Abstract
We studied the tetrabromobisphenol A (TBBPA) and bisphenol A (BPA) patterns and their sources and transport in different land-use soils from Longtang, South China, a typical electronic waste recycling center. We also studied the reductive debromination of TBBPA in paddy soils. TBBPA and BPA concentrations (on a dry weight basis) were undetected–220 and 0.50–325 ng/g, respectively, and both increased, by similar factors, in the following order: pond sediments < paddy soils = vegetable soils < wasteland < dismantling sites < former open burning sites. BPA concentrations were higher than TBBPA concentrations in all six land-use soils, and they correlated significantly. TBBPA and BPA were transported through the soil profiles, being found at relatively high concentrations in soil 0–40 cm deep, but only at low concentrations in soil 40–80 cm deep. The surface soil concentrations appear to have been strongly affected by crude recycling activities, and former open burning and dismantling sites were the main point sources. The areas surrounding the open burning sites and dismantling sites have been contaminated not only by the dumping of waste residues but also by fly ash deposition, even though the agricultural soils are far from the point pollution sources. Some BPA in the soils is likely to be the reductive debromination product of TBBPA because the long rainy season promotes TBBPA transformation and because BPA can persist for a long time. Incubation experiments confirmed that TBBPA could be transformed into BPA and that BPA could accumulate in waterlogged paddy soils, and this may be why BPA concentrations were higher than TBBPA concentrations in the Longtang area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
More electronic waste (e-waste) recycling is currently performed in China than in any other part of the world. It is estimated that approximately 90 % of the global e-waste imported into Asia is legally or illegally imported into China (Chen et al. 2009). Low labor costs and weak environmental regulations and enforcement led to primitive e-waste recycling techniques, including manual dismantling, open incineration, and acid dipping, being widely used from the 1990s to the early 2000s, and these processes led to the release of large quantities of toxic metals and organic pollutants into the surrounding environment (Luo et al. 2011; Wang et al. 2011). For example, Cd, Pb, Cu, polychlorinated biphenyls (PCBs), and polybrominated diphenyl ethers (PBDEs) have been detected in abiotic and biotic samples around e-waste recycling sites in the provinces of Guangdong and Zhejiang, the two main e-waste recycling areas in China (Luo et al. 2011; Wong et al. 2007; Zhao et al. 2009).
It is well known that PBDEs and tetrabromobisphenol A (TBBPA) are widely used in electronic products, and they have been used for more than three decades as brominated flame retardants (BFRs). PBDEs can be released into soil and the ambient environment from uncontrolled e-waste recycling activities and threaten local ecosystems and the health of recycling workers and local residents (Qu et al. 2007; Wang et al. 2011). There has been an increasing amount of research into PBDEs in e-waste recycling areas in China (Leung et al. 2007; Liu et al. 2008; Luo et al. 2007; Wong et al. 2007; Wu et al. 2008). However, there has been little research on TBBPA concentrations in soils in these areas, and in particular, there has been little research on its natural degradation in agricultural soils. Bisphenol A (BPA), the final reductive debromination product of TBBPA, is known to show estrogenic activity (Huang et al. 2012; Renner 1997), and its fate in agricultural soils remains to be elucidated.
According to previous reports, the reductive dehalogenation of highly halogenated compounds is a key step in their degradation in the environment. For example, TBBPA (Arbeli and Ronen 2003; Ronen and Abeliovich 2000) and PCBs (Wiegel and Wu 2000) have been found to be reductively dehalogenated and then further degraded by microorganisms. Likewise, the microbial degradation rates of polychlorinated benzenes and phenols can be enhanced if the chlorine atoms are first anaerobically removed from the aromatic ring (Suflita and Townsend 1995). Biogeochemical process and remediation research has only recently started to be conducted on BFRs in soil in China (Liu et al. 2013), despite the fact that BFRs have been released into the environment from e-waste recycling activities for more than 20 years (Chen et al. 2009; Luo et al. 2009; Wang et al. 2011; Wong et al. 2007).
South China is one of the main acid deposition regions in China (Huang et al. 2009), and it has a subtropical monsoon climate with a rainy season from March to October. The agricultural fields in South China are often covered with water, and the soils are predominantly red soil, containing abundant iron and manganese oxides, which are considered to be important sources of reductants for the reduction of halogenated hydrocarbons (Lin et al. 2009).
