Abstract
Due to the increasing pollution of tetrabromobisphenol A (TBBPA) in paddy soils, it is of great importance to explore the degradation of TBBPA under repeated anoxic-oxic conditions. In the present study, the degradation of TBBPA (kinetics, metabolites and potential pathways) and the influence of low molecular weight organic acid i.e., lactic acid were investigated in a paddy soil during sequential anoxic-oxic incubations. Under the anoxic condition, TBBPA in the non-sterile soils was efficiently debrominated into three intermediates (including tri-BBPA, di-BBPA and mono-BBPA) and bisphenol A (BPA) with a rate constant (k) of 0.0371 d−1 and a half-life (t1/2) of 60.8 d. The debromination end product (BPA) steadily accumulated. Next, turning to the oxic conditions, the anaerobically accumulated BPA degraded rapidly, while the intermediates and TBBPA were desorbed from the bound residues and were persistent. The detection of tri-BBPA followed by di-BBPA and mono-BBPA thereafter indicated that the dehalogenation of TBBPA was likely a stepwise removal of bromine atoms. A pathway of TBBPA → tri-BBPA → di-BBPA → mono-BBPA → BPA was thus proposed for TBBPA degradation. The degradation of TBBPA and its metabolites was biologically mediated. Moreover, the biodegradation of TBBPA could be significantly accelerated by the addition of lactic acid as an exogenous carbon source and electron donor, with k being increased to 0.0766 d−1 and t1/2 being shortened to 31.9 d. The information will improve our understanding of biotic process associated with agronomic practices (such as applying organic fertilizers) contributing to TBBPA attenuation in the natural soil environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Tetrabromobisphenol A [4,4′-isopropylidenebis(2,6-dibromophenol); TBBPA] is one of the most commonly used brominated flame retardants in the world. Given the frequent detection, high persistence and potential toxicity of TBBPA1,2, it is of great interest to investigate the dissipation of TBBPA in the environment.
According to previous studies, the reductive dehalogenation is a critical step in the degradation of multi-halogenated compounds3,4,5. The dehalogenated products might be more efficiently degraded by the microorganisms by comparison with their multi-halogenated parent compounds6. Because of the significantly faster degradation of the dehalogenated products under oxic conditions compared to anoxic conditions7, a multistage process, including both anoxic and oxic stages, may be the best solution for the detoxification of TBBPA in the environment.
Due to the alternation of rainy-dry seasons and various agronomic practices (including flooding and draining, plowing and fallow in winter), the paddy soils are subjected to repeated redox cycles between anoxic and oxic states8,9,10. In view of the reality of increasing TBBPA contamination and repeated redox cycles in the paddy fields, it is of great importance to investigate the biodegradation of TBBPA in soils during the sequential anoxic-oxic process and to develop abatement measures. The biodegradation of halogenated pollutants in soils may be affected by various factors such as the pH, salinity, temperature, availability of organic carbon, mineral composition and microbial community11,12,13. In soils, particularly in iron-rich red paddy soils, ferrous iron plays an important role in the reductive transformation of chlorinated compounds14,15. With the participation of soil microorganisms, chlorinated compounds may undergo faster transformation due to both of the reductive ability of the microorganisms and the biogenic Fe(II) formed by the microorganisms16. For example, iron-reducing bacteria in soils can reduce iron minerals into biogenic Fe(II) and thereby enhance the dechlorination rate of DDX under anoxic conditions17,18. Additionally, applying organic fertilizers containing abundant low molecular weight organic acids usually increases the content of soil organic carbon19. Utilized as the carbon and energy source for the microorganisms20, the soil organic carbon may have a significant effect on the activities of microbes and consequently affect the biodegradation of halogenated pollutants. Low-molecular-weight organic acids added as exogenous carbon sources have been shown to stimulate the anoxic dechlorination of pentachlorophenol and DDT in soils4,18,21. However, the influence of low molecular weight organic acids (e.g., lactic acid) introduced by agronomic practices, such as fertilization, on the degradation of TBBPA in soils is still unclear and needs to be investigated further.
In the present study, the sequential anoxic-oxic incubations of TBBPA were performed in a paddy soil from the Longtang area of South China, a main e-waste recycling area which was extensively polluted by TBBPA and bisphenol A (BPA), supplemented by lactic acid as an exogenous carbon source. The aims are (i) to elucidate the degradation kinetics, metabolites and potential pathways of TBBPA in the paddy soil and (ii) to illustrate the influence of lactic acid on the degradation of TBBPA. The information will improve our understanding of biotic process associated with agronomic practices (such as applying organic fertilizers) contributing to TBBPA attenuation in the natural soil environment.
