Abstract
The sensitivity of animal photoreceptors to different wavelengths of light strongly influence the perceived visual contrast of objects in the environment. Outside of the human visual wavelength range, ultraviolet sensitivity in many species provides important and behaviourally relevant visual contrast between objects. However, at the opposite end of the spectrum, the potential advantage of red sensitivity remains unclear. We investigated the potential benefit of long wavelength sensitivity by modelling the visual contrast of a wide range of jewel beetle colours against flowers and leaves of their host plants to hypothetical insect visual systems. We find that the presence of a long wavelength sensitive photoreceptor increases estimated colour contrast, particularly of beetles against leaves. Moreover, under our model parameters, a trichromatic visual system with ultraviolet (λmax = 355 nm), short (λmax = 445 nm) and long (λmax = 600 nm) wavelength photoreceptors performed as well as a tetrachromatic visual system, which had an additional medium wavelength photoreceptor (λmax = 530 nm). When we varied λmax for the long wavelength sensitive receptor in a tetrachromatic system, contrast values between beetles, flowers and leaves were all enhanced with increasing λmax from 580 nm to at least 640 nm. These results suggest a potential advantage of red sensitivity in visual discrimination of insect colours against vegetation and highlight the potential adaptive value of long wavelength sensitivity in insects.
Similar content being viewed by others
Introduction
Visual cues are perceived differently by different visual systems. For example, the UV sexual ornaments of male swordtails are attractive to females but are less visible to the swordtail’s main predators, Mexican tetras, because these predators lack a UV photoreceptor1. Variation in the detectable wavebands and perceived contrast in a visual scene is due, in part, to the number of retinal photoreceptor types and their peak sensitivities. The UV photoreceptor in birds, for instance, enhances the modelled contrast between upper and lower leaf surfaces2 because leaves reflect more UV light than they transmit3. Enhanced UV contrast could possibly facilitate leaf-localisation tasks such as prey searching. While the ecological function of UV sensitivity has been widely researched, the potential adaptive value of sensitivity to long wavelengths (600–750 nm) has received less attention (but see e.g.4,5,6).
Sensitivity to red (600–700 nm) or far red (700–750 nm) wavelengths requires the presence of a long wavelength sensitive (LWS) photoreceptor. The peak sensitivity, λmax, of LWS photoreceptors is generally below 600 nm, but an LWS photoreceptor often enables sensitivity to wavelengths well beyond the photoreceptor’s peak sensitivity, depending on the shape of the photoreceptor sensitivity function. For example, the human red photoreceptor peaks at 562 nm but still has 30% relative absorbance at 635 nm7. LWS photoreceptors that provide sensitivity beyond 600 nm are ancestral in vertebrates and common in diurnal vertebrates8; whereas they are usually absent in insects9. Nevertheless, red sensitivity has evolved independently and repeatedly in several insect families and can be found in certain species of beetles, bees, moths, and butterflies9,10. λmax of the LWS photoreceptor is most commonly around 560–600 nm in insects9, with the longest known λmax occurring in the butterfly, Colias erate, at 660 nm11. In the swallowtail butterfly, Papilio aegeus, red sensitivity enables discrimination of the young leaves preferred as oviposition sites from older, less favourable leaves12. Sensitivity to red or far-red could also enhance discrimination of leaves from fruit13, flowers, conspecific coloration or other objects in the environment which differ spectrally at long wavelengths.
Constraints on the evolution of red sensitivity depend on the mechanism used to shift photoreceptor sensitivity to longer wavelengths. Photoreceptors contain an opsin protein coupled to a light sensitive vitamin A derivative, the chromophore, which together comprise the visual pigment. Insect photoreceptors can also contain coloured filtering or screening pigments. In some insect groups such as Nymphalidae and Pieridae butterflies11,14,15, moths16,17, fireflies18, and click beetles19, a subset of photoreceptors with an LWS visual pigment have a red filter that red-shifts the receptor sensitivity to give a separate and additional red receptor. Colias erate butterflies, for instance, have perirhabdomal pigments that shift the peak sensitivity of the photoreceptor from around 570 nm to over 600 nm11. Some species use different filtering pigments with a single type of opsin to create multiple LWS photoreceptors (e.g. LWS receptors of Pieris rapae crucivora peak at 620 nm and 640 nm15). By narrowing and red-shifting the spectral sensitivity of these photoreceptors, filtering pigments inevitably reduce total light capture (i.e. absolute sensitivity). Modification to the opsin sequence can also shift peak sensitivity to longer wavelengths, but in insects this has only been documented in butterflies so far20,21,22. Changes in chromophore type, specifically use of an A2 rather than A1 chromophore, is one of the main mechanisms of increasing long wavelength sensitivity in vertebrates23,24, but has not been found in insects. The latter two mechanisms shift the absorption spectra of visual pigments to longer wavelengths and do not decrease absolute sensitivity. However, these mechanisms increase thermal noise because the activation energy threshold decreases with increasing λmax25,26,27,28,29,30. This increase in noise is believed to restrict the upper limit of visual pigment absorption31.
Here, we investigated the potential adaptive benefit of long wavelength sensitivity in insects by modelling the visual contrast of natural objects when systematically modifying visual sensitivities. To generalise the study across insect taxa, the visual sensitivities used in the models were based on the median photoreceptor peak sensitivities observed in insects. We modelled the effect of the presence or absence of an LWS photoreceptor in a trichromatic visual system and compared these to a tetrachromatic visual system. We also modelled the effect of systematically increasing the peak sensitivity of the LWS photoreceptor. We modelled increases in peak sensitivity caused by filtering pigments, as commonly found in insects, and the shifts due to modified opsin sequences for comparison. Additionally, we modelled the effect of light environment by running the same models using both daylight and civil twilight (when the sun is 0°–6° below the horizon) illumination spectra because these periods differ markedly in the spectral distribution and represent the lowest light level for colour discrimination of most diurnal insects32. These modelled contrasts provide an indication of how variation in visual sensitivities may influence visual discrimination, although actual discrimination for any given organism depends on colour opponent mechanisms and neural processing and can be context dependent33.
We chose target spectra for a system where red sensitivity is likely to be ecologically relevant, namely jewel beetles (Coleoptera: Buprestidae) and their host plants. Jewel beetles are diurnally active and have diverse colours that span a broad spectral range (Fig. 1a)34,35,36,37. They have both pigment and structural colours that are spectrally similar to colours commonly found in other insects and are representative of the diversity of insect colours. Many species are specialists and feed on flowers or leaves of specific plant genera38 but the flowers of their host plants also span a diverse spectral range. Spectral data on jewel beetles and the leaves and flowers of their host plants are therefore likely to be representative of ecologically relevant visual stimuli for jewel beetles and other diurnally active insect groups. Moreover, jewel beetles have relatively large eyes and use visual signals to locate mates and host plants39,40,41. Intracellular recordings, opsin expression and electroretinogram data all indicate that jewel beetles have an LWS photoreceptor with sensitivity potentially extending into the far-red42,43,44. We first conducted a behavioural assay to confirm long wavelength sensitivity in living jewel beetles, then tested if long wavelength sensitivity enhances visual contrast using the modelling approach described above. Our spectral dataset comprises a wide range of natural spectra relevant for diurnal insects and the hypothetical visual systems represent general tri- and tetrachromatic insect vision. Hence, our results are likely to have broader relevance to tri- and tetrachromatic diurnal insects.