This study was carried out in the Longtang area (23° 34′ N, 113° 0′ E), in northern Guangdong Province, southern China, which has an area of approximately 150 km2 and approximately 100,000 residents, approximately half of whom are engaged in dismantling e-waste. e-waste recycling in Longtang and its surrounding area can be traced back to the 1980s, and Longtang is now one of the largest e-waste recycling centers in China, with more than 1,000 workshops processing approximately one million tonnes of e-waste per year (Luo et al. 2011; Ni and Zeng 2009). However, traditional agricultural activities, such as rice and vegetable cultivation, fish farming, and poultry rearing, are still practiced in this area. Unfortunately, most of the e-waste recycling methods have remained primitive, and there are many former open e-waste burning sites and current e-waste dismantling workshops dispersed in or close to agricultural fields. The objectives of the study presented here were to (1) study TBBPA and BPA contamination, released from primitive e-waste recycling activities, in the surrounding soils and (2) investigate whether TBBPA could be naturally transformed into BPA in waterlogged paddy soils. The results of the study will be helpful in planning management measures and in evaluating potential health risks to the workers and local residents in e-waste recycling areas.
Materials and methods
Standards and reagents
A mixture of N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) and trimethylchlorosilane (TMCS) (99:1, v/v), TBBPA, and BPA (all with purity >98 %) were purchased from AccuStandard (New Haven, CT, USA). 13C12-labeled TBBPA and 13C12-labeled BPA (both with purity >99 %) were provided by Cambridge Isotope Laboratories (Andover, MA, USA). All other chemicals were purchased from Guangzhou Chemical Reagent Factory (Guangzhou, China). Acetone, ethyl acetate, and n-hexane were of HPLC grade, and other chemicals were of analytical grade.
Soil sampling
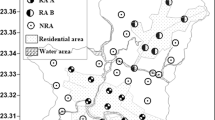
The sampling locations are shown in Fig. 1 and were mainly concentrated in nine villages (BC, BHT, DA, DG, JL, NY, SB, XB, and YMY). The sites were classified into six land-use groups: former open burning sites, dismantling sites (including open air sites and workshops), paddy fields, vegetable fields, wasteland, and pond sediments. Seven soil profiles 1(100 cm long × 50 cm wide × 80 cm deep) were collected from a large former open burning site (BC village, n = 2), three paddy fields (BC village, SB village, and YMY village; n = 3), and two wasteland sites (BHT village, n = 2). The number of collected surface samples and soil profiles in different villages is shown in Table 1. Soil and its associated water were collected for the anaerobic incubation experiments from a waterlogged paddy field in YMY village.
All samples were collected in January 2011, which is in the dry season. Topsoil (0–20 cm) samples were collected from the sites using a stainless steel scoop, including pond areas, waterlogged paddy fields, and dismantling sites. Soil profile samples were collected from the top layer to the bottom layer (0–20, 20–40, 40–60, and 60–80 cm). Each sample was a composite of five subsamples taken from an approximately square area with each subsample site being about 15 m from the central subsample site. The samples were wrapped in aluminum foil, put in polythene bags, and transported to the laboratory, where they were freeze-dried, ground in an agate mortar, passed through a 2.0-mm sieve, and stored at −18 °C. Water from a waterlogged paddy field was collected in a glass bottle with a lid.
Soil incubation
Anoxic incubation experiments were performed using 200-mL serum bottles. To avoid the effects of background TBBPA and BPA in the original soil, a paddy soil collected in YMY village, containing only trace concentrations of TBBPA (2.34 ± 0.23 ng/g) and BPA (4.32 ± 0.27 ng/g), was chosen for the incubation experiments. The soils in the Longtang area are Ferric Acrisols. The properties of the paddy soil used in the incubation study are given in Table 2. The mean pH value was approximately 6.0, and the total organic matter content was approximately 3.0 %. The active Fe2O3 and Mn species reached approximately 3.0 % and 240 μg/g, respectively. The H2O content was approximately 70 %.
An aliquot of dry soil (5 g) was spiked with TBBPA (1.05 mL, 500 μg/mL, dissolved in acetone), placed under a fume hood for 2 h to allow the solvent to evaporate, and then mixed thoroughly. The spiked soil sample was mixed with 100 g of unspiked soil, to bring the final TBBPA concentration in the soil to 5 μg/g. Each soil aliquot (10 g) was then incubated in a serum bottle with 100 mL of sterilized medium, and three replicates were performed in the batch samples. The medium was the water collected from the waterlogged paddy field, to which had been added 3 g of NaCl, 0.15 g of K2HPO4, 0.06 g of MgSO4, 0.05 g of peptone, 0.05 g of tryptone, 0.1 g of glucose, and 0.1 g of yeast extract. The pH was adjusted to 6.1 with 2 N NaOH(aq). Each bottle was flushed with N2 gas for 10 min and then sealed, to simulate the anoxic environment in a waterlogged paddy field. The bottles were incubated at 25 °C in the dark and without shaking. After incubation, the samples in the bottles were freeze-dried, ground in an agate mortar, and stored at −18 °C. Samples sterilized in an autoclave were used as control samples. For further comparison, three aliquots (5 g) of the soil spiked with TBBPA were kept dry. The incubated samples were analyzed after incubation for 18 and 30 days, respectively, and the sterilized and dry samples were analyzed at the end of the experiments.