Results
Degradation of TBBPA and formation of its metabolites in a paddy soil
In the sterile soils, the concentration of TBBPA was found to be reduced by 22.4% (from 20.1 ± 0.14 to 15.6 ± 0.05 mg·L−1), while no metabolites were detectable in the first 10 days of the anoxic incubations. After 10 d, the concentration of TBBPA rose back to 16.2 ± 0.07 mg·L−1 and later varied insignificantly from 15.4 ± 0.08 to 16.2 ± 0.07 mg·L−1. However, the lower brominated metabolites and BPA were still undetectable throughout the 150 d incubations (Fig. 1a).
Degradation of TBBPA and formation of metabolites in non-sterile and sterile paddy soil during the incubations of TBBPA under the static anoxic conditions (210 d for non-sterile soil; 150 d for sterile soil) or sequential anoxic-oxic conditions (0–150 d: anoxic, hollow symbols; 151–210 d: oxic, solid symbols). (a) The degradation of TBBPA and formation of BPA in non-sterile and sterile soils, respectively. (b) The formation of intermediates (mono-, di-, and tri-BBPA) in the non-sterile soil. No metabolites were detectable in the sterile soil. Data based on three replicates were presented as mean ± standard deviation (SD).
In the non-sterile soils, the concentration of TBBPA also dropped by 22.8% (from 20.3 ± 0.41 to 15.7 ± 0.17 mg·L−1) during the first 10 days of the anoxic incubations (Fig. 1a). After 10 d, unlike the sterile treatment, the concentration of TBBPA in the non-sterile soils declined continuously, decreased sharply after 50 d and was reduced by 94.0% at 130 d. Correspondingly, the concentration of BPA was 0.06 ± 0.01 mg·L−1 at 20 d, increased significantly after 50 d, and maximized at 14.2 ± 0.38 mg·L−1 (70.1% of the initially applied TBBPA concentrations) at 130 d. The intermediates were temporarily detectable at 1.07–7.06%, 1.02–8.21% and 1.25–5.78% of the initially applied TBBPA during 20–110 d for the tri-BBPA, di-BBPA, and mono-BBPA, respectively (Fig. 1b). After 130 d, the concentrations of TBBPA and BPA remained stable at 1.22 ± 0.02 mg·L−1 and 13.9 ± 0.06 mg·L−1, respectively, and the three intermediates were undetectable, leaving BPA as the only measurable degradation product (Fig. 1). The degradation of TBBPA and production of BPA were almost complete at 130 d.
As depicted in Fig. 1b, the tri-BBPA, di-BBPA, and mono-BBPA were first detectable at 20 d, 40 d and 50 d, respectively, simultaneously increased to maxima at 90 d, and decreased significantly and were undetectable after 130 d. During the first 10–50 d, the concentrations of the intermediates were arranged in order of tri-BBPA > di-BBPA > mono-BBPA, and the trend changed to di-BBPA > tri-BBPA > mono-BBPA during 50–130 d (Fig. 1b).
For the non-sterile soil treatments, after the anoxic incubations for 150 d, some of the bottles were exposed to the air and subsequently incubated for 60 d under the oxic conditions. The change of incubation conditions from the anoxic to the oxic state strongly affected the degradation of BPA (Fig. 1a), where the anaerobically accumulated BPA decreased rapidly by 81.1%. However, TBBPA remained stable from 1.24 ± 0.03 to 1.39 ± 0.04 mg·L−1 (Fig. 1a). The less brominated intermediates were detected at the ranges of 2.59–3.68%, 3.74–4.56% and 1.17–2.29% of the initially applied TBBPA for tri-BBPA, di-BBPA, and mono-BBPA, respectively. The concentrations of intermediates were in the order of di-BBPA > tri-BBPA > mono-BBPA (Fig. 1b).
Degradation of TBBPA in a paddy soil with the addition of lactic acid
Time-dependent profiles of TBBPA and its metabolites in the sterile and non-sterile soils with the exogenous lactic acid addition are depicted in Fig. 2. During the first 10 d of the anoxic incubations, the concentrations of TBBPA decreased both in the sterile (from 19.2 ± 0.05 to 14.6 ± 0.88 mg·L−1) and non-sterile (from 19.8 ± 0.36 to 15.1 ± 1.02 mg·L−1) soils, with no detection of the debrominated products (Fig. 2a). The reductions of TBBPA in the lactic acid treatments were 24.0% and 24.1% for the sterile and non-sterile soils, respectively.
Degradation of TBBPA and production of metabolites in non-sterile and sterile paddy soil with the addition of lactic acid during the incubations of TBBPA under static anoxic conditions (210 d for non-sterile soil; 150 d for sterile soil) or sequential anoxic-oxic conditions (0–150 d: anoxic, hollow symbols; 151–210 d: oxic, solid symbols). (a) The degradation of TBBPA and formation of BPA in non-sterile and sterile soils, respectively. (b) The formation of intermediates (mono-, di-, and tri-BBPA) in the non-sterile soil. No metabolites were found in the sterile soil. Data based on three replicates were presented as mean ± SD.