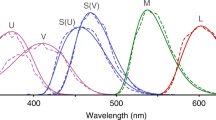
Spectral data used in visual modelling. (a) Reflectance spectra of representative beetle samples. Blue: Stigmodera gratiosa; yellow: Cyria imperialis; red: Castiarina erythroptera; violet: Temognatha obscuripennis; pink: Melobasis cuprifera; black: Merimna atrata; dark green: Melobasis propinqua; green: Pseudotaenia gigas. (b) Reflectance spectra of representative leaf and flower samples. Orange: Chorizema cordatum; purple: Olearia homolepis; pink: Chamelaucium uncinatum; yellow: Senna artemisioides; red: Eremophila maculata; white: Leptospermum liversidgei; green: Gastrolobium bilobus. Shaded regions in (a, b) show 95% confidence intervals of three replicates from the same species. (c) Sensitivity curves of photoreceptors with different peak wavelengths. (a–c) were created using R software version 3.6.3 (https://www.r-project.org) and modified using Inkscape version 1.0.2 (https://inkscape.org). Photographs by L.-Y.W.
Materials and methods
Specimen collection
To obtain a broad spectral dataset of colours relevant to insects, we used jewel beetles and their host plants. Living jewel beetles were collected on bushes by both foliage beating and by hand in Victoria, Australia between November 2018 to January 2019 and October 2019. Among them, 28 individuals were collected from Wombargo (− 36.926667, 148.211111) and used in the behavioural experiment (Permit no. 10009115). We pinned the beetles soon after they deceased and measured their reflectance properties. We also used specimens of Australian jewel beetles from the Australian National Insect Collection (ANIC) to increase the colour diversity in our spectral dataset. By including both locally caught jewel beetles and samples from the ANIC collection, we could measure a broad range of jewel beetle colours from jewel beetles found across Australia. In total, we measured reflectance spectra of 34 jewel beetle species from 2 subfamilies, 8 tribes, and 13 genera with relatively even representation of different colours including human-perceived red, pink, yellow, green, blue, purple, and black (Fig. 1a; Table S2). For plant reflectance, we collected flower and leaf samples from the known host plants of Australian jewel beetles38 from botanic gardens in Victoria. This allowed us to obtain a diverse sample of host plants found across Australia with a broad range of flower colours. In total we sampled 46 species from 22 genera with relatively even representation across different colours, including human-perceived red, pink, yellow, purple, white, and orange. (Fig. 1b; Table S2). No blue flowers were included because we could not find any host plants with blue flowers.
Behavioural experiments
To test the behavioural response of jewel beetles to long wavelengths, we conducted bi-choice behavioural experiments based on their positive phototaxis45. Full details of the experiment are presented in Supplementary Information 1. Briefly, we used a neutral-grey Y-maze as an arena with a light stimulus at the end of one arm and no stimulus at the end of the other. Beetles were tested for a response to narrow band LED lights with peak intensity at 590, 645, 680, 700, 720, and 735 nm (Marubeni, Japan; see Supplementary Information 1 for LED details). In total, 28 individuals from 2 species (16 Castiarina dimidiata and 12 C. flavopicta) completed the 6-wavelength trial series. We used the beetles’ responses (attracted/not attracted) to obtain a response curve over 590–735 nm by fitting a logistic regression for the 2 species, separately. In the model, we set the response of the beetles as the dependent variable, peak wavelength of the light source as a fixed factor, and individual ID as a random factor to account for repeated trials for each individual (see Supplementary Information 1 for results and discussion).
Reflectance measurements and spectral processing
We collected the reflectance spectra (300–800 nm) of the beetles and host plants to model visual contrasts. Specifically, we measured the reflectance of the beetles’ elytra and major colour patches on flowers as well as the leaves of the same species using an integrating sphere (see Supplementary Information 2 for measurement details). Because of the limitation of minimum measured area, we only measured the colour patches that were larger than a 4-mm diameter circle. For each colour patch, we measured 3 different samples and averaged the 3 measurements to represent the reflectance of the colour patch. Five beetles and one flower had two colours measured because each colour patch was large enough for measurement (Fig. S4; Table S2). In total, we measured 37, 47, and 46 colour patches from beetles, flowers, and leaves, respectively.
Hypothetical visual systems
To maximise the generality of the results across insect taxa, we created different visual systems based on the median sensitivities of insect photoreceptor types documented in a recently published review9. We first grouped the photoreceptors into four types based on their peak sensitivities—ultraviolet sensitive (UVS): ≤ 400 nm; short wavelength sensitive (SWS): 400–480 nm; medium wavelength sensitive (MWS): 480–560 nm; LWS: > 560 nm. Next, to avoid taxonomic bias, we calculated the median peaks of tri- and tetrachromats from each family, then calculated the median λmax from the family medians. The representative λmax of UVS, SWS, MWS, LWS were 355 nm, 445 nm, 530 nm, and 600 nm, respectively, and these values were used to derive spectral sensitivity functions (see below).
All spectral sensitivities were generated from peak wavelengths using an A1 chromophore template46, the most common chromophore in animals47. The longest known peak of A1-based visual pigments in animals is ca. 570 nm48,49,50,51. In most insects with peak sensitivities beyond 570 nm, the shift to longer sensitivity is achieved through red or orange pigments which act as filters11,44,52,53 (but see Papilio and Eumaeus that shift through opsin sequence modification21,22). Therefore, we generated LWS spectral sensitivity curves by applying cut-off filters to the 570 nm A1-based profile (Fig. S5; Supplementary Information 3). The cut-off filter was selected to achieve a similar spectral profile as the LWS photoreceptor of other insects11,44,52,53. Additionally, we generated another set of LWS spectral sensitivities with the same λmax values directly from the template. This equates to shifts in λmax due to changes in the sequence of the opsin protein and results in much broader LWS sensitivity curves (Supplementary Information 3). This enabled us to compare the effect of sensitivity shifts generated from different mechanisms on visual contrast, though the opsin shifts that we have modelled (λmax = 580 nm, 600 nm, 620 nm, 640 nm, 660 nm) are longer peak sensitivities than recorded for any insect opsins.
