa Plateau were all higher than 10. These inconsistent results may be due to variation among ecosystem types and environmental factors45.
Conclusions
Total plant biomass and the biomass of different organs significantly increased with a range of N addition treatments (0–9.2 g m−2 year−1), and root biomass increased more rapidly than the leaf and stem biomass, resulting in higher belowground:aboveground ratios. N addition enhanced soluble sugar concentrations, but reduced the starch concentrations of all organs, and root NSC concentrations significantly increased under all N addition, but leaf and stem NSC concentrations only increased significantly under the N1 and N2 treatments. Plant N concentrations increased under higher N addition treatments (9.2–13.8 g m−2), but treatments had no significant effect on C and P concentrations, thus resulted in significantly reduced C:N ratios. N addition significantly increased the available N (AN), available P (AP) and total phosphorus (TP) of rhizosphere soil, and these soil nutrient variables were significantly positively correlated with root NSC concentrations. However, rhizosphere soil nutrients had stronger relationships with root stoichiometric characteristics than leaf and stem stoichiometric characteristics. This indicates that N addition alters the biomass, NSC concentration, N concentration, C:N ratio, and N:P ratio of different organs, though roots responded more strongly than stems or leaves, and rhizosphere soil AP, AN and TP concentrations were important factors affecting the NSC concentrations and stoichiometric characteristics of R. soongorica.
Materials and methods
Plant materials and growth conditions
R. soongorica is plentiful in the Gobi experimental fields at the Linze Inland River Basin Research Station (39°21′ N, 100°02′ E, 1400 m asl), Chinese Academy of Sciences. We harvested and dried fruiting branches of R. soongorica with mature seeds in October 2017. We beat the dried fruiting branches and collected the fallen seeds. After the seeds were soaked in water for 5–10 min, filled seeds would sink to the bottom, and we collected and air-dried these seeds, saving them in sealed plastic bags to nurture seedlings in the following year.
The experiment was also conducted at the Linze Inland River Basin Research Station. Average annual precipitation at the study site is 117 mm, and the mean annual temperature is 7.6 °C. Before the start of the experiment, soil samples (0–20 cm, 20–40 cm soil layer) without vegetation were collected at the Gobi experimental field in October 2017, and we removed visible plant residues, roots, stones and other materials, crushing the larger clods of soil. After air-drying, the soil samples were sieved through a 5 mm mesh and each soil layer was mixed. Initial soil chemical properties are presented in Table 2.
Experimental design
In March 2018, we added the collected soil samples to pots with a diameter of 34 cm and a height of 40 cm, first adding the 20–40 cm soil layer and then adding the 0–20 cm soil layer at the top. We sowed full seeds of R. soongorica into the pots, and soil water content was maintained at > 80% of field capacity during seedling establishment. After emergence, two seedlings with heights around 5 cm were retained in each pot for one year of growth. We initiated the experiment in May 2019, and one seedling was maintained in each pot after removal of the excess plant.
Nitrogen levels were designed according to nitrogen deposition levels in the desert region18, and four treatments received additional N in the form of urea at the rates of 0 (N0), 4.6 (N1), 9.2 (N2), and 13.8 (N3) g N m−2 year−1. Each treatment had six replicates. Urea [CO(NH 2)2] has an N content of 46.7%, so the amount of urea applied was 0.8865, 1.7730, and 2.6595 g for treatments N1, N2, and N3 treatments, respectively. N was applied equally in the beginning of May, June, July, August, September and October in 2019, as a solution of urea in the same volume of deionised water, and the N0 treatment received the same volume of water at the same times.
Sampling and chemical analysis
The six seedlings of R. soongorica for each treatment were sampled at the end of October. The whole seedlings of R. soongorica were taken out of the pots, the roots were wrapped with plastic wrap to prevent water loss, and all samples were taken back to the laboratory. We divided the seedlings into 3 parts: leaves, stems, and roots. Each part was washed with distilled water, and excess water at the surface was removed with blotting paper. We weighed all samples, placed them in labeled envelopes, and oven-dried each sample at 80 °C in an oven until they reached a constant weight to assess dry mass. Finally, the dried samples were ground using a Wiley Mill until they were sufficiently fine to pass through a 40-mesh sieve and stored for chemical analysis. Upon removal of each seedling from the pot, we collected the soil that was adhered to the root system using a brush and considered these samples rhizospheric soil. Each of these soil samples was air-dried and passed through a 2-mm-mesh sieve for chemical analysis.