An insignificant variation of TBBPA (from 15.6 ± 0.18 to 16.2 ± 1.15 mg·L−1), as well as no detection of the intermediates and BPA, were observed during 10–150 d of the anoxic incubations in the sterile soils with the addition of lactic acid (Fig. 2a). However, the amount of TBBPA in the non-sterile soils steadily decreased after 10 d, reduced by 93.0% at 70 d, and varied insignificantly from 1.25 ± 0.13 to 1.45 ± 0.13 mg·L−1 during 70–210 d. The reductive debromination of TBBPA was almost complete at 70 d. Meanwhile, BPA was first detected at 0.25 ± 0.10 mg·L−1 at 20 d, the concentration of BPA increased sharply after 30 d and maximized at 13.3 ± 0.50 mg·L−1 (accounting for 67.1% of the initially applied TBBPA) at 90 d, and remained stable from 13.3 ± 0.50 to 13.4 ± 0.45 mg·L−1 during 90–210 d. In comparison with the non-sterile treatments without lactic acid, the dissipation of TBBPA and production of BPA were quickened by 60 d and 40 d, respectively, with the addition of lactic acid.
As shown in Fig. 2b, the three intermediates were all detected for the first time at 20 d, increased significantly and reached their maxima at 60 d, then decreased markedly to undetectable levels leaving BPA as the only detectable degradation product after 100 d. The production and disappearance of the intermediates were 30 d early with the addition of lactic acid compared to the lactic acid-free experiment. Tri-BBPA, di-BBPA, and mono-BBPA were individually quantified at 1.05–9.53%, 1.13–11.2% and 0.66–7.88% of the initially applied TBBPA during 20–90 d. The concentrations of the intermediates were initially in the following order of tri-BBPA > di-BBPA > mono-BBPA during 10–25 d, and then changed to di-BBPA > tri-BBPA > mono-BBPA during 25–100 d (Fig. 2b).
For the subsequent oxic incubations of 60 d in the non-sterile soil with the addition of lactic acid, TBBPA varied from 1.40 ± 0.07 to 1.90 ± 0.08 mg·L−1, while BPA decreased sharply by 95.7% (from 13.4 ± 0.24 to 0.57 ± 0.05 mg·L−1) (Fig. 2a). The lower brominated intermediates were formed again and detected at the ranges of 2.49–4.08%, 4.21–6.55% and 1.06–2.14% of the initially applied TBBPA for tri-BBPA, di-BBPA, and mono-BBPA, respectively. The concentrations of the intermediates were in the order of di-BBPA > tri-BBPA > mono-BBPA (Fig. 2b).
Fe(II) generation during the anoxic incubations
The paddy soil used in the present study is iron-rich soil collected from South China with a high content of iron oxides (31.8 g·kg−1). Adsorbed Fe(II) species on mineral surfaces are critical to accelerating the reductive process of organic pollutants18,22. Consequently, 0.5 M HCl-extracted Fe(II) has been shown to be effective in extracting produced Fe(II), including adsorbed and dissolved forms23. Dissolved Fe(II) species were increased from 0.95 ± 0.002 to 40.0 ± 0.006 mg·L−1 and from 0.82 ± 0.001 to 20.1 ± 0.005 mg·L−1 in the non-sterile soils with and without lactic acid addition, respectively (Fig. 3a). However, the concentrations of dissolved Fe(II) were stable at ranges of 0.48 ± 0.001–0.51 ± 0.002 mg·L−1 and 0.47 ± 0.004–0.51 ± 0.001 mg·L−1 for the sterile treatments with and without lactic acid, respectively (Fig. 3a). For the sterile soils, the concentrations of HCl-extracted Fe(II), including the forms of adsorbed and dissolved Fe(II), varied from 115 ± 0.13 to 184 ± 8.42 mg·L−1 and from 116 ± 0.08 to 183 ± 0.07 mg·L−1 with and without lactic acid, respectively, showing no significant difference (p > 0.05) (Fig. 3b). For the non-sterile treatments, the concentrations of HCl-extracted Fe(II) increased markedly and varied from 116 ± 0.13 to 417 ± 8.42 mg·L−1 and from 116 ± 0.07 to 318 ± 9.05 mg·L−1 with and without lactic acid, respectively. The concentrations of dissolved Fe(II) and HCl-extracted Fe(II) were both significantly higher in the non-sterile treatments compared to the sterile treatments, regardless of whether lactic acid was added (p < 0.05). Moreover, the concentrations of dissolved Fe(II) and HCl-extracted Fe(II) in the non-sterile treatments with lactic acid were significantly higher than the concentrations in the treatments without lactic acid (p < 0.05). The concentrations of adsorbed Fe(II) under different treatments (Fig. 3c) were in accordance with TBBPA transformation efficiencies. In sterile soils, approximately 160 mg·L−1 adsorbed Fe(II) was generated, which was substantially lower than those obtained in the non-sterile soils (Fig. 3c). The highest concentrations of adsorbed Fe(II) were generated in the non-sterile soils with the presence of lactic acid. The results showed significant iron reduction in the non-sterile soils, which indicated that most of the Fe(II) species were microbially generated by the soil microorganisms, where the addition of lactic acid might promote the formation of Fe(II) species during the non-sterile incubations. Thess findings are in good agreement with the above results showing that TBBPA obtained higher transformation rates in the non-sterile soils with the addition of lactic acid.