To test whether the presence of an LWS photoreceptor enhances visual contrast, we created three trichromatic visual systems that varied in their photoreceptor composition, based on common insect photoreceptor combinations [Fig. 1c; USM (UVS, SWS, MWS); UML (UVS, MWS, LWS); USL (UVS, SWS, LWS)] we identified from van der Kooi et al.9. We did not create a visual system without the UV photoreceptor because few, if any, tri- or tetrachromatic insects do not have a UV receptor9. Additionally, we modelled a tetrachromatic visual system USML (UVS, SWS, MWS, LWS) to compare with the three trichromatic visual systems because discrimination has been reported to increase by tetrachromacy, especially an additional LWS photoreceptor54.
Next, we tested if increasing the peak sensitivity of the LWS photoreceptor increased discriminability between targets. We created five tetrachromatic visual systems (VS), where we systematically filter-shifted the LWS photoreceptor 20 nm from 580 nm to the longest known peak (660 nm in a butterfly)—VS 580, VS 600, VS 620, VS 640, VS 660 (Fig. 1c). Shifting LWS peak sensitivity while keeping other photoreceptors constant changes the overlap of sensitivity curves. To evaluate the effect of overlap in sensitivities on results, we ran the model using an evenly spaced visual system (λmax of receptors: 355 nm, 455 nm, 560 nm, 660 nm) and compared the contrasts with that of VS 660 (Supplementary Information 4).
Visual modelling
For each of the above visual systems, we estimated visual contrast of pairs of reflectance spectra (comparison groups: ‘flower vs. leaf’, ‘beetle vs. leaf’, ‘beetle vs. flower’) using the receptor-noise limited model55,56 implemented in the R package ‘pavo’57. This model assumes that colour discrimination is limited only by photoreceptor noise and ignores effects of higher level processing. Colour contrasts are expressed in units of just-noticeable-differences (JND), where 1 JND is the theoretical threshold of colour discrimination for stimuli viewed simultaneously under ideal viewing conditions56 (see “Discussion” section regarding factors affecting actual discrimination thresholds).
We first calculated the quantum catch of each photoreceptor under standard daylight illumination (D65; Commission internationale de l’éclairage—CIE) using the ‘vismodel’ function with von Kries chromatic adaptation and average leaf reflectance as the adapting background. Next, we calculated the chromatic contrast between pairs of spectra using the function ‘coldist’ (with signal in each photoreceptor proportional to the natural logarithm of the quantum catch, in accordance with Fechner’s law). For the tetrachromat, we used a Weber fraction of 0.1256 to account for the receptor noise of the LWS photoreceptor. We used relative photoreceptor density of 1.14, 1, 1.26, and 1.38 for the four photoreceptors (UVS, SWS, MWS, and LWS, respectively) based on relative opsin gene expression reported in a jewel beetle, the Emerald ash borer (Agrilus planipennis)43. For the trichromatic visual systems, we ensured the same number of photoreceptors within a neurally integrative unit as the tetrachromatic visual system. Specifically, we proportionally redistributed photoreceptors from the missing receptor class between the remaining three classes while keeping the noise level of individual photoreceptors the same across visual systems, thereby improving their signal-to-noise ratios54 (Supplementary Information 5).
To test whether our results were sensitive to the photoreceptor ratios used, we repeated analyses using published ratios that represent the known variation in tetrachromatic insects, which happen to be two butterflies (Papilio xuthus—1.00: 1.00: 4.08: 2.9258; Heliconius sp. type III—1.00: 1.44: 2.22: 11.1159; details in Supplementary Information 6). To test if the contrast values change in different light environments, we ran the same vision models but using civil twilight for illumination (Supplementary Information 7). We chose twilight illumination because it has the greatest difference in spectral distribution from daylight and has proportionally more blue and red light compared with daylight illumination in the 300–800 nm range (300–500 nm, daylight: 37%, twilight: 42%; 650–800 nm, daylight: 38%, twilight: 41%). Moreover, it is likely close to the dimmest light level for colour discrimination of diurnal insects32. We took photon shot noise into account when using twilight illumination and converted the absolute irradiance of twilight into photon flux for twilight models.
Statistical analysis
To test how different photoreceptor combinations influence visual contrast between beetles, flowers and leaves, we compared chromatic contrast values among the four visual systems—USM, UML, USL and USML for each comparison group (‘flower vs. leaf’, ‘beetle vs. leaf’, ‘beetle vs. flower’). For each comparison group, we ran a generalised linear mixed model (GLMM; ‘lmer’ function in the R package ‘lme4’60) with JNDs for each pairwise combination of spectra as the dependent variable and visual system as the fixed factor. Since each spectrum was compared to multiple others (e.g. each beetle spectrum to each of the flower spectra in the ‘beetle vs. flower’ comparison group) we included two random effects in each model, one for each type of spectra being compared (e.g. beetle ID and flower ID). Subsequent post hoc Tukey honest significant differences (Tukey HSD) with Bonferroni correction were applied to test for significant differences between each pair of visual systems. We used the same approach to test the effect of visual systems with an increase in λmax from 580 to 680 nm (20 nm increments). All statistical analyses were performed in R 3.6.361.
Results
Visual sensitivity at long wavelengths in living beetles
Behavioural experiments confirmed red sensitivity in both species of jewel beetle and possibly far-red sensitivity in C. dimidiata. 96% of jewel beetles were attracted by light at 590 nm and ca. 87% of them still responded to light at 645 nm (C. dimidiata: 100% and 96%; C. flavopicta, 93% and 78%; at 590 nm and 645 nm) (Fig. S1). The 50% response rate was at ca. 704 nm for C. dimidiata and ca. 685 nm for C. flavopicta. There was only one C. dimidiata individual that responded to light at 735 nm, which might be random movement. Overall, compared to the dark arm, C. dimidiata and C. flavopicta beetles reached the ’choice zone’ of the lit arm 19 and 5 times more often, respectively. This indicated that the valid choices of the beetles were not random movement.
Varying photoreceptor combinations
We found contrast values were significantly different between visual systems for all comparison groups (Fig. 2, Table 1). The presence of both short and long wavelength sensitive receptors together improved the contrast of beetles against flowers and flowers against leaves. The USL and USML visual systems, which have both short and long wavelength sensitive receptors, had the same and significantly higher contrasts than the other two visual systems (USM and UML) for these two comparison groups (Tukey HSD, all p < 0.001; except UML-USML in ‘flower vs. leaf’, p < 0.01). USL and USML increased contrast values by 7% on average. There was no difference between USM and UML in comparisons of beetles against flowers and flowers against leaves (p = 0.09 and 1.00, respectively).