Sample preparation
Briefly, 10 g of each freeze-dried soil sample was extracted with 150 mL of ethyl acetate in a Soxhlet apparatus for 48 h, with activated copper added to remove sulfur. Each soil extract was concentrated to about 1 mL using a rotary evaporator (Laborota 4000 efficient, Heidolph, Germany) and then cleaned by passing it through a multilayer silica gel/alumina column (i.d. 1 cm) containing, from the top to the bottom, anhydrous Na2SO4 (2 cm), neutral silica (12 cm), neutral alumina (6 cm), and anhydrous Na2SO4 (2 cm). TBBPA and BPA were eluted with 20 mL ethyl acetate. Each extract was concentrated using a rotary evaporator and then reduced to 200 μL under a gentle stream of purified nitrogen. Known amounts of 13C12-labeled TBBPA and 13C12-labeled BPA were then added to each sample, to act as internal standards. Two microliters of 2 μg/mL 13C12-labeled TBBPA and 1 μL of 2 μg/mL 13C12-labeled BPA (less BPA relative to TBBPA was used because BPA gives a higher detector response) were added to the environmental (survey) samples, and 10 μL of 13C12-labeled TBBPA and 5 μL of 13C12-labeled BPA (both at 4 μg/mL) were added to the incubated samples.
Derivatization
Derivatization is necessary to enhance the chromatographic peaks of the highly polar TBBPA and BPA. An aliquot (100 μL) of each standard or extract solution was transferred into a 2-mL reaction vial, and 50 μL of BSTFA containing 1 % TMCS was added. The vial was then filled with N2, closed, and left for 30 min at 60 °C. Finally, an aliquot (1 μL) of the solution was analyzed by gas chromatography-mass spectrometry (GC-MS).
Instrumental analysis
GC-MS (Agilent 6890 GC coupled to an Agilent 5973 N mass selective detector; Agilent Technologies Inc., Santa Clara, CA, USA) was used to determine TBBPA and BPA in the extracts, with separation achieved using a DB5-MS capillary column (length 30 m, i.d. 0.25 mm, and film thickness 0.25 mm; J&W Scientific, Inc., Folsom, NM, USA). The operating conditions were similar to those described elsewhere (Sánchez-Brunete et al. 2009). Briefly, the injector port temperature was 280 °C, helium (purity 99.995 %) was used as the carrier gas, and splitless mode was used. The column temperature was 75 °C for 1 min; then, it was increased at 20 °C/min to 310 °C, which was held for 5 min. The ion source temperature was 300 °C, and the quadrupole temperature was 150 °C. The limit of quantification (LOQ) is defined as the concentration of a standard that gives a peak with a signal/noise ratio of 10. The TBBPA and BPA LOQs were 0.30 and 0.10 ng/g, respectively. For the analysis of the environmental (survey) samples, a series of 12 calibration standards were prepared, with a concentration range of 2–350 ng/mL, each containing 20 ng/mL of the 13C12-labeled TBBPA and 10 ng/mL of the 13C12-labeled BPA. For the analysis of the incubated samples, a series of six calibration standards were prepared, with a concentration range of 1–6 μg/mL, each containing 200 ng/mL of the 13C12-labeled TBBPA and 100 ng/mL of the 13C12-labeled BPA. The standard solutions were derivatized with BSTFA before GC injection.
Quality assurance and quality control
The quality assurance and quality control (QA/QC) measures used included analyzing a duplicate sample, a laboratory blank (solvent), and a laboratory spiked sample in each set of 15 samples. A laboratory blank and a duplicate sample were run with each set of samples to assess the potential for sample contamination and the repeatability of the analysis. No TBBPA or BPA was detected in the laboratory blanks.
We performed analyte recovery studies, in which laboratory soil samples (n = 8) were spiked with 13C12-labeled internal standards (equivalent to 10 ng/g of each analyte in the soil) and left at room temperature in a fume hood for 2 h to allow the solvent to evaporate. The internal standards in these samples were determined using the external standards method, and their recoveries ranged from 92.8 to 108.5 % for 13C12-labeled TBBPA and from 91.6 to 106.0 % for 13C12-labeled BPA. Their relative standard deviations were 6.3 and 5.8 %, respectively. The analyte concentrations were not corrected for the surrogate recoveries, and all of the data are expressed on a dry weight basis (nanograms per gram dw).