Variation of pH values during the anoxic incubations
During the anoxic incubations in non-sterile soils with and without lactic acid, pH values both slightly increased and varied in a range of 6.09–7.11, exhibiting a little higher in the lactic acid treatments (Fig. 4). It has been documented that TBBPA could biodegrade effectively in neutral and mildly acidic pH media24. Apparently, the slightly increased pH values, a result of the dissociation of protein and urea by microorganisms to generate alkaline substances during the anoxic incubations, showed no adverse impact on the microorganisms.
Discussion
Degradation dynamics and potential pathway of TBBPA in the paddy soil
As neither BPA nor intermediates of TBBPA were detectable in both sterile and non-sterile soils during the first 10 d, the dissipation of TBBPA could be ascribed to the sequestration of TBBPA in soils, rather than its degradation25. No metabolites were found in the sterile treatments throughout the incubations, indicating that TBBPA was not degraded. The increase and insignificant fluctuation of TBBPA after 10 d in the sterile treatments (p > 0.05) was likely a consequence of the equilibrium of soil adsorption and desorption. The TBBPA concentration decreased continuously with the concurrent production of the lower brominated intermediates and BPA after 10 d in the non-sterile soils, which suggested that the transformation process of TBBPA was reductive debromination under the anoxic conditions. The reductive debromination of TBBPA was demonstrated to be biologically mediated, which had also been documented previously3,26,27.
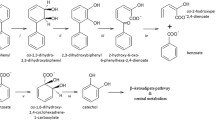
The detection of tri-BBPA was the earliest followed by di-BBPA and mono-BBPA thereafter, indicating that debromination of TBBPA was probably a stepwise removal of bromine atoms. Therefore, we propose the following biotransformation pathway: TBBPA → tri-BBPA → di-BBPA → mono-BBPA → BPA (Fig. 5). This pathway indicates that the debromination was dominated by the debromination of TBBPA and tri-BBPA during 10–90 d, and then was governed by the debromination of di-BBPA and mono-BBPA during 90–150 d. Both of the OH substituents on the aromatic rings of BPA may be possible sites for degradation, but the two rings joined by a quaternary carbon may inhibit its degradation by anoxic microbes11. The steady accumulation of the debromination end product (BPA) during the anoxic incubations confirms that BPA is difficult to degrade. These results provide a good support for the debromination of TBBPA to BPA in anoxic paddy soils and thus explain the levels of BPA higher than TBBPA in paddy soils from the Longtang area28. The reductive debromination of TBBPA to the lower brominated intermediates and BPA was also reported in the paddy soils from the Yangtze River Delta3.
The rate constant k for the reductive debromination of TBBPA in non-sterile lactic acid-free experiments was estimated at 0.0371 d−1 (R2 = 0.97, p < 0.05; Fig. 6). Thus, the half-life (t1/2) was 60.8 d, which was less than the reported degradation half-life of TBBPA under the anoxic conditions in the salt marshes (t1/2 70 to >130 d)29 and the heavy clay soil (t1/2 430 d)30 and greater than the reported degradation half-life of TBBPA under the anoxic conditions in the rice paddy soils (t1/2 36 d)3, the estuarine sediments (t1/2 30–40 d)31, and the submerged soils planted with reed (t1/2 11.4 d) and without any plant growth (t1/2 20.8 d)26.
The rapid degradation of BPA under the subsequent oxic incubations was because of the existence of BPA-degraders in the non-sterile soils, which was in good accordance with the ubiquitous occurrence of BPA-degrading bacteria in the oxic environments32, and the rapid acclimation of the microbial population to degrade BPA33. Consequently, the rapid dissipation of BPA in the oxic soils even after long-term anoxic incubations further confirmed the widespread existence and survival of BPA degrading bacteria under different environmental conditions.
The formation of substantial bound residues during the anoxic incubations has been documented, which strongly hinted at the potential release of TBBPA and its metabolites from the bound residues3,26,34,35. Considerable amounts of TBBPA and the intermediates were observed during the subsequent oxic incubations, although TBBPA and the intermediates were at a low level (TBBPA) or undetectable (the intermediates) at the end of the preceding anoxic incubations3. TBBPA and the intermediates during the subsequent oxic incubations were likely released from the bound residues3. The increased levels of TBBPA and the intermediates during the oxic incubations (Fig. 1) showed that no degradation or slower degradation rates compared with the release rates from bound residues. Owing to the formation of bound residues and the release of brominated BPAs, it is strongly recommended the significance of the repeated anoxic-oxic incubations for the complete reductive debromination and mineralization of TBBPA in polluted soils.