Comparison of chromatic contrast for visual systems with different photoreceptor combinations: USM (UVS, SWS, MWS), UML (UVS, MWS, LWS), USL (UVS, SWS, LWS), USML (UVS, SWS, MWS, LWS). Black dots show the means of the representative contrast values in each visual system. Each coloured dot represents the average contrast value of each flower pattern to all leaves (left panel), each beetle colour to all leaves (middle panel) or each flower colour to all beetle colours (right panel). Colours of the dots correspond to the human-visible colour of the flower (left and right panels) or beetle (middle panel) with the lines of the same colour connecting the results between different visual systems. This is for graphical representation only; statistical tests are based on all pairwise combinations of spectra and not averages. Letters on the top of each panel show the significant difference in contrast value between visual systems. Three contrast values > 10 JND are from flowers (2 white and 1 light pink) that have high UV—blue chroma compared to beetles and leaves. The Figure was created using R software version 3.6.3 (https://www.r-project.org) and modified using Inkscape version 1.0.2 (https://inkscape.org).
The presence of a long wavelength sensitive receptor improved the contrast of beetles against leaves. In this comparison (‘beetle vs. leaf’), there was no significant difference between UML, USL, and USML (all p = 1.00; Fig. 2) and USM had a significantly lower contrast value than the other three visual systems by around 29% (all p < 0.001). Additionally, we found no advantage to tetrachromacy over trichromacy where USL had the same contrast values as USML for all comparison groups (‘flower vs. leaf’, p = 0.12; ‘beetle vs. leaf’, p = 1.00; ‘beetle vs. flower’, p = 0.28; Fig. 2).
Models where changes in the spectral absorbance of the long wavelength sensitive receptor were due to modified opsin sequences rather than the optical influence of screening pigments showed qualitatively similar results for the comparison of beetles against leaves, with a long wavelength sensitive receptor improving contrast values (Fig. S12). However, only the presence of a short wavelength sensitive receptor (rather than both short and long wavelength sensitive receptors together) improved contrast values of beetles against flowers and flowers against leaves (full results in Supplementary Information 8).
The contrast values obtained from the four visual systems in the models using civil twilight showed different patterns and were substantially lower than the values obtained from the models using daylight (Fig. S9). In twilight illumination, USM performed significantly worse than the other visual systems and the tetrachromat performed significantly better than the trichromats but all contrast values were below 2 JND (Supplementary Information 7).
There were two white and one pink flower (Eremophila laanii, Actinotus helianthi, and Chamelaucium uncinatum) with exceptionally high contrast values when compared against beetles and leaves (Fig. 2). Such high contrast was the result of a steep rise in reflectance at ca. 400 nm in these three flowers, which was not present in beetles and leaves.
Shifts in the peak of long wavelength sensitivity
Contrast values increased as the peak sensitivity of the long wavelength sensitive photoreceptor increased from 580 nm to at least 640 nm for all comparison groups (Fig. 3; Table 1). In the ‘beetle vs. flower’ comparison, the contrast value steadily increased from VS 580 to VS 660 (stepwise increase in average contrast value by 6%, 7%, 5%, 4% as the long wavelength sensitive peak increased from 580 to 660 nm; p < 0.001 for all pairwise comparisons). Similarly, in the ‘beetle vs. leaf’ comparison, the contrast value increased from VS 580 to VS 660 but more steeply (stepwise increase in average contrast value by 13%, 13%, 9%, 3% as the long wavelength sensitive peak increased from 580 to 660 nm; p < 0.001 for all pairwise comparisons). The average contrast value of VS 640 was 39% higher than that of VS 580. For the ‘flower vs. leaf’ comparison, contrast values increased steeply from VS 580 to VS 640 (stepwise increase in average contrast value by 7%, 12%, 9%, as the long wavelength sensitive peak increased from 580 to 640 nm; p < 0.001 for all pairwise comparisons) but there was no significant difference between VS 640 and VS 660 (p = 1.00). Results were qualitatively the same for different photoreceptor ratios (Supplementary Information 6), indicating that different ratios did not influence the modelling results. When compared with the evenly spaced visual system, VS 660 (unevenly spaced) had significantly higher contrast values in all comparison groups (Supplementary Information 4). This indicates that even spacing of the photoreceptors does not improve contrast in our dataset and therefore the results are not biased by uneven spacing of spectral sensitivities in our hypothetical visual systems.
Comparison of chromatic contrast between visual systems (VS) with the long wavelength sensitive photoreceptor peaking at different wavelengths. Black dots show the means of the representative contrast values in each visual system. Each coloured dot represents the average contrast value of each flower pattern to all leaves (left panel), each beetle colour to all leaves (middle panel) or each flower colour to all beetle colours (right panel). Colours of the dots correspond to the human-visible colour of the flower (left and right panels) or beetle (middle panel) with the lines of the same colour connecting the results between different visual systems. This is for graphical representation only; statistical tests are based on all pairwise combinations of spectra and not averages. Letters on the top of each panel show the significant difference in contrast value between visual systems. Three contrast values > 10 JND are from flowers (2 white and 1 light pink) that have high UV—blue chroma compared to beetles and leaves. The Figure was created using R software version 3.6.3 (https://www.r-project.org) and modified using Inkscape version 1.0.2 (https://inkscape.org).
Similar to the results of filter-shifted models, for opsin-shifted models the contrast value increased from VS 580 to at least VS 640 but the differences in contrast value between visual systems were smaller (Fig. S13; full results in Supplementary Information 8).
The contrast values in the corresponding comparisons were qualitatively similar in twilight models, but many contrast values were substantially lower and all below 2 JND (Fig. S10; Supplementary Information 7).
Discussion
Our results highlight the potential benefit of red to far red sensitivity in resource targeting for jewel beetles and insects more generally. Taken together with intracellular recordings42, opsin expression43, and electroretinogram data44, our behavioural experiments suggest that jewel beetles have visual sensitivity to red wavelengths, possibly extending into the far-red (700–750 nm) in C. dimidiata. Vision models using hypothetical insect visual systems indicate that having both short and long wavelength sensitive receptors together enhances the chromatic contrast of beetles with flowers and flowers with leaves, and a long wavelength sensitive (LWS) receptor enhances the chromatic contrast of beetles against leaves. The diversity of spectral profiles among our beetle and flower samples, and the robustness of the results to specific model parameter values (e.g. receptor ratios) suggest that red sensitivity may be important in mate location, mate recognition and/or foraging not only for jewel beetles but also other diurnal insects with an LWS receptor. Additionally, the optimal peak sensitivity of the LWS photoreceptor depends on what is being viewed. When comparing beetle colours with both leaves and flowers, the mean contrast values increase with increasing λmax of the LWS receptor up to 660 nm; whereas when comparing flowers against leaves, the mean contrast values stop increasing beyond 640 nm. Physiological constraints, such as a reduction in absolute sensitivity (filter-shifted) or an increase in thermal noise (opsin-shifted), may limit the upper peak sensitivity of the long wavelength receptor. Our modelling results suggest that a limited increase in visual contrast may also contribute to an upper limit in peak sensitivity for diurnal insects.