Statistical analyses
Statistical analyses, including variance analysis and Pearson’s correlations, were carried out using the statistical package SPSS 13.0 for Windows (SPSS, Inc., Chicago, IL, USA). When the average concentration in a surface soil or soil profile sample was lower than the LOQ, it was considered undetected.
Results
Concentrations and distributions of TBBPA and BPA in surface soils
The TBBPA and BPA concentrations found in the Longtang soils (0–20 cm deep, excluding the soil profile samples) are given in Table 3. The TBBPA concentrations ranged from undetected, in an SB village pond sediment, to 219.99 ng/g, in soil from the largest former open burning site in BC village. The BPA concentrations ranged from 0.50 ng/g, also from a pond sediment in SB village, and 324.56 ng/g, also from the largest former open burning site in BC village. It is clear from Table 3 that former open burning sites and dismantling sites contained high TBBPA and BPA concentrations, and wastelands, paddy soils, vegetable soils, and pond sediments contained relatively low concentrations in all villages. TBBPA and BPA concentrations were approximately 2 orders of magnitude higher at burning sites than in agricultural fields. Both TBBPA and BPA concentrations found in the different land-use soils decreased in the following order: former open burning site > dismantling site > wasteland > paddy field = vegetable filed > pond sediment. The BPA concentrations were generally higher than the TBBPA concentrations at all of the sampling sites.
Both the TBBPA and BPA concentrations were higher in most of the soil samples from BC village than in the soil samples from the other villages. The TBBPA and BPA concentrations were also relatively high in the soil samples from YMY and BHT villages. The TBBPA and BPA concentrations in soil samples from the other villages (e.g., DG and JL villages) far from BC village were relatively low. There was a general trend that the farther from the open burning and dismantling sites and the farther from BC village the soils samples were taken, the lower the TBBPA and BPA concentrations were.
TBBPA and BPA distributions in soil profiles
As is shown in Fig. 2, both TBBPA and BPA could be detected at 0–40-cm-deep soil samples in each of the soil profiles. However, TBBPA and BPA could be detected in only a few of the 40–80-cm-deep soil samples. The TBBPA and BPA concentrations decreased sharply from top to bottom in each soil profile. TBBPA and BPA were mainly found in the 0–40-cm-deep soils, which accounted for more than 95 % of the total in each profile, and their concentrations in the 40–80-cm-deep soils were very low (<1.00 ng/g), accounting for less than 5 % of the total. The TBBPA and BPA concentrations in most of the 60–80-cm-deep soil samples were lower than the instrument LOQs. In general, as in the surface soils, the BPA concentrations were higher than the TBBPA concentrations in the 20–40-cm-deep soils in each soil profile.
Correlation between TBBPA and BPA
There were significant correlations between TBBPA and BPA concentrations in the soil samples. The Pearson correlation coefficient between the TBBPA and BPA concentrations was 0.91, which was significant at the 0.01 level (two-tailed).
Debromination of TBBPA in anoxic waterlogged paddy soil
As is shown in Fig. 3, although there were no significant differences in concentrations between dry and sterilized experiments, or between 18 and 30 days of incubation, we can compare their average values. The spiked TBBPA and initial BPA concentrations in the control samples showed almost no change after incubation. However, incubation in serum bottles for 18 days caused the TBBPA concentration to decrease by approximately 8 %, from 5.01 ± 0.24 μg/g before incubation to 4.62 ± 0.24 μg/g after 18 days of incubation. The BPA concentration increased from 4.32 ± 0.27 ng/g before incubation to 0.11 ± 0.03 μg/g after 18 days of incubation. Incubation for 30 days caused the TBBPA concentration to decrease further to 4.14 ± 0.23 μg/g and the BPA concentration to increase further to 0.23 ± 0.04 μg/g.
Reductive debromination of TBBPA in simulated waterlogged paddy soil. Average values (±standard deviations, n = 3) that have the same lowercase letters are not significantly different at P ≥ 0.05. Dry, dry soils with no water; Sterilized, autoclaved mixture of dry soils and water from the paddy field
Discussion
Sources and transport of TBBPA and BPA in the Longtang surface soils
As is shown in Table 3, the highest TBBPA and BPA concentrations were only detected in soils from former open burning sites, and the next highest concentrations were found at dismantling sites. However, relatively low TBBPA and BPA concentrations were also detected in soils of other land-use type which are still used for traditional agriculture. The dismantling sites are important point pollution sources. There are more than 1,000 workshops in the Longtang area, and large amounts of e-waste have been dismantled each day at the dismantling sites since the end of the 1980s. Valuable components were previously reclaimed using acid dipping and coal-fire baking, to melt circuit boards, and some plastic waste was burned in and around some of the recycling workshops. TBBPA and BPA can be released during dismantling activities, and the ash, dust, and e-waste residues produced have caused the high TBBPA and BPA concentrations found in the dismantling site soil samples. The former open burning sites are the other important point pollution sources. In the past, especially at the end of the last century, piles of e-waste were burned at the open burning sites, without any treatment of the fumes or residues. The thick smoke released and the ash residues produced at the open burning sites will be the reason why the highest TBBPA and BPA concentrations were found in the open burning site soil samples. It is clear that the former open burning sites and dismantling sites are still point pollution sources, even though the open burning of e-waste and acid dipping and coal-fire baking of circuit boards have been forbidden for more than 10 years.
