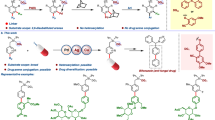
Abstract
Although aromatic rings are common elements in pharmaceutically active compounds, the presence of these motifs brings several liabilities with respect to the developability of a drug1. Nonoptimal potency, metabolic stability, solubility and lipophilicity in pharmaceutical compounds can be improved by replacing aromatic rings with non-aromatic isosteric motifs2. Moreover, whereas aromatic rings are planar and lack three-dimensionality, the binding pockets of most pharmaceutical targets are chiral. Thus, the stereochemical configuration of the isosteric replacements may offer an added opportunity to improve the affinity of derived ligands for target receptors. A notable impediment to this approach is the lack of simple and scalable catalytic enantioselective syntheses of candidate isosteres from readily available precursors. Here we present a previously unknown palladium-catalysed reaction that converts hydrocarbon-derived precursors to chiral boron-containing nortricyclanes and we show that the shape of these nortricyclanes makes them plausible isosteres for meta disubstituted aromatic rings. With chiral catalysts, the Pd-catalysed reaction can be accomplished in an enantioselective fashion and subsequent transformation of the boron group provides access to a broad array of structures. We also show that the incorporation of nortricyclanes into pharmaceutical motifs can result in improved biophysical properties along with stereochemistry-dependent activity. We anticipate that these features, coupled with the simple, inexpensive synthesis of the functionalized nortricyclane scaffold, will render this platform a useful foundation for the assembly of new biologically active agents.
Similar content being viewed by others
Main
Bioactive compounds, from natural products to clinically relevant active ingredients, often contain aromatic rings. The reasons for incorporation of this motif are multifold1: (1) the rigidity of aromatic rings often restricts available molecular conformations thereby lessening the entropic cost for the aromatic ligand to bind to a target receptor; (2) aromatic rings participate in unique non-covalent interactions that can provide enhanced attraction to receptor binding pockets; and (3) the methods used to attach substituents to aromatic rings include efficient cross-coupling reactions3 that facilitate construction and screening of diverse compound collections. These notable benefits are counter-balanced by the ready oxidation of π-electron-rich aromatic rings that can severely diminish metabolic stability4. Moreover, relative to their C(sp3) counterparts, aromatic molecules have decreased aqueous solubility5 and increased log D (ref. 6). A particular challenge arising from the increased lipophilicity of an aromatic compound is that this feature increases off-target promiscuity7 that is problematic for drug development. To address this limitation, bicyclic hydrocarbon frameworks have been advanced as C(sp3)-based isosteric replacements for aromatic rings8, with the expectation that the saturated bicyclic analogues might offer enhanced biophyscial properties while retaining biological activity9. When appropriately designed, these molecular scaffolds can retain the conformational restriction of the benzenoid ring while simultaneously avoiding associated liabilities. Following this model, an array of small bicyclic motifs have been developed that position substituents at a similar distance and with similar exit vectors compared with substituted arenes, with selected examples of meta benzene isosteres shown in Fig. 1a (refs. 10,11,12,13,14,15,16,17,18,19,20,21,22).
In the context of ligand development, two important features arise when planar C(sp2)-based aromatic rings are replaced with C(sp3)-based bicyclic isosteres. The first is that of retaining the efficient, reliable, modular and scalable synthesis techniques that accompany modern catalytic cross-coupling methods. The second issue is that of three-dimensional shape. Even if the isostere positions its substituents in a manner that would superimpose them onto those of an aromatic nucleus, the tetrahedral nature of the saturated carbon centres in the isostere automatically results in a three-dimensionality that is absent from the flat aromatic ring system. As the preponderance of biologically relevant receptors are chiral, the handedness of a nonsymmetric isostere may either enhance or impede the binding of the derived ligand to its cognate receptor18,23. The way MK-5108 binds to the inactive DFG-out state of the mitotic kinase Aurora A is illustrative24,25. When bound, the meta disubstituted arene in MK-5108 is sandwiched in a hydrophobic pocket with Leu139 positioned 3.9 Å above the arene (carbon-to-carbon distance) and Gly216 3.5 Å below (Fig. 1b). Thus, when a nonsymmetric benzene isostere occupies this binding pocket, it would be expected to fit better if it occupies more volume in the upper region of the receptor rather than the lower. Such a lock-and-key fit of ligands to their complementary receptors can result in enhanced specificity and ligand promiscuity has been shown to diminish as the number of chiral centres in a compound increases7. From this vantage point, it is clear that methods are needed to control the absolute configuration of the overall molecular ensemble. In this report, we describe a reaction that retains all the practical advantages of contemporary cross-coupling and that allows conversion of simple starting materials into enantiomerically enriched 3,5-disubstituted nortricyclane frameworks (Fig. 1c). Of note, comparison of the metrical parameters of the nortricyclane with those of benzene show that carbons 3 and 5 are equidistant with the meta carbons of benzene. Moreover, the exit vectors for connection to substituents at carbons 3 and 5 have an angle between them that is nearly the same as in benzene (127° versus 120°), and the vectors are essentially co-planar as in the aromatic framework. Last, the process that we describe for the production of 3,5-disubstituted nortricyclanes occurs by a heretofore unknown catalytic reaction. This reaction occurs with low loadings of a chiral palladium complex and delivers malleable boron-containing isosteres, holding marked potential for further modification.
a, Selected recent examples of meta benzene isosteres. b, The kinase inhibitor MK-5108 bound to the chiral binding pocket in the kinase domain of AurA. c, Structural comparison of a meta disubstituted arene and a 3,5-disubstituted nortricyclane. d, Metal-promoted reactions for the construction of nortricyclanes from simple bicycloheptane precursors. pin, pinacolato group.
The bicyclic norbornadiene skeleton (1; Fig. 1d) has provided an instructive framework for studying the nature of non-classical carbocations and for probing the ability of homoconjugation to stabilize organic structures and influence the course of their chemical reactions26,27. Owing to the through-space interaction between the two π-bonds, the conversion of the bicyclo[2.2.1] framework to nortricyclanes (that is, 2) readily occurs on the addition of suitable electrophilic reagents28. Similar electrophilic activation of bicyclic organometallics (that is, 3) can result in ring-closing C–C bond formation to furnish the nortricyclane skeleton (4) (ref. 29). In contrast to these stoichiometric reactions, we herein demonstrate that a catalytic synthesis of nortricyclanes operates when the mercury salts used in the synthesis of 2 and 4 are replaced with substoichiometric quantities of palladium complexes in conjunction with an organic electrophile. The reaction that occurs results in ring closure with the concomitant addition of the organic electrophile to the bicyclic framework. An important finding for the application of this reaction to the modular construction of enantiomerically