Spectral characteristics of vegetation likely explain the visual contrast benefits of an LWS and SWS receptor for flower discrimination and an LWS receptor for leaf discrimination. In chromatic vision, each class of photoreceptor detects the relative difference in reflectance spectra of two objects across the photoreceptor’s absorption span. The reflectance of flowers varies greatly; but due to the characteristics of floral pigments, many have sigmoidal spectra with a sharp rise at shorter wavelengths and plateau at longer wavelengths (Fig. 1b;62). By comparison, due to the absorbance profile of chlorophyll, leaves have a characteristic reflectance peak between 500 and 600 nm and a sharp rise in reflectance at ca. 690 nm (the ‘red edge’63). The combination of a short and long wavelength receptor picks up differences between sigmoidal flower spectra and either leaves or diverse beetle spectra while an LWS receptor spans wavelengths where leaves differ most strongly from many other natural objects. UV signals are known to play a critical role in flower detection and discrimination in insects, particularly for pollinators64. Here, we did not test the contribution of the UV sensitive photoreceptor because almost all trichromatic and tetrachromatic insect visual systems have a UV photoreceptor9,10. Therefore, our results do not indicate that the UV receptor is unimportant, but suggest that short and long wavelength sensitive receptors together provide additional benefits for discriminating flowers from leaves and insect colours from vegetation.
Shifting the peak of the long wavelength sensitive receptor improved contrast of beetles against leaves and flowers up to 660 nm, whereas contrast of flowers against leaves stopped increasing beyond 640 nm. Almost all variation in beetle spectra occurs beyond 500 nm and often beyond 600 nm. Increasing λmax of the long wavelength sensitive receptor captures the variation in long wavelengths between beetle and leaves/flowers spectra more effectively. By comparison, the 640 nm long wavelength sensitive receptor provides the greatest contrast between flowers and leaves because it minimizes the relative stimulation by leaf reflectance (not capturing the reflectance at green wavelengths or the increase at ca. 690 nm) but maximises the relative stimulation by flower reflectance (capturing both high flower reflectance and low leaf reflectance before 700 nm). This conclusion is based on the average results in each comparison group, though may differ for specific pairs of spectra.
We employed a receptor noise model of colour discrimination to provide an indication of the effect of variation in visual sensitivities. In reality, however, discrimination is influenced by post-receptor processing, can differ between closely related species and even within species and can be context dependent65,66. Therefore, the actual detection threshold requires empirical validation and is likely higher than the theoretical threshold of 1 JND, even under ideal viewing conditions e.g. a threshold of 2.3 for honeybees67. Recent evidence also suggests that the relationship between JND value and conspicuousness is non-linear such that high JND values (> 10 JND) may be perceived as equally conspicuous66,68. Mean contrast values in daylight generally ranged between 5 and 10 JND suggesting that the differences in contrast values among hypothetical visual systems provide a meaningful indication of differences in discriminability. By contrast, under twilight illumination, mean contrast values were generally < 1 JND and all were < 2 JND, indicating that most colours would be indistinguishable in these conditions.
Vision models with hypothetical opsin-shifted long wavelength sensitive photoreceptors indicate similar patterns but smaller contrast differences between visual systems than the filter-shifted equivalent. Moreover, shifting the peaks of the long wavelength sensitive photoreceptor to longer wavelengths produced a greater increase in contrast value for the filter-shifted models than for the opsin-shifted models. For example, the filter-shifted VS 640 has on average 22% higher contrast value than opsin-shifted VS 640. The lower contrast value for opsin-shifted visual systems is likely due to their much broader sensitivity and greater overlap between photoreceptors. Greater overlap between photoreceptors can decrease colour discrimination69,70. Most known insect LWS receptors with a peak beyond 600 nm are due to filtering by coloured pigments. These pigments act as long pass filters and shift photoreceptor sensitivity to longer wavelengths with a narrower span than the underlying opsin absorption spectrum, which also has a beta peak. For example, the perirhabdomal pigments of Colias erate butterflies shift the peak sensitivity from around 570 nm to 610–660 nm depending on the pigment type and butterfly sex11. The same shifting mechanism has been reported in several other butterflies and is likely the mechanism causing red-shift in a jewel beetle, a bumble bee, and a scarab beetle14,44,48,52.
The two mechanisms to shift photoreceptor sensitivity (filter-shift and opsin-shift) may entail different trade-offs. The optimal peak sensitivity of LWS receptors may be limited by dark noise (spontaneous fluctuations in the electrical signals of photoreceptors) because spontaneous pigment activation increases with increasing λmax, particularly beyond 620 nm25,26,29,30. Dark noise will be greater for opsin-shifted photoreceptors due to their broader absorption span that extends to longer wavelengths. By contrast, the use of filters to shift peak sensitivity reduces absolute sensitivity, thereby reducing the signal to noise ratio70. Due to our limited knowledge of the effect of reduced sensitivity on perceived chromatic contrast, we did not consider the reduction in absolute sensitivity from filters in the colour discrimination models. Nevertheless, the cost of noise for both opsin and filtering pigment mechanisms is likely one of the important factors limiting far-red sensitivity in insects.
Another potential fundamental constraint on increasing peak sensitivity of the visual pigment is associated with chromophore types. The chromophore is a carotenoid derivative of vitamin A and one of the two components of a visual pigment. Different chromophore types have varied peak sensitivities and their own unique spectral absorption functions. Each of them can bind to different opsin proteins, leading to diverse spectral tuning. Use of an A2 chromophore has been implicated in shifting sensitivity to longer wavelengths in fish and lizards23,24. For example, in the Nile tilapia, using a mix of A1 and A2 chromophores leads to longer λmax of long wavelength sensitive photoreceptors than using A1 alone71. However, only A1 and A3 but not A2 chromophore types are used by insects10,72, and the absorbance spectra of A1- and A3-visual pigments are similar73. Limitations associated with the biomolecular nature of chromophores could restrict the increase in λmax of A1-/A3-visual pigment. Therefore, filter-shifts may be the only way for insects to achieve longer peak sensitivity.
Red sensitivity has been described in numerous insect groups, yet its ecological functions remain unclear. Many insects have been shown to have colour vision that they use in essential visual tasks, such as foraging74,75, sexual selection50,76,77, and oviposition12. Insects with long wavelength sensitive photoreceptors are mainly lepidopterans (butterflies and moths) and coleopterans (beetles), though only a minority have long wavelength sensitive receptors9,10. We identified that around 70% of insect species with a long wavelength photoreceptor in Briscoe and Chittka10 have prominent colour patterns, and it is possible that long wavelength sensitivity may play a role in detection of conspecifics for these species too. Benefits of having a long wavelength sensitive receptor may extend to other visual behaviours, especially tasks requiring discrimination against prevailing foliage background such as finding prey, host plants, young leaves, and shelter or oviposition sites. For example, female Lycaena butterflies use long wavelength sensitivity to locate their host plants for laying eggs76. Our results suggest visual advantages of having red sensitivity in visually guided behaviours such as foraging and mate search or recognition. However, there is limited enhancement in perceived contrast beyond the peak of 640 nm for flower-leaf discrimination, indicating that an upper limit of peak sensitivity in terms of visual benefit may apply in some contexts. This supports the view that the optimal long wavelength sensitivity may be related to the essential visual tasks of an animal.