It is clear that the TBBPA and BPA released from dismantling and open burning activities had diffused and been transported from the point pollution sources. The soils surrounding the open burning and dismantling sites usually had relatively high TBBP and BPA concentrations, but in the paddy and vegetable fields, relatively far from the point pollution sources, the TBBPA and BPA concentrations were relatively low. It is interesting that the farther the samples were taken from BC village, the lower the TBBPA and BPA concentrations in the agricultural soils were. BC village was the main point pollution source in the Longtang area, and this is where e-waste recycling activities began earlier than elsewhere in the area and where former open burning sites, including the largest open incineration plant, were mainly situated. The areas surrounding the open burning sites and dismantling sites are expected to have been contaminated not only by the dumping of waste residues but also by fly ash deposition.
Brominated flame retardants (including PBDEs), PCDD/Fs, and PBDD/Fs have previously been detected in air and in deposition around e-waste dismantling areas (Chen et al. 2009; Li et al. 2007; Tian et al. 2011, 2012). It is reasonable to suggest that TBBPA and BPA could also be deposited onto agricultural land by short- and long-range atmospheric transport from former open burning sites and dismantling sites, even though TBBPA and BPA have low volatilities. Fly ash and other suspended particulates containing TBBPA and BPA, generated at open burning sites and dismantling sites, may have led to their detectable concentrations in the paddy and vegetable soils, and even in the pond sediment samples, through transport and deposition. The mean TBBPA and BPA concentrations were lower in the pond sediments (outside the villages) than in the paddy and vegetable soils, possibly because of the ponds being drained and the sediments being dug out during the dry season. However, the mean TBBPA and BPA concentrations were higher in the wasteland soils than in the paddy and vegetable soils, which may have been caused by the dumping of waste residues at many wasteland sites.
It is well known that TBBPA can be debrominated into BPA under anoxic condition, while BPA is not biodegraded and can persist in anaerobic sediments (Ronen and Abeliovich 2000; Voordeckers et al. 2002). The Longtang area has a typical subtropical monsoon climate, with a rainy season from March to October, a mean annual temperature of approximately 21 °C, and an annual rainfall of approximately 1,700 mm. Soils in the area are often wet and anoxic. Paddy and vegetable soils are often wet or waterlogged for most of the rainy season. These conditions may promote the anaerobic reductive debromination of TBBPA. It is clear that the mean BPA concentrations were higher than the mean TBBPA concentrations in all of the land-use soils (Table 3). This may have been caused by the transformation of TBBPA in the soils. TBBPA and BPA have been widely used in electronic equipment, and both can be released during primitive dismantling activities. e-waste has been recycled in the Longtang area for more than 20 years. Some of the TBBPA may have been transformed into BPA by reductive debromination in soils. It is, therefore, possible that BPA has been accumulating in the Longtang soils, and it degrades more slowly than TBBPA, especially in waterlogged soils. This may be an important reason why the BPA concentrations were higher than the TBBPA concentrations in all of the land-use soils. In addition, according to the Pearson correlation test, the TBBPA and BPA concentrations correlated significantly. This also implies that some of the TBBPA had been degraded after its release during the e-waste recycling processes.