Effects of lactic acid on the transformation of TBBPA in the paddy soil
After adding lactic acid to the sterile soils, there were no detectable metabolites of TBBPA in the sterile soils, which showed that TBBPA did not degrade. However, for the non-sterile soils, TBBPA was debrominated into the lower brominated BPA and BPA, further demonstrating that the microbial activity was the critical factor governing the degradation of TBBPA. The initial dissipation, subsequent increase and insignificant fluctuation of TBBPA in the sterilized soils were in response to the adsorption and desorption of TBBPA to and from soil particles.
With the addition of lactic acid, the degradation in the non-sterile soils was initially dominated by the debromination of TBBPA and tri-BBPA during 10–60 d, and then was governed by the debromination of di-BBPA and mono-BBPA during 60–100 d. Apparently, the reductive transformation of TBBPA and production of BPA, as well as the formation and dissipation of the intermediates, were all accelerated by lactic acid. The transformation of TBBPA was complete at 70 d while the production of BPA was maximum at 90 d (Fig. 2), possibly due to the stimulating effects of lactic acid on the debromination of TBBPA and the lower brominated intermediates. The addition of lactic acid enhanced the TBBPA transformation, with k being increased to 0.0766 d−1 and t1/2 being shortened to 31.9 d (R2 = 0.98, p < 0.05; Fig. 6).
The lactic acid, a low-molecular-weight organic acid, can be utilized directly by dehalogenating bacteria as a carbon source18,36. Furthermore, the lactic acid can also be decomposed into H2, acetate, and propionate by the soil indigenous microorganisms, and the H2 that is produced can act as an electron donor for the dehalogenation of the halogenated pollutants. For example, lactic acid has been reported to stimulate dechlorinating microorganisms, and lactic acid might have stimulated the reductive dechlorination of pentachlorophenol in paddy soils4. The debromination of TBBPA in the sediments was significantly stimulated by the pyruvate, ethanol, and acetate +H2, which might be utilized as electron donors and carbon sources for the microorganisms37. In the present study, the addition of lactic acid might stimulate the growth of bacteria that are responsible for the transformation of TBBPA.
Under the anoxic conditions in soils, the main inorganic electron acceptor Fe(III) could be biologically reduced to Fe(II) by a series of dissimilatory iron-reducing bacteria by coupling the energy generated from the oxidation of organic substrates38. The Fe(II) species that are formed have been widely recognized as reducers for reductive degradation of soil pollutants39. The reduction of Fe(III) to Fe(II) depends mainly on the availability of organic carbon for microbial growth, where organic acids, such as lactic acid, acetic acid and oxalic acid, can reductively dissolve Fe(III)40 and serve as the electron donors for the dissimilatory iron-reducing bacteria during the Fe(III) reduction38. Lactic acid has been confirmed to have a stimulated effect on iron-reducing and dechlorinating microorganisms and consequently accelerates the transformation of pentachlorophenol in paddy soils4. In the present study, significantly positive correlations were observed between the degradation rates of TBBPA and the concentrations of adsorbed Fe(II) in the non-sterile soils with and without the addition of lactic acid (R2 = 0.94 and 0.91, respectively, Fig. 7). It is indicated that the adsorbed Fe(II) was an important factor affecting the reductive degradation of TBBPA under anoxic conditions. Moreover, iron reduction in the non-sterile soils is stimulated to form more adsorbed Fe(II) with the addition of lactic acid, and subsequently enhances the reductive debromination of TBBPA.
Methods
Chemicals and materials
The standard substances of TBBPA and BPA (purity >98%), and a mixture of N,O-bis(trimethylsilyl)trifluoroacetamide and trimethylchlorosilane (99:1, v-v) were purchased from AccuStandard (New Haven, CT, USA). The solid phase extraction columns (cleanert S C18, 500 mg/6 mL) were purchased from Agela Technologies (Wilmington, NC, USA). The acetone, ethyl acetate and n-hexane (HPLC grade solvents) were from Honeywell International Inc (Morristown, New Jersey, USA). Other chemicals were obtained from the Chemical Reagent Factory (Guangzhou, China).
Paddy soil
The surface paddy soil used in the present study was sampled from a rice field located in Longtang Town (23°33′39.53′′N, 113°1′59.35′′E), Qingyuan City, Guangdong Province, China. The soil collection method was described previously28. The paddy soil was a ferric acrisol containing 3.24% organic matter and 0.20% nitrogen with a pH (0.01 M CaCl2) of 6.1 and a cation exchange capacity of 17.6 cmol(+)·kg−1. TBBPA, the lower brominated intermediates and BPA were undetectable in the soil. The paddy soil was air-dried, separated from plant residues and stones and later passed through a 100-mesh (0.15 mm) sieve prior to use. Soil for the control experiment was sterilized with high energy γ-rays released from a 60Co radioactive isotope and was sealed and stored below −20 °C before use.