Data availability
The dataset and R code are available from Dryad Digital Repository (https://doi.org/10.5061/dryad.w6m905qnz). An HTML report showing all the code used for visual modelling and visualisation of results can be viewed online at https://luyiwangtw.github.io/LongWavelengthSensitivity/.
References
Cummings, M. E., Rosenthal, G. G. & Ryan, M. J. A private ultraviolet channel in visual communication. Proc. R. Soc. B-Biol. Sci. 270, 897–904. https://doi.org/10.1098/rspb.2003.2334 (2003).
Tedore, C. & Nilsson, D. E. Avian UV vision enhances leaf surface contrasts in forest environments. Nat. Commun. https://doi.org/10.1038/s41467-018-08142-5 (2019).
Qi, Y. D., Bai, S. J. & Heisler, G. M. Changes in ultraviolet-B and visible optical properties and absorbing pigment concentrations in pecan leaves during a growing season. Agric. For. Meteorol. 120, 229–240. https://doi.org/10.1016/j.agrformet.2003.08.018 (2003).
Mollon, J. D. “Tho’ she kneel’d in that place where they grew…” The uses and origins of primate colour vision. J. Exp. Biol. 146, 21–38. https://doi.org/10.1242/jeb.146.1.21 (1989).
Osorio, D. & Vorobyev, M. Photoreceptor sectral sensitivities in terrestrial animals: Adaptations for luminance and colour vision. Proc. R. Soc. B Biol. Sci. 272, 1745–1752. https://doi.org/10.1098/rspb.2005.3156 (2005).
Osorio, D. & Vorobyev, M. A review of the evolution of animal colour vision and visual communication signals. Vis. Res. 48, 2042–2051. https://doi.org/10.1016/j.visres.2008.06.018 (2008).
Bowmaker, J. K. & Dartnall, H. J. A. Visual pigments of rods and cones in a human retina. J. Physiol. Lond. 298, 501–511. https://doi.org/10.1113/jphysiol.1980.sp013097 (1980).
Bowmaker, J. K. & Hunt, D. M. Evolution of vertebrate visual pigments. Curr. Biol. 16, R484–R489. https://doi.org/10.1016/j.cub.2006.06.016 (2006).
van der Kooi, C. J., Stavenga, D. G., Arikawa, K., Belušič, G. & Kelber, A. Evolution of insect color vision: From spectral sensitivity to visual ecology. Annu. Rev. Entomol. 66, 435–461 (2021).
Briscoe, A. D. & Chittka, L. The evolution of color vision in insects. Annu. Rev. Entomol. 46, 471–510. https://doi.org/10.1146/annurev.ento.46.1.471 (2001).
Ogawa, Y., Kinoshita, M., Stavenga, D. G. & Arikawa, K. Sex-specific retinal pigmentation results in sexually dimorphic long-wavelength-sensitive photoreceptors in the eastern pale clouded yellow butterfly, Colias erate. J. Exp. Biol. 216, 1916–1923. https://doi.org/10.1242/jeb.083485 (2013).
Kelber, A. Ovipositing butterflies use a red receptor to see green. J. Exp. Biol. 202, 2619–2630 (1999).
Osorio, D. & Vorobyev, M. Colour vision as an adaptation to frugivory in primates. Proc. R. Soc. B Biol. Sci. 263, 593–599. https://doi.org/10.1098/rspb.1996.0089 (1996).
Zaccardi, G., Kelber, A., Sison-Mangus, M. P. & Briscoe, A. D. Color discrimination in the red range with only one long-wavelength sensitive opsin. J. Exp. Biol. 209, 1944–1955. https://doi.org/10.1242/jeb.02207 (2006).
Wakakuwa, M., Stavenga, D. G., Kurasawa, M. & Arikawa, K. A unique visual pigment expressed in green, red and deep-red receptors in the eye of the small white butterfly, Pieris rapae crucivora. J. Exp. Biol. 207, 2803–2810. https://doi.org/10.1242/jeb.01078 (2004).
Satoh, A. et al. Red-shift of spectral sensitivity due to screening pigment migration in the eyes of a moth, Adoxophyes orana. Zool. Lett. https://doi.org/10.1186/s40851-017-0075-6 (2017).
Pirih, P. et al. The giant butterfly-moth Paysandisia archon has spectrally rich apposition eyes with unique light-dependent photoreceptor dynamics. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 204, 639–651. https://doi.org/10.1007/s00359-018-1267-z (2018).
Cronin, T. W., Jarvilehto, M., Weckstrom, M. & Lall, A. B. Tuning of photoreceptor spectral sensitivity in fireflies (Coleoptera: Lampyridae). J. Comp. Physiol. A Sens. Neural Behav. Physiol. 186, 1–12. https://doi.org/10.1007/s003590050001 (2000).
Lall, A. B. et al. Vision in click beetles (Coleoptera: Elateridae): pigments and spectral correspondence between visual sensitivity and species bioluminescence emission. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 196, 629–638. https://doi.org/10.1007/s00359-010-0549-x (2010).
Frentiu, F. D. et al. Adaptive evolution of color vision as seen through the eyes of butterflies. Proc. Natl. Acad. Sci. U.S.A. 104, 8634–8640. https://doi.org/10.1073/pnas.0701447104 (2007).
Liénard, M. A. et al. The evolution of red color vision is linked to coordinated rhodopsin tuning in lycaenid butterflies. Proc. Natl. Acad. Sci. U. S. A. https://doi.org/10.1073/pnas.2008986118 (2021).
Saito, T. et al. Spectral tuning mediated by helix III in butterfly long wavelength-sensitive visual opsins revealed by heterologous action spectroscopy. Zool. Lett. https://doi.org/10.1186/s40851-019-0150-2 (2019).
Enright, J. M. et al. Cyp27c1 red-shifts the spectral sensitivity of photoreceptors by converting vitamin A1 into A2. Curr. Biol. 25, 3048–3057. https://doi.org/10.1016/j.cub.2015.10.018 (2015).
Martin, M., Le Galliard, J. F., Meylan, S. & Loew, E. R. The importance of ultraviolet and near-infrared sensitivity for visual discrimination in two species of lacertid lizards. J. Exp. Biol. 218, 458–465. https://doi.org/10.1242/jeb.115923 (2015).
