Abstract
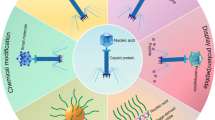
Successful delivery of nucleic acid therapeutics to diseased sites would present a pivotal advancement in cancer treatment. However, progress has been hindered by the lack of efficient tumor-selective vectors via clinical systemic routes, the blood–brain barrier for brain tumors and problems with repeated administrations. We present a new generation of M13 phage-based vectors termed transmorphic phage/adeno-associated virus (AAV) (TPA), wherein the phage genome has been excised to facilitate exclusive packaging of human AAV DNA by phage coat proteins. Here we provide a detailed protocol for the molecular cloning of DNA into the TPA construct, display of disease-specific ligands on the helper phage capsid for cell targeting and entry, and packaging of TPA DNA by helper phage coat proteins in a bacterial host. Furthermore, we provide methods for mammalian cell transduction and assessment of transgene expression in vitro as well as in vivo application of TPA particles in tumor-bearing mice. Unlike other similar methods, our protocol enables high-yield production and control of helper phage quantity in TPA preparations. Moreover, compared with existing M13 phage vectors, TPA particles can accommodate large size transgene inserts, despite being considerably more compact, providing superior gene delivery through enhanced diffusion across the extracellular matrix, improved cellular binding and entry and increased vector DNA accumulation in the nucleus. The protocol encompasses a timeline of 4–5 months, including construction and production of TPA particles with transgene and targeted ligand and in vitro/in vivo testing. This protocol can be conducted by researchers trained in basic molecular biology/bacteriology research techniques.
Key points
-
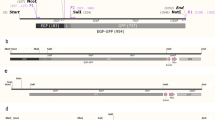
This protocol describes the construction and production of transmorphic phage/adeno-associated virus particles for the targeted delivery of nucleic acid payloads, in vitro and in vivo.
-
Transmorphic phage/adeno-associated virus particles ensure effective and safe delivery of nucleic acids (for example, luciferase, green fluorescent protein or cytokine genes) to solid tumors, thanks to their greater diffusion across the extracellular matrix and improved internalization and intracellular trafficking to the nucleus of target cells.







Similar content being viewed by others
References
Edelstein, M. L., Abedi, M. R. & Wixon, J. Gene therapy clinical trials worldwide to 2007—an update. J. Gene Med. 9, 833–42 (2007).
Ginn, S. L. et al. Gene therapy clinical trials worldwide to 2017: an update. J. Gene Med. 20, e3015 (2018).
Pranjol, M. Z. & Hajitou, A. Bacteriophage-derived vectors for targeted cancer gene therapy. Viruses. 7, 268–84 (2015).
Bouard, D., Alazard-Dany, D. & Cosset, F. L. Viral vectors: from virology to transgene expression. Br. J. Pharmacol. 157, 153–65 (2009).
Nayak, S. & Herzog, R. W. Progress and prospects: immune responses to viral vectors. Gene Ther. 17, 295–304 (2010).
Larocca, D. et al. Evolving phage vectors for cell targeted gene delivery. Curr. Pharm. Biotechnol. 3, 45–57 (2002).
Smith, G. P. & Petrenko, V. A. Phage display. Chem. Rev. 97, 391–410 (1997).
Barderas, R. & Benito-Peña, E. The 2018 Nobel Prize in Chemistry: phage display of peptides and antibodies. Anal. Bioanal. Chem. 411, 2475–2479 (2019).
Stoneham, C. A., Hollinshead, M. & Hajitou, A. Clathrin-mediated endocytosis and subsequent endo-lysosomal trafficking of adeno-associated virus/phage. J. Biol. Chem. 287, 35849–59 (2012).
Endersen, L. et al. Phage therapy in the food industry. Annu. Rev. Food Sci. Technol. 5, 327–49 (2014).
Asavarut, P. & Hajitou, A. The phage revolution against antibiotic resistance. Lancet Infect. Dis. 14, 686 (2014).
Hajitou, A. et al. A hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell 125, 385–98 (2006).
Hajitou, A. et al. Design and construction of targeted AAVP vectors for mammalian cell transduction. Nat. Protoc. 2, 523–31 (2007).
Koivunen, E., Wang, B. & Ruoslahti, E. Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD-directed integrins. Nat Biotechnol 13, 265–270 (1995).
Arap, W., Pasqualini, R. & Ruoslahti, E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 279, 377–80 (1998).
Brooks, P. C., Clark, R. A. & Cheresh, D. A. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 264, 569–71 (1994).
Przystal, J. M. et al. Efficacy of systemic temozolomide-activated phage-targeted gene therapy in human glioblastoma. EMBO Mol. Med. 11, e8492 (2019).
Paoloni, M. C. et al. Launching a novel preclinical infrastructure: comparative oncology trials consortium directed therapeutic targeting of TNFalpha to cancer vasculature. PLoS ONE. 4, e4972 (2009).
Suwan, K. et al. Next-generation of targeted AAVP vectors for systemic transgene delivery against cancer. Proc. Natl Acad. Sci. USA 116, 18571–18577 (2019).
Przystal, J. M. et al. Proteasome inhibition in cancer is associated with enhanced tumor targeting by the adeno-associated virus/phage. Mol. Oncol. 7, 55–66 (2013).
Kia, A. et al. Inhibition of histone deacetylation and DNA methylation improves gene expression mediated by the adeno-associated virus/phage in cancer cells. Viruses. 5, 2561–72 (2013).