Incubation under the anoxic conditions
Approximately 2 g of soil (dry weight) were placed into a 50 mL serum glass bottle and submerged with 20 mL oxygen-free medium containing NH4Cl (2.7 g·L−1), MgCl2·6H2O (0.1 g·L−1), CaCl2·2H2O (0.1 g·L−1), FeCl2·4H2O (0.02 g·L−1), K2HPO4 (0.27 g·L−1) and KH2PO4 (0.35 g·L−1). Resazurin was added to three of the bottles as redox indicator at 20 mg·L−1. The anoxic incubation treatments included (1) sterile soil + TBBPA; (2) sterile soil + lactic acid + TBBPA; (3) non-sterile soil + TBBPA, and (4) non-sterile soil + lactic acid + TBBPA, where the final concentrations of TBBPA and lactic acid were 20 mg·L−1 and 0.1 mmol·L−1, respectively. Each treatment was performed in triplicate. After adding the designated reagents, the bottles were purged with N2 (99.99%) for 30 min, and sealed with silicone-lined septa and aluminum caps. The serum bottles were placed in the dark in a biochemical incubator at 25 ± 1 °C for incubation for 150 days. At each of the designated sampling times, three bottles were randomly taken for the analysis of dissolved and HCl-extracted Fe(II), TBBPA and its metabolites, respectively.
Incubation under the oxic conditions
For the non-sterile soil treatments (noted above 3 and 4), after the anoxic incubations for 150 days, several bottles were continuously incubated under anoxic incubations, while the other bottles were transferred into triangular flasks (50 mL) to undergo oxic incubations. The water layer of the mixture was decanted after centrifugation (3000 rpm), and the soil (pellet) was mixed with a stainless spatula and exposed to the air. Next, the flask was closed with a rubber stopper, placed in a shaker at 100 rpm, and kept in a dark room at 25 °C for further incubation. Each treatment was performed in triplicate. At each of the defined sampling times (0, 30 and 60 days), three flasks were randomly taken for the analysis of TBBPA and its metabolites, respectively.
Analysis of iron species and TBBPA and its metabolites
The dissolved Fe(II) and HCl-extracted Fe(II) were quantified by 1,10-phenanthroline spectrophotometry after direct filtration and extraction of reaction mixtures with 0.5 M HCl for 1.5 h, respectively41. The difference between HCl-extracted Fe(II) and dissolved Fe(II) was defined as adsorbed Fe(II)23. TBBPA and its products were identified by gas chromatography/maass spectrometry as previously described28,42,43. Due to the unavailability of commercial standards for the intermediates, i.e., tri-, di-, and monobromobisphenol A (tri-BBPA, di-BBPA, and mono-BBPA, respectively), their concentrations were estimated from the peak areas of the chromatograms based on the standard curves of TBBPA and BPA on a molar basis with compensation for the number of bromine atoms43. Recoveries of the added TBBPA and BPA in the extraction and determination process were 83.4 ± 6.85% and 86.3 ± 8.19%, respectively.
Data analysis
A kinetics model modified from the microbial logistic growth Eq. (1) has usually been applied to describe the TBBPA transformation process, depicted as Eq. (2)21,44,45.
where A is the maximal transformation amount of TBBPA, B is the regression coefficient, k is the rate constant of TBBPA transformation, and C0 and Ct are the concentrations of TBBPA at the reaction time of 0 and t, respectively. The half-life time (t1/2) can be calculated as
where C50% is the concentration of TBBPA when half of the applied TBBPA is gone46.
References
Darnerud, P. O. Toxic effects of brominated flame retardants in man and in wildlife. Environment International 29, 841–853 (2003).
Liu, K. et al. A review of status of tetrabromobisphenol A (TBBPA) in China. Chemosphere 148, 8–20 (2016).
Liu, J. et al. Degradation, metabolism, and bound-residue formation and release of tetrabromobisphenol A in soil during sequential anoxic-oxic incubation. Environmental Science & Technology 47, 8348–8354 (2013).
Liang, X. L. et al. The variation of cationic microstructure in Mn-doped spinel ferrite during calcination and its effect on formaldehyde catalytic oxidation. Journal of Hazardous Materials 306, 305–312 (2016).
Chen, M. J. et al. A humic substance analogue AQDS stimulates Geobacter sp abundance and enhances pentachlorophenol transformation in a paddy soil. Chemosphere 160, 141–148 (2016).
Suflita, J. M. & Townsend, G. T. The microbial ecology and physiology of aryl dehalogenation and implications for bioremediation in Microbial transformation and degradation of toxic organic chemicals, edited by Young, L. Y. & Cerniglia, C. E., pp. 243–268 (Wiley-Liss, New York, 1995).