Transport of TBBPA and BPA in the Longtang soil profiles
The Longtang area not only has a subtropical climate, with abundant rainfall, but also has obvious alternating dry and rainy seasons, and these factors may help to explain TBBPA and BPA transport through soil profiles. As ionizable compounds, a part of TBBPA and BPA can be desorbed from soils at a pH of approximately 6.0 (Han et al. 2013; Sun et al. 2008) and then leached with water along the soil profiles. In addition, e-waste recycling activities have been carried out in the Longtang area for many years, and it is clear that the soils in the area have been greatly affected by the pollutants released during e-waste recycling. In terms of groundwater pollution, as can be seen from Fig. 2, TBBPA and BPA were mainly found in soils less than 40 cm deep (and were mostly found in the surface soils, <20 cm deep), and their concentrations in the 40–80-cm-deep soils were low (<1.00 ng/g). These results indicate that most of the TBBPA and BPA were sorbed to the soil particles but that they could be gradually transported from the surface soil to deeper soils. Plowing is a basic agricultural activity in paddy and vegetable fields in the Longtang area, and this could have enhanced the degradation of BPA because plowing can increase soil porosity, and BPA can be oxidized in aerobic environments (Ronen and Abeliovich 2000). These results imply that the effects of TBBPA and BPA on groundwater are likely to be very low. To summarize, the diffusion of TBBPA and BPA farther from the point pollution sources could be controlled if it is possible to manage the former open burning sites and dismantling sites well in the future.
Degradation of TBBPA in the waterlogged paddy soils
As can be seen from Fig. 3, the TBBPA concentrations in the spiked sterilized and dry soils showed almost no change during incubation, even though there were abundant iron and manganese oxides which can be used as sources of chemical reductants in the soils. There may be reducing materials, such as Fe(II) and reduced humic substances, in the sterilized soils, but they may have no effect on the degradation of TBBPA without microbial activity which is the driving force for the recycling of these redox species. In addition, the lack of TBBPA degradation in the dry soils indicated that water content can be a key factor governing the degradation of TBBPA in the anoxic environment in our study.
Previous studies using anoxic environments have shown that ferric respiration and humus respiration are two important types of microorganism metabolism (Coates et al. 2002; Lovley et al. 1996; Straub et al. 2001; Van Trump et al. 2006), and the electrons produced during metabolism can be finally transferred to a high valency metal or halogenated organic compound, such as Fe(III), Cr(VI), U(VI), Pb(II), 2,4-dichlorophenoxyacetic acid, or a member of the dichlorodiphenyltrichloroethane group of compounds (Li et al. 2009, 2010; Lovley et al. 2004; Wang et al. 2009). However, it is clear that the main driving force for these processes is the metabolic activity of microorganisms.
The TBBPA concentration gradually decreased in the test soils during the incubation period. In addition, as can be seen from Fig. 3, BPA was produced, and its concentration clearly increased after 18 days, further increasing up to the end of the experiment. This indicates that TBBPA in the Longtang soils could be transformed into BPA under anoxic conditions and confirms that some of the BPA found in the soils was likely to be the debromination product of TBBPA. TBBPA degradation and BPA accumulation have also been found in an anaerobic sediment slurry from a wet ephemeral desert stream bed in Israel (Ronen and Abeliovich 2000), and, according to that report, TBBPA was completely debrominated in the anaerobic slurry after 45 days, and BPA persisted and was not degraded further even after 3 months of incubation. Other studies have shown that TBBPA can be reductively debrominated, and BPA has been found to accumulate in estuarine sediments (Voordeckers et al. 2002). The degradation of TBBPA in anaerobic environments has also been shown to be enhanced by adding certain substrates, such as an enrichment culture (Arbeli and Ronen 2003), humic acid, zerovalent Fe, and vitamin B12 (Chang et al. 2012).
Fe, Mn, and organic matter are ubiquitous and abundant in Longtang agricultural soils (Table 2) and are usually considered to be redox-reactive compounds (Kappler and Haderlein 2003; Lin et al. 2009; Lovley et al. 2004), which could provide important substrate conditions for reductive debromination. Paddy fields are usually drained, plowed, and aired in the winter in South China, which would promote the degradation of BPA by creating an aerobic environment (Ronen and Abeliovich 2000). Therefore, if the pollution point sources can be controlled, and suitable management procedures can be implemented, the TBBPA and BPA contamination in the Longtang agricultural soils could be gradually ameliorated.
Conclusions
In this study, the following conclusions have been made:
-
1.
Soils in the Longtang area have been contaminated because of crude e-waste recycling activities in the area, and TBBPA and BPA were detected not only in former open burning and dismantling sites but also in agricultural fields and pond sediments. The TBBPA and BPA concentrations increased in the following order: pond sediments < paddy soils = vegetable soils < wasteland < dismantling sites < former open burning sites. TBBPA and BPA have been transported through the soil profiles at each of the study sites, including in agricultural fields.
-
2.
TBBPA and BPA found in the soils were mainly derived from the previous incomplete combustion of e-waste in open burning and dismantling sites, which were their main point sources. It is highly likely that some of the BPA was derived from the reductive debromination of TBBPA in the soils and that BPA can persist for longer than TBBPA in anoxic soils. This may be the main reason for the BPA concentrations being higher than the TBBPA concentrations in soils from all six sampling site types in the Longtang area.