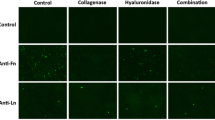
Yata, T. et al. Modulation of extracellular matrix in cancer is associated with enhanced tumor cell targeting by bacteriophage vectors. Mol. Cancer. 14, 110 (2015).
Yata, T. et al. Hybrid nanomaterial complexes for advanced phage-guided gene delivery. Mol. Ther. Nucleic Acids. 3, e185 (2014).
Tsafa, E. et al. Doxorubicin improves cancer cell targeting by filamentous phage gene delivery vectors. Int. J. Mol. Sci. 21, 7867 (2020).
Tandle, A. et al. Tumor vasculature-targeted delivery of tumor necrosis factor-alpha. Cancer. 115, 128–39 (2009).
Asavarut, P. et al. Systemically targeted cancer immunotherapy and gene delivery using transmorphic particles. EMBO Mol. Med. 14, e15418 (2022).
Larocca, D. et al. Receptor-targeted gene delivery using multivalent phagemid particles. Mol. Ther. 3, 476–84 (2001).
Monjezi, R. et al. Purification of bacteriophage M13 by anion exchange chromatography. J. Chromatogr. B 878, 1855–9 (2010).
Chongchai, A. et al. Targeting human osteoarthritic chondrocytes with ligand directed bacteriophage-based particles. Viruses. 13, 2343 (2021).
Zhu, J. et al. Preparation of a bacteriophage T4-based prokaryotic–eukaryotic hybrid viral vector for delivery of large cargos of genes and proteins into human cells. Bio. Protoc. 10, e3573 (2020).
Zhu, J. et al. Design of bacteriophage T4-based artificial viral vectors for human genome remodeling. Nat. Commun. 14, 2928 (2023).
Zhu, J. et al. A prokaryotic-eukaryotic hybrid viral vector for delivery of large cargos of genes and proteins into human cells. Sci. Adv. 5, eaax0064 (2019).
Al-Bahrani, M. et al. Transmorphic phage-guided systemic delivery of TNFα gene for the treatment of human pediatric medulloblastoma. FASEB J. 37, e23038 (2023).
Chongchai, A. et al. Bacteriophage-mediated therapy of chondrosarcoma by selective delivery of the tumor necrosis factor alpha (TNFα) gene. FASEB J. 35, e21487 (2021).
Li, Y. et al. Harnessing filamentous phages for enhanced stroke recovery. Front Immunol. 14, 1343788 (2024).
González-Mora, A. et al. Bacteriophage-based vaccines: a potent approach for antigen delivery. Vaccines (Basel) 8, 504 (2020).
Chang, C. et al. Engineered M13 phage as a novel therapeutic bionanomaterial for clinical applications: From tissue regeneration to cancer therapy. Mater Today Bio. 20, 100612 (2023).
Arap, W. et al. Steps toward mapping the human vasculature by phage display. Nat. Med. 8, 121–127 (2002).
Hajitou, A., Pasqualini, R. & Arap, W. Vascular targeting: recent advances and therapeutic perspectives. Trends Cardiovasc. Med. 16, 80–88 (2006).
Zhou, J. Y., Suwan, K. & Hajitou, A. Initial steps for the development of a phage-mediated gene replacement therapy using CRISPR–Cas9 technology. J Clin Med. 9, 1498 (2020).
Krag, D. N. et al. Selection of tumor-binding ligands in cancer patients with phage display libraries. Cancer Res. 66, 7724–33 (2006).
Jurač, K., Nabergoj, D. & Podgornik, A. Bacteriophage production processes. Appl. Microbiol. Biotechnol. 103, 685–694 (2019).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Danielak, D. et al. A novel open source tool for ELISA result analysis. J. Pharm. Biomed. Anal. 189, 113415 (2020).
Acknowledgements
We thank the UK Medical Research Council for their support with a grant MR/T029226/1, Cancer Research UK for the C31277/A25887 award, Brain Research UK for the 202021-34 award and Children with Cancer UK for the 16-230 grant. We also thank P. Asavarut, S. Waramit, P. Vila-Gomez and T. Yata at Imperial College London. Source data are provided with this paper.
Author information
Authors and Affiliations
Contributions
L.G., K.S. and A.H. designed the methods and analyses, and performed experiments. A.H. founded, designed and supervised the whole study, and secured research funding. All authors wrote and edited the manuscript, and approved the protocol.
Corresponding author
Ethics declarations
Competing interests
K.S. and A.H. are inventors on patent applications describing the vector constructs described here.
Peer review
Peer review information
Nature Protocols thanks Alfonso Jaramillo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Asavarut, P. et al. EMBO Mol. Med. 14, e15418 (2022): https://doi.org/10.15252/emmm.202115418
Al-Bahrani, M. et al. FASEB J. 37, e23038 (2023): https://doi.org/10.1096/fj.202300045R
Chongchai, A. et al. FASEB J. 35, e21487 (2021): https://doi.org/10.1096/fj.202002539R
Supplementary information
Source data
Source Data Figs. 4, 6a and 7b,d–f
. Statistical source data for Figs. 4, 6a and 7b,d–f.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gay, L., Suwan, K. & Hajitou, A. Construction and utilization of a new generation of bacteriophage-based particles, or TPA, for guided systemic delivery of nucleic acids to tumors. Nat Protoc (2024). https://doi.org/10.1038/s41596-024-01040-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41596-024-01040-9
- Springer Nature Limited