Ala-Laurila, P., Donner, K. & Koskelainen, A. Thermal activation and photoactivation of visual pigments. Biophys. J. 86, 3653–3662. https://doi.org/10.1529/biophysj.103.035626 (2004).
Ala-Laurila, P., Pahlberg, J., Koskelainen, A. & Donner, K. On the relation between the photoactivation energy and the absorbance spectrum of visual pigments. Vis. Res. 44, 2153–2158. https://doi.org/10.1016/j.visres.2004.03.031 (2004).
Barlow, H. B. Purkinje shift and retinal noise. Nature 179, 255–256. https://doi.org/10.1038/179255b0 (1957).
Koskelainen, A., Ala-Laurila, P., Fyhrquist, N. & Donner, K. Measurement of thermal contribution to photoreceptor sensitivity. Nature 403, 220–223. https://doi.org/10.1038/35003242 (2000).
Luo, D. G., Yue, W. W. S., Ala-Laurila, P. & Yau, K. W. Activation of visual pigments by light and heat. Science 332, 1307–1312. https://doi.org/10.1126/science.1200172 (2011).
Rieke, F. & Baylor, D. A. Origin and functional impact of dark noise in retinal cones. Neuron 26, 181–186. https://doi.org/10.1016/s0896-6273(00)81148-4 (2000).
Cronin, T. W., Johnsen, S., Marshall, N. J. & Warrant, E. J. Visual pigments and photoreceptors. In Visual Ecology, pp. 37–65: Princeton University Press.
Kelber, A., Yovanovich, C. & Olsson, P. Thresholds and noise limitations of colour vision in dim light. Philos. Trans. R. Soc. B-Biol. Sci. https://doi.org/10.1098/rstb.2016.0065 (2017).
Kemp, D. J. et al. An integrative framework for the appraisal of coloration in nature. Am. Nat. 185, 705–724. https://doi.org/10.1086/681021 (2015).
Hawkeswood, T. Observations on some Buprestidae (Coleoptera) from the Blue Mountains, N.S.W.. Aust. Zool. 19, 257–275 (1978).
Hawkeswood, T. Observations on two sympatric species of Buprestidae (Coleoptera) from sand dunes on the north coast of New South Wales. Victorian Naturalist 98, 146–151 (1981).
Hawkeswood, T. Observations on some jewel beetles (Coleoptera Buprestidae) from the Armidale district, North-eastern New South Wales. Vic. Nat. 98, 152–155 (1981).
Poland, T. M., Chen, Y. G., Koch, J. & Pureswaran, D. Review of the emerald ash borer (Coleoptera: Buprestidae), life history, mating behaviours, host plant selection, and host resistance. Can. Entomol. 147, 252–262. https://doi.org/10.4039/tce.2015.4 (2015).
Bellamy, C. L., Williams, G., Hasenpusch, J. & Sundholm, A. A summary of the published data on host plants and morphology of immature stages of Australian jewel beetles (Coleoptera: Buprestidae), with additional new records. Insecta Mundi, 1–172 (2013).
Domingue, M. J. et al. Field observations of visual attraction of three European oak buprestid beetles toward conspecific and heterospecific models. Entomol. Exp. Appl. 140, 112–121. https://doi.org/10.1111/j.1570-7458.2011.01139.x (2011).
Domingue, M. J. et al. Differences in spectral selectivity between stages of visually guided mating approaches in a buprestid beetle. J. Exp. Biol. 219, 2837–2843 (2016).
Pureswaran, D. S. & Poland, T. M. Effects of visual silhouette, leaf size and host species on feeding preference by adult emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae). Great Lakes Entomol. 42, 4 (2018).
Crook, D. J. et al. Laboratory and field response of the emerald ash borer (Coleoptera: Buprestidae), to selected regions of the electromagnetic spectrum. J. Econ. Entomol. 102, 2160–2169 (2009).
Lord, N. P. et al. A cure for the blues: Opsin duplication and subfunctionalization for short-wavelength sensitivity in jewel beetles (Coleoptera: Buprestidae). BMC Evol. Biol. 16, 107 (2016).
Meglič, A., Ilić, M., Quero, C., Arikawa, K. & Belušič, G. Two chiral types of randomly rotated ommatidia are distributed across the retina of the flathead oak borer Coraebus undatus (Coleoptera: Buprestidae). J. Exp. Biol. 223, jeb225920. https://doi.org/10.1242/jeb.225920 (2020).
Chen, Y. G. & Poland, T. M. Biotic and abiotic factors affect green ash volatile production and emerald Ash borer adult feeding preference. Environ. Entomol. 38, 1756–1764. https://doi.org/10.1603/022.038.0629 (2009).
Govardovskii, V. I., Fyhrquist, N., Reuter, T., Kuzmin, D. G. & Donner, K. In search of the visual pigment template. Vis. Neurosci. 17, 509–528. https://doi.org/10.1017/s0952523800174036 (2000).
Dartnall, H. J. A. Visual pigment. Trans. Zool. Soc. Lond. 33, 147–152. https://doi.org/10.1111/j.1096-3642.1976.tb00047.x (1976).
Arikawa, K., Scholten, D. G. W., Kinoshita, M. & Stavenga, D. G. Tuning of photoreceptor spectral sensitivities by red and yellow pigments in the butterfly Papilio xuthus. Zool. Sci. 16, 17–24. https://doi.org/10.2108/zsj.16.17 (1999).
Das, D., Wilkie, S. E., Hunt, D. M. & Bowmaker, J. K. Visual pigments and oil droplets in the retina of a passerine bird, the canary Serinus canaria: microspectrophotometry and opsin sequences. Vision. Res. 39, 2801–2815. https://doi.org/10.1016/s0042-6989(99)00023-1 (1999).
Sison-Mangus, M. P., Bernard, G. D., Lampel, J. & Briscoe, A. D. Beauty in the eye of the beholder: The two blue opsins of lycaenid butterflies and the opsin gene-driven evolution of sexually dimorphic eyes. J. Exp. Biol. 209, 3079–3090. https://doi.org/10.1242/jeb.02360 (2006).
Bernard, G. D. Red-absorbing visual pigment of butterflies. Science 203, 1125. https://doi.org/10.1126/science.203.4385.1125 (1979).
Martínez-Harms, J. et al. Evidence of red sensitive photoreceptors in Pygopleurus israelitus (Glaphyridae: Coleoptera) and its implications for beetle pollination in the southeast Mediterranean. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 198, 451–463. https://doi.org/10.1007/s00359-012-0722-5 (2012).
Stavenga, D. G. & Arikawa, K. Photoreceptor spectral sensitivities of the Small White butterfly Pieris rapae crucivora interpreted with optical modeling. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 197, 373–385. https://doi.org/10.1007/s00359-010-0622-5 (2011).