References
Arbeli Z, Ronen Z (2003) Enrichment of a microbial culture capable of reductive debromination of the flame retardant tetrabromobisphenol-A, and identification of the intermediate metabolites produced in the process. Biodegradation 14:385–295
Chang B-V, Yuang S-Y, Ren Y-L (2012) Anaerobic degradation of tetrabromobisphenol-A in river sediment. Ecol Eng 49:73–76
Chen DH, Bi XH, Zhao JP, Chen LG, Tan JH, Mai BX, Sheng GY, Fu JM, Wong MH (2009) Pollution characterization and diurnal variation of PBDEs in the atmosphere of an E-waste dismantling region. Environ Pollut 157:1051–1057
Coates JD, Cole KA, Chakraborty R (2002) Diversity and ubiquity of bacteria capable of utilizing humic substances as electron donors for anaerobic respiration. Appl Environ Microb 68:2445–2452
Han W, Luo L, Zhang S (2013) Adsorption of tetrabromobisphenol A on soils: contribution of soil components and influence of soil properties. Colloid Surface A 428:60–64
Huang D-Y, Xu Y-G, Peng PA, Zhang HH, Lan JB (2009) Chemical composition and seasonal variation of acid deposition in Guangzhou, South China: comparison with precipitation in other major Chinese cities. Environ Pollut 157:35–41
Huang YQ, Wong CKC, Zheng JS, Bouwman H, Barra R, Wahlstrom B, Neretin L, Wong MH (2012) Bisphenol A (BPA) in China: a review of sources, environmental levels, and potential human health impacts. Environ Int 42:91–99
Kappler A, Haderlein SB (2003) Natural organic matter as reductant for chlorinated aliphatic pollutants. Environ Sci Technol 37:2714–2719
Leung AOW, Luksemburg WJ, Wong AS, Wong MH (2007) Spatial distribution of polybrominated diphenyl ethers and polychlorinated dibenzo-p-dioxins and dibenzofurans in soil and combusted residue at Guiyu, an electronic waste recycling site in southeast China. Environ Sci Technol 41:2730–2737
Li H, Yu L, Sheng G, Fu J, Peng P (2007) Severe PCDD/F and PBDD/F pollution in air around an electronicwaste dismantling area in China. Environ Sci Technol 41:5641–5646
Li XM, Zhou SG, Li FB, Wu CY, Zhuang L, Xu W, Liu L (2009) Fe(III) oxide reduction and carbon tetrachloride dechlorination by a newly isolated Klebsiella pneumoniae strain L17. J Appl Microbiol 106:30–139
Li FB, Li XM, Zhou SG, Zhuang L, Cao F, Huang DY, Xu W, Liu TX, Feng CH (2010) Enhanced reductive dechlorination of DDT in an anaerobic system of dissimilatory iron-reducing bacteria and iron oxide. Environ Pollut 158:1733–1740
Lin K, Liu W, Gan J (2009) Reaction of tetrabromobisphenol A (TBBPA) with manganese dioxide: kinetics, products, and pathways. Environ Sci Technol 43:4480–4486
Liu HX, Zhou QF, Wang YW, Zhang QH, Cai ZW, Jiang GB (2008) E-waste recycling induced polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins and dibenzo-furans pollution in the ambient environment. Environ Int 34:67–72
Liu J, Wang Y, Jiang B, Wang L, Chen J, Guo H, Ji R (2013) Degradation, metabolism, and bound-residue formation and release of tetrabromobisphenol A in soil during sequential anoxic-oxic incubation. Environ Sci Technol 47:8348–8354
Lovley DR, Coates JD, Blunt-Harris EL, Phillips EJP, Woodward JC (1996) Humic substances as electron acceptors for microbial respiration. Nature 382:445–448
Lovley DR, Holems DE, Nevin KP (2004) Dissimilatory Fe(III) and Mn(IV) reduction. Adv Microb Physiol 49:221–270
Luo Q, Cai ZW, Wong MH (2007) Polybrominated diphenyl ethers in fish and sediment from river polluted by electronic waste. Sci Total Environ 383:115–127
Luo Y, Luo XJ, Lin Z, Chen SJ, Liu J, Mai BX, Yang ZY (2009) Polybrominated diphenyl ethers in road and farmland soils from an e-waste recycling region in Southern China: concentrations, source profiles, and potential dispersion and deposition. Sci Total Environ 407:1105–1113
Luo C-L, Liu C-P, Wang Y, Liu X, Li F-B, Zhang G, Li X-D (2011) Heavy metal contamination in soils and vegetables near an e-waste processing site, south China. J Hazard Mater 186:481–490
Ni HG, Zeng EY (2009) Law enforcement and global collaboration are the keys to containing e-waste tsunami in China. Environ Sci Technol 43:3991–3994