Armenante, P. M., Kafkewitz, D., Lewandowski, G. A. & Jou, C. J. Anaerobic-aerobic treatment of halogenated phenolic compounds. Water Research 33, 681–692 (1999).
Eggleton, J. & Thomas, K. V. A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environment International 30, 973–980 (2004).
Kogel-Knabner, I. et al. Biogeochemistry of paddy soils. Geoderma 157, 1–14 (2010).
Ronen, Z. & Abeliovich, A. Anaerobic-aerobic process for microbial degradation of tetrabromobisphenol A. Applied and Environmental Microbiology 66, 2372–2377 (2000).
Chang, B. V., Yuan, S. Y. & Ren, Y. L. Anaerobic degradation of tetrabromobisphenol-A in river sediment. Ecological Engineering 49, 73–76 (2012).
Faulwetter, J. L. et al. Microbial processes influencing performance of treatment wetlands: A review. Ecological Engineering 35, 987–1004 (2009).
Field, J. A., Cervantes, F. J., van der Zee, F. P. & Lettinga, G. Role of quinones in the biodegradation of priority pollutants: a review. Water Science and Technology 42, 215–222 (2000).
Glass, B. L. Relation between the degradation of DDT and the iron redox system in soils. Journal of Agricultural and Food Chemistry 20, 324–327 (1972).
Li, F. B. et al. Reductive transformation of pentachlorophenol on the interface of subtropical soil colloids and water. Geoderma 148, 70–78 (2008).
Amonette, J. E., Workman, D. J., Kennedy, D. W., Fruchter, J. S. & Gorby, Y. A. Dechlorination of carbon tetrachloride by Fe(II) associated with goethite. Environmental Science & Technology 34, 4606–4613 (2000).
Li, F. B. et al. Enhanced reductive dechlorination of DDT in an anaerobic system of dissimilatory iron-reducing bacteria and iron oxide. Environmental Pollution 158, 1733–1740 (2010).
Chen, M. J. et al. Anaerobic transformation of DDT related to iron(III) reduction and microbial community structure in paddy soils. Journal of Agricultural and Food Chemistry 61, 2224–2233 (2013).
Liang, X. L. et al. Adsorption isotherm, mechanism, and geometry of Pb(II) on magnetites substituted with transition metals. Chemical Geology 470, 132–140 (2017).
Johnsen, A. R., Wick, L. Y. & Harms, H. Principles of microbial PAH-degradation in soil. Environmental Pollution 133, 71–84 (2005).
Liu, Y., Li, F. B., Xia, W., Xu, J. M. & Yu, X. S. Association between ferrous iron accumulation and pentachlorophenol degradation at the paddy soil-water interface in the presence of exogenous low-molecular-weight dissolved organic carbon. Chemosphere 91, 1547–1555 (2013).
Strathmann, T. J. & Stone, A. T. Mineral surface catalysis of reactions between FeII and oxime carbamate pesticides. Geochimca et Cosmochimica Acta 67, 2775–2791 (2003).
Fredrickson, J. K. et al. Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochimica Et Cosmochimica Acta 62, 3239–3257 (1998).
Peng, X. X. & Jia, X. S. Optimization of parameters for anaerobic co-metabolic degradation of TBBPA. Bioresource Technology 148, 386–393 (2013).
Gevao, B., Semple, K. T. & Jones, K. C. Bound pesticide residues in soils: a review. Environmental Pollution 108, 3–14 (2000).
Sun, F. F. et al. Degradation and metabolism of tetrabromobisphenol A (TBBPA) in submerged soil and soil-plant systems. Environmental Science & Technology 48, 14291–14299 (2014).
Li, G. Y., Xiong, J. K., Wong, P. K. & An, T. C. Enhancing tetrabromobisphenol A biodegradation in river sediment microcosms and understanding the corresponding microbial community. Environmental Pollution 208, 796–802 (2016).
Huang, D. Y., Zhao, H. Q., Liu, C. P. & Sun, C. X. Characteristics, sources, and transport of tetrabromobisphenol A and bisphenol A in soils from a typical e-waste recycling area in South China. Environmental Science and Pollution Research 21, 5818–5826 (2014).
Ravit, B., Ehrenfeld, J. G. & Haggblom, M. M. Salt marsh rhizosphere affects microbial biotransformation of the widespread halogenated contaminant tetrabromobisphenol-A (TBBPA). Soil Biology & Biochemistry 37, 1049–1057 (2005).
Nyholm, J. R., Lundberg, C. & Andersson, P. L. Biodegradation kinetics of selected brominated flame retardants in aerobic and anaerobic soil. Environmental Pollution 158, 2235–2240 (2010).