Vorobyev, M., Osorio, D., Bennett, A. T. D., Marshall, N. J. & Cuthill, I. C. Tetrachromacy, oil droplets and bird plumage colours. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 183, 621–633. https://doi.org/10.1007/s003590050286 (1998).
Vorobyev, M. & Osorio, D. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. Ser. B Biol. Sci. 265, 351–358. https://doi.org/10.1098/rspb.1998.0302 (1998).
Vorobyev, M., Brandt, R., Peitsch, D., Laughlin, S. B. & Menzel, R. Colour thresholds and receptor noise: Behaviour and physiology compared. Vis. Res. 41, 639–653. https://doi.org/10.1016/s0042-6989(00)00288-1 (2001).
Maia, R., Gruson, H., Endler, J. A. & White, T. E. pavo 2: new tools for the spectral and spatial analysis of colour in R. Methods in Ecology and Evolution 10, 1097–1107 (2019).
Matsushita, A., Awata, H., Wakakuwa, M., Takemura, S. Y. & Arikawa, K. Rhabdom evolution in butterflies: insights from the uniquely tiered and heterogeneous ommatidia of the Glacial Apollo butterfly, Parnassius glacialis. Proc. R. Soc. B Biol. Sci. 279, 3482–3490. https://doi.org/10.1098/rspb.2012.0475 (2012).
McCulloch, K. J. et al. Sexual dimorphism and retinal mosaic diversification following the evolution of a violet receptor in butterflies. Mol. Biol. Evol. 34, 2271–2284. https://doi.org/10.1093/molbev/msx163 (2017).
Bates, D., Mächler, M., Bolker, B. M. & Walker, S. C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. https://doi.org/10.18637/jss.v067.i01 (2015).
R: A Language and environment for statistical computing (R Foundation for Statistical Computing, 2018).
van der Kooi, C. J., Elzenga, J. T. M., Staal, M. & Stavenga, D. G. How to colour a flower: On the optical principles of flower coloration. Proc. R. Soc. B Biol. Sci. 283, 20160429. https://doi.org/10.1098/rspb.2016.0429 (2016).
Horler, D. N. H., Dockray, M. & Barber, J. The red edge of plant leaf reflectance. Int. J. Remote Sens. 4, 273–288. https://doi.org/10.1080/01431168308948546 (1983).
Silberglied, R. E. Communication in the Ultraviolet. Annu. Rev. Ecol. Syst. 10, 373–398. https://doi.org/10.1146/annurev.es.10.110179.002105 (1979).
Lind, O. Colour vision and background adaptation in a passerine bird, the zebra finch (Taeniopygia guttata). R. Soc. Open Sci. 3, 160383. https://doi.org/10.1098/rsos.160383 (2016).
Santiago, C. et al. Does conspicuousness scale linearly with colour distance? A test using reef fish. Proc. R. Soc. B Biol. Sci. 287, 20201456. https://doi.org/10.1098/rspb.2020.1456 (2020).
Giurfa, M., Vorobyev, M., Brandt, R., Posner, B. & Menzel, R. Discrimination of coloured stimuli by honeybees: Alternative use of achromatic and chromatic signals. J. Comp. Physiol. A. 180, 235–243. https://doi.org/10.1007/s003590050044 (1997).
Garcia, J. E., Spaethe, J. & Dyer, A. G. The path to colour discrimination is S-shaped: Behaviour determines the interpretation of colour models. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 203, 983–997. https://doi.org/10.1007/s00359-017-1208-2 (2017).
Hart, N. S., Bailes, H. J., Vorobyev, M., Marshall, N. J. & Collin, S. P. Visual ecology of the Australian lungfish (Neoceratodus forsteri). BMC Ecol. 8, 21. https://doi.org/10.1186/1472-6785-8-21 (2008).
Vorobyev, M. Coloured oil droplets enhance colour discrimination. Proc. R. Soc. B Biol. Sci. 270, 1255–1261. https://doi.org/10.1098/rspb.2003.2381 (2003).
Carleton, K. L. et al. Visual sensitivities tuned by heterochronic shifts in opsin gene expression. Bmc Biol. https://doi.org/10.1186/1741-7007-6-22 (2008).
Seki, T. & Vogt, K. Evolutionary aspects of the diversity of visual pigment chromophores in the class Insecta. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 119, 53–64. https://doi.org/10.1016/s0305-0491(97)00322-2 (1998).
Stavenga, D. G., Smits, R. P. & Hoenders, B. J. Simple exponential functions describing the absorbance bands of visual pigment spectra. Vis. Res. 33, 1011–1017. https://doi.org/10.1016/0042-6989(93)90237-q (1993).
Kinoshita, M. & Arikawa, K. Color and polarization vision in foraging Papilio. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 200, 513–526. https://doi.org/10.1007/s00359-014-0903-5 (2014).
Vorobyev, M. & Menzel, R. Flower advertisement for insects: Bees, a case study. In Adaptive Mechanisms in the Ecology of Vision (eds S. N. Archer et al.) 537–553 (Springer Netherlands, 1999).
Bernard, G. D. & Remington, C. L. Color vision in Lycaena butterflies: Spectral tuning of receptor arrays in relation to behavioral ecology. Proc. Natl. Acad. Sci. U.S.A. 88, 2783–2787. https://doi.org/10.1073/pnas.88.7.2783 (1991).
McCulloch, K. J., Osorio, D. & Briscoe, A. D. Sexual dimorphism in the compound eye of Heliconius erato: A nymphalid butterfly with at least five spectral classes of photoreceptor. J. Exp. Biol. 219, 2377–2387. https://doi.org/10.1242/jeb.136523 (2016).
Acknowledgements
We thank Katrina Rankin for fieldwork assistance; Laura Ospina-Rozo for technical assistance with spectral measurement and the design of the Y-maze setup; Casper J. van der Kooi and anonymous reviewers for the helpful comments on previous versions of the manuscript. We also thank Maranoa and Melton Botanic Gardens for the approval of plant collection.
Funding
This study was supported by Australian Research Council (DP190102203 to DS-F and NWR and FT180100216 to DS-F).
Author information
Authors and Affiliations
Contributions
D.S.-F., L.-Y.W., and A.M.F. conceived and designed the study and analytical approaches. L.-Y.W., G.W., A.M.F. conducted field sampling. L.-Y.W. collected and analysed the data and drafted the manuscript. L.-Y.W., N.W.R., G.W., D.S.-F. and A.M.F. contributed to interpretation, edited the manuscript and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, LY., Stuart-Fox, D., Walker, G. et al. Insect visual sensitivity to long wavelengths enhances colour contrast of insects against vegetation. Sci Rep 12, 982 (2022). https://doi.org/10.1038/s41598-021-04702-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-04702-w
- Springer Nature Limited