We calculated NSC concentrations by summing soluble sugar concentrations and starch concentrations4, and the concentrations of soluble sugars and starch were determined using an anthrone method46. Total N concentrations were measured using the Kjeldahl method. Total P concentrations were determined using the molybdenum blue colorimetric method. Total C concentrations were determined using the dichromate oxidation method. Available N was measured using the alkaline diffusion method, and available P was measured using the sodium bicarbonate leaching-molybdenum antimony anti-colorimetric method47.
Data analysis
The relative growth rate (RGR, g d−1) was calculated as follows:
where W1 is the initial biomass, W2 is the biomass under the N addition treatment (N0–N3), and t is the time interval between the two measurements.
We used one-way analysis of variance (ANOVA) to test the effects of nitrogen on biomass, NSC concentrations, C, N and P stoichiometry of different organs. Duncan’s multiple range tests were used for multiple comparisons among each treatment. Data analyses were performed using SPSS Statistics procedure (Version 13.0) and figures were drawn using Origin 8.
Research involving plants
Experimental research and field studies on Reaumuria soongorica plants complied with relevant institutional guidelines.
Data availability
All datasets generated and/or analyzed during the current study are included in this article.
References
Galloway, J. N., Townsend, W. H., Erisman, J. W., Bekunda, M. & Cai, Z. Transformation of the nitrogen cycle: Recent trends, questions and potential solutions. Science 320, 889–892 (2008).
Phoenix, G. K. et al. Impacts of atmospheric nitrogen deposition: Responses of multiple plant and soil parameters across contrasting ecosystems in long-term field experiments. Glob. Change Biol. 18, 1197–1215 (2012).
Pons, T. L., van der, Werf, A. & Lambers, H. Photosynthetic nitrogen use efficiency of inherently slow- and fast-growing species: Possible explanations for observed differences. In A Whole Plant Perspective on Carbon-Nitrogen Interactions (eds Roy, J., Garnier, E.) 61–77 (SPB Academic Publishing, The Hague, 1994).
Ai, Z. M., Xue, S., Wang, G. L. & Liu, G. B. Responses of Non-structural carbohydrates and C:N: P stoichiometry of Bothriochloa ischaemum to nitrogen addition on the Loess Plateau, China. J. Plant Growth Regul. 36, 714–722 (2017).
Marklein, A. R. & Houlton, B. Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 193, 696–704 (2012).
Dietze, M. C. et al. Nonstructural carbon in woody plants. Annu. Rev. Plant Biol. 65, 667–687 (2014).
Hartmann, H. & Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees -from what we can measure to what we want to know. New Phytol. 211, 386–403 (2016).
Yang, Q. P. et al. Different responses of non-structural carbohydrates in above-ground tissues/organs and root to extreme drought and re-watering in Chinese fir (Cunninghamia lanceolata) saplings. Trees 30, 1863–1871 (2016).
Peng, Z. T. et al. Non-structural carbohydrates regulated by nitrogen and phosphorus fertilization varied with organs and fertilizer levels in Moringa oleifera Seedlings. J. Plant Growth Regul. 40, 1777–1786 (2021).
Nardini, A. et al. Rooting depth, water relations and non-structural carbohydrate dynamics in three woody angiosperms deferentially affected by an extreme summer drought. Plant Cell Environ. 39, 618–627 (2016).
Wang, F. C. et al. Effects of experimental nitrogen addition on nutrients and nonstructural carbohydrates of dominant understory plants in a Chinese Fir plantation. Forests 10, 155 (2019).
Elser, J. J. et al. Growth rate-stoichiometry couplings in diverse biota. Ecol. Lett. 6, 936–943 (2003).
Jinm, X. M. et al. Ecological stoichiometry and biomass response of Agropyron michnoi under simulated N deposition in a sandy grassland, China. J. Arid Land. 12, 741–751 (2020).
Jing, H. et al. Nitrogen addition changes the stoichiometry and growth rate of different organsin pinus tabuliformis seedlings. Front. Plant Sci. 8, 1922 (2017).
Zhan, S. X., Wang, Y., Zhu, Z. C., Li, W. H. & Bai, Y. F. Nitrogen enrichment alters plant N: P stoichiometry and intensifies phosphorus limitation in a steppe ecosystem. Environ. Exp. Bot. 134, 21–32 (2017).
Stiling, P. & Cornelissen, T. How does elevated carbon dioxide (CO2) affect plant–herbivore interactions? A field experiment and meta-analysis of CO2 -mediated changes on plant chemistry and herbivore performance. Glob. Change Biol. 13, 1823–1842 (2007).
Wang, X. G. et al. Responses of C:N: P stoichiometry of plants from a Hulunbuir grassland to salt stress, drought and nitrogen addition. Phyton-Int. J. Exp. Bot. 87, 123–132 (2018).
Liu, X. J. et al. Enhanced nitrogen deposition over China. Nature 494, 459–462 (2013).
Huang, W. J., Houlton, B. Z., Marklein, A. R., Liu, J. X. & Zhou, G. Y. Plant stoichiometric responses to elevated CO2 vary with nitrogen and phosphorus inputs: Evidence from a global-scale meta-analysis. Sci. Rep. 5, 18225 (2015).
Wang, X. et al. Effects of nutrient addition on nitrogen, phosphorus and non-structural carbohydrates concentrations in leaves of dominant plant species in a semiarid steppe. Chin. J. Ecol. 33, 1795–1802 (2014).
Yang, D. X., Song, L. & Jin, G. Z. The soil C:N: P stoichiometry is more sensitive than the leaf C:N: P stoichiometry to nitrogen addition: A four-year nitrogen addition experiment in a Pinus koraiensis plantation. Plant Soil 442, 183–198 (2019).
Chong, P. F., Zhan, J., Li, Y. & Jia, X. Y. Carbon dioxide and precipitation alter Reaumuria soongorica root morphology by regulating the levels of soluble sugars and phytohormones. Acta Physiol. Plant 41, 184 (2019).
Ma, X. Z. & Wang, X. P. Biomass partitioning and allometric relations of the Reaumuria soongorica shrub in Alxa steppe desert in NW China. For. Ecol. Manag. 468, 118–178 (2020).
He, F. L., Bao, A. K., Wang, S. M. & Jin, H. X. NaCl stimulates growth and alleviates drought stress in the salt-secreting xerophyte Reaumuria soongorica. Environ. Exp. Bot. 162, 433–443 (2019).
Xu, D. H. et al. Photosynthetic parameters and carbon reserves of a resurrection plant Reaumuria soongorica during dehydration and rehydration. Plant Growth Regul. 60, 183–190 (2010).
Zhang, H. et al. miRNA–mRNA integrated analysis reveals roles for miRNAs in a typical halophyte, Reaumuria soongorica, during seed germination under salt stress. Plants 9, 351 (2020).
Bai, Y. M., Li, Y., Shan, L. S., Su, M. & Zhang, W. T. Effects of precipitation change and nitrogen addition on root morphological characteristics of Reaumuria soongorica. Arid Zone Res. 37, 1284–1292 (2020).
Hedwall, P. O., Nordin, A., Strengbom, J., Brunet, J. & Olsson, B. Does background nitrogen deposition affect the response of boreal vegetation to fertilization?. Oecologia 173, 615–624 (2013).
Wang, G., Fahey, T. J., Xue, S. & Liu, F. Root morphology and architecture respond to N addition in Pinus tabuliformis, west China. Oecologia 171, 583–590 (2013).
Grechi, I. et al. Effect of light and nitrogen supply on internal C: N balance and control of root-to-shoot biomass allocation in grapevine. Environ. Exp. Bot. 59, 139–149 (2007).
Xiao, L., Liu, G., Li, P. & Xue, S. Nitrogen addition has a stronger effect on stoichiometries of non-structural carbohydrates, nitrogen and phosphorus in Bothriochloa ischaemum than elevated CO2. Plant Growth Regul. 83, 325–334 (2017).
Quentin, A. G. et al. Non-structural carbohydrates in woody plants compared among laboratories. Tree Physiol. 35, 1146–1165 (2015).
White, L. M. Carbohydrate reserves of grasses: A review. J. Range Manag. 26, 13–18 (1973).
Millard, P., Sommerkorn, M. & Grelet, G. A. Environmental change and carbon limitation in trees: A biochemical, ecophysiological and ecosystem appraisal. New Phytol. 175, 11–28 (2007).
Zhang, T., Cao, Y., Chen, Y. M. & Liu, G. B. Non-structural carbohydrate dynamics in Robinia pseudoacacia saplings under three levels of continuous drought stress. Trees 29, 1837–1849 (2015).
Chapin, F. S., Schulze, E. D. & Mooney, H. A. The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 21, 423–447 (1990).
Sardans, J., Rivas-Ubach, A. & Peñuelas, J. The C:N: P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. Evolut. Syst. 14, 33–47 (2012).
Xia, J. Y. & Wan, S. Q. Global response patterns of terrestrial plant species to nitrogen addition. New Phytol. 179, 428–439 (2008).
Yang, Y. H., Luo, Y. Q., Lu, M., Schädel, C. & Han, W. X. Terrestrial C: N stoichiometry in response to elevated CO2 and N addition: a synthesis of two meta-analyses. Plant Soil 343, 393–400 (2011).
Mayor, J. R., Wright, S. J. & Turner, B. L. Species-specific responses of foliar nutrients to long-term nitrogen and phosphorus additions in a lowland tropical forest. J. Ecol. 102, 36–44 (2014).
Koerselman, A. & Meuleman, A. F. The vegetation ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 33, 1441–1450 (1996).
Gusewell, S. N: P ratios in terrestrial plants: variation and functional significance. New Phytol. 164, 243–266 (2004).
Wang, S., Shan, L. S., Li, Y., Zhang, Z. Z. & Ma, J. Effect of Precipitation on the Stoichiometric Characteristics of Carbon, Nitrogen and Phosphorus of Reaumuria soongarica and Salsola passerina. Acta Bot. Boreal. Occident. Sin. 40, 0335–0344 (2020).
Niu, D. C., Li, Q., Jiang, S. G., Chang, P. J. & Fu, H. Seasonal variations of leaf C:N: P stoichiometry of six shrubs in desert of China’s Alxa Plateau. Chin. J. Plant Ecol. 37, 317–325 (2013).
Kleyer, M. & Minden, V. Why functional ecology should consider all plant organs: An allocation-based perspective. Basic Appl. Ecol. 16, 1–9 (2015).
Yemm, E. & Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 57, 508–514 (1954).
Bao, S. D. Soil and Agricultural Chemistry Analysis 3rd edn. (China Agriculture Press, Beijing, 2000).
Acknowledgements
This work was financially supported by the National Natural Sciences Foundation of China (31960245), the scientific research start-up funds for openly-recruited doctors (GAU-KYQD-2018-07), and the Discipline construction fund project of Gansu Agricultural University (GAU-XKJS-2018-104, GAU-XKJS-2018-108). We thank Linze Inland River Basin Research Station, Chinese Academy of Sciences, for providing the experiment site. We would like to thank the High Edit company for assistance with English language editing of this manuscript.
Author information
Authors and Affiliations
Contributions
T.T.X. analyzed the data and wrote the manuscript. W.T.Z. conducted the experiment and performed the measurements. S.L.S. participated in the planning of the study, data analysis, and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, T., Shan, L. & Zhang, W. N addition alters growth, non-structural carbohydrates, and C:N:P stoichiometry of Reaumuria soongorica seedlings in Northwest China. Sci Rep 12, 15390 (2022). https://doi.org/10.1038/s41598-022-19280-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19280-8
- Springer Nature Limited