Voordeckers, J. W., Fennell, D. E., Jones, K. & Haggblom, M. M. Anaerobic biotransformation of tetrabromobisphenol A, tetrachlorobisphenol A, and bisphenol A in estuarine sediments. Environmental Science & Technology 36, 696–701 (2002).
Kang, J. H., Y., K. & Kondo, F. Biodegradation or metabolism of bisphenol A: From microorganisms to mammals. Toxicology 217, 81–90 (2006).
Staples, C. A., Dorn, P. B., Klecka, G. M., O’Block, S. T. & Harris, L. R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 36, 2149–2173 (1998).
Li, F. J. et al. Fate and metabolism of tetrabromobisphenol A in soil slurries without and with the amendment with the alkylphenol degrading bacterium Sphingomonas sp strain TTNP3. Environmental Pollution 193, 181–188 (2014).
Li, F. J. et al. Fate of tetrabromobisphenol A (TBBPA) and formation of ester- and ether-linked bound residues in an oxic sandy soil. Environmental Science & Technology 49, 12758–12765 (2015).
Brenner, A., Mukmenev, I., Abeliovich, A. & Kushmaro, A. Biodegradability of tetrabromobisphenol A and tribromophenol by activated sludge. Ecotoxicology 15, 399–402 (2006).
Arbeli, Z., Ronen, Z. & Diaz-Baez, M. C. Reductive dehalogenation of tetrabromobisphenol-A by sediment from a contaminated ephemeral streambed and an enrichment culture. Chemosphere 64, 1472–1478 (2006).
Lovley, D. R., Holmes, D. E. & Nevin, K. P. Dissimilatory Fe(III) and Mn(IV) reduction. Advances in Microbial Physiology, Vol. 49 49, 219–286 (2004).
Li, F. B. et al. Enhancement of the reductive transformation of pentachlorophenol by polycarboxylic acids at the iron oxide-water interface. Journal of Colloid and Interface Science 321, 332–341 (2008).
Jones, D. L. Organic acids in the rhizosphere - a critical review. Plant and Soil 205, 25–44 (1998).
Fredrickson, J. K. & Gorby, Y. A. Environmental processes mediated by iron-reducing bacteria. Current Opinion in Biotechnology 7, 287–294 (1996).
Sánchez-Brunete, C., Miguel, E. & Tadeo, J. L. Determination of tetrabromobisphenol-A, tetrachlorobisphenol-A and bisphenol-A in soil by ultrasonic assisted extraction and gas chromatography-mass spectrometry. Journal of Chromatography A 1216, 5497–5503 (2009).
Huang, Q., Liu, W., Peng, P. A. & Huang, W. L. Reductive debromination of tetrabromobisphenol A by Pd/Fe bimetallic catalysts. Chemosphere 92, 1321–1327 (2013).
Yang, Y., Zhang, N., Xue, M., Lu, S. T. & Tao, S. Effects of soil organic matter on the development of the microbial polycyclic aromatic hydrocarbons (PAHs) degradation potentials. Environmental Pollution 159, 591–595 (2011).
Chen, M. J. et al. Biostimulation of indigenous microbial communities for anaerobic transformation of pentachlorophenol in paddy soils of Southern China. Journal of Agricultural and Food Chemistry 60, 2967–2975 (2012).
Gimsing, A. L., Sorensen, J. C., Tovgaard, L., Jorgensen, A. M. F. & Hansen, H. C. B. Degradation kinetics of glucosinolates in soil. Environmental Toxicology and Chemistry 25, 2038–2044 (2006).
Acknowledgements
We gratefully acknowledge the financial support from GDAS’ Project of Science and Technology Development (Grant No. 2017GDASCX-0106), the National Natural Science Foundation of China (Grant Nos 41471267, 41601527 and 21277034), the Natural Science Foundation of Guangdong Province, China (Grant No. 2014A030313704), the Technology Program of Guangzhou, China (Grant No. 201607010236), and the Science and Technology Planning Project of Guangdong Province (Grant No. 2017A070702015).
Author information
Authors and Affiliations
Contributions
G.L.W. and D.Y.H. conceived and designed the experiments. G.L.W. and H.Q.Z. analyzed the data. G.L.W., H.Q.Z. and D.Y.H. wrote the manuscript. M.F.H. was involved in the related discussion and helped to improve the quality of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wei, G., Zhao, H., Huang, D. et al. Degradation of tetrabromobisphenol A in a paddy soil during sequential anoxic-oxic incubation: Kinetics, metabolites, and potential pathways. Sci Rep 8, 13435 (2018). https://doi.org/10.1038/s41598-018-31723-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-31723-9
- Springer Nature Limited
This article is cited by
-
Two halogenated flame retardants and cadmium in the soil-rice system: sorption, root uptake, and translocation
Environmental Science and Pollution Research (2023)
-
What drives Tetrabromobisphenol A degradation in biotreatment systems?
Reviews in Environmental Science and Bio/Technology (2021)