Qu WY, Bi XH, Sheng GY, Lu SY, Fu H, Yuan J, Li LP (2007) Exposure to polybrominated diphenyl ethers among workers at an electronic waste dismantling region in Guangdong, China. Environ Int 33:1029–1034
Renner R (1997) European bans on surfactant trigger transatlantic debate. Environ Sci Technol 31:316A–320A
Ronen Z, Abeliovich A (2000) Anaerobic-aerobic process for microbial degradation of tetrabromobisphenol A. Appl Environ Microb 66:2372–2377
Sánchez-Brunete C, Miguel E, Tadeo JL (2009) Determination of tetrabromobisphenol-A, tetrachlorobisphenol-A and bisphenol-A in soils by ultrasonic assisted extraction and gas chromatography-mass spectrometry. J Chromatogr A 1216:5497–5503
Straub KL, Benz M, Schink B (2001) Iron metabolism in anoxic environments at near neutral pH. FEMS Microbiol Ecol 34:181–186
Suflita JM, Townsend GT (1995) The microbial ecology and physiology of aryl dehalogenation and implications for bioremediation. In: Young LY, Cerniglia CE (eds) Microbial transformation and degradation of toxic organic chemicals. Wiley, New York, pp 243–268
Sun Z, Yu Y, Mao L, Feng Z, Yu H (2008) Sorption behavior of tetrabromobiphenol A in two soils with different characteristics. J Hazard Mater 160:456–461
Tian M, Chen SJ, Wang J, LuoY LXJ, Mai BX (2011) Brominated flame retardants in the atmosphere of e-waste and rural sites in southern China: seasonal variation, temperature dependence, and gas-particle partitioning. Environ Sci Technol 45:8819–8825
Tian M, Chen SJ, Wang J, Luo Y, Luo XJ, Mai BX (2012) Plant uptake of atmospheric brominated flame retardants at e-waste site in southern China. Environ Sci Technol 46:2708–2714
Van Trump JI, Sun Y, Coates JD (2006) Microbial interactions with humic substances. Adv Appl Microbiol 60:55–96
Voordeckers JW, Fennell DE, Jones K, Häggblom MM (2002) Anaerobic biotransformation of tetrabromobisphenol A, tetrachlorobisphenol A, and bisphenol A in estuarine sediments. Environ Sci Technol 36:696–701
Wang YB, Wu CY, Wang XJ, Zhou SG (2009) The role of humic substances in the anaerobic reductive dechlorination of 2,4-dichlorophenoxyacetic acid by Comamonas koreensis strain CY01. J Hazard Mater 164:941–947
Wang Y, Luo C-L, Li J, Yin H, Li X-D, Zhang G (2011) Characterization of PBDEs in soils and vegetations near an e-waste recycling site in South China. Environ Pollut 159:2443–2448
Wiegel J, Wu Q (2000) Microbial reductive dehalogenation of polychlorinated biphenyls. FEMS Microb Ecol 32:1–15
Wong MH, Wu SC, Deng WJ, Yu XZ, Luo Q, Leung AOW, Wong CSC, Luksemburg WJ, Wong AS (2007) Export of toxic chemicals—a review of the case of uncontrolled electronic-waste recycling. Environ Pollut 149:131–140
Wu JP, Luo XJ, Zhang Y, Luo Y, Chen SJ, Mai BX, Yang ZY (2008) Bioaccumulation of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in wild aquatic species from an electronic waste (e-waste) recycling site in South China. Environ Int 34:1109–1113
Zhao XR, Qin ZF, Yang ZZ, Zhao Q, Zhao YX, Qin XF, Zhang YC, Ruan XL, Zhang YF, Xu XB (2009) Dual body burdens of polychlorinated biphenyls and polybrominated diphenyl ethers among local residents in an e-waste recycling region in Southeast China. Chemosphere 78:659–666
Acknowledgments
This study was supported by the National Natural Science Foundation of China (NSFC, no. 21277034), the NSFC-Guangdong Province Science Foundation (no. U1133004), the Guangdong Natural Science Foundation (nos. S2011010005901 and S2012010010128), and the Guangzhou City Science-Technology Project (no. 2013 J4100063).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Leif Kronberg
Rights and permissions
About this article
Cite this article
Huang, DY., Zhao, HQ., Liu, CP. et al. Characteristics, sources, and transport of tetrabromobisphenol A and bisphenol A in soils from a typical e-waste recycling area in South China. Environ Sci Pollut Res 21, 5818–5826 (2014). https://doi.org/10.1007/s11356-014-2535-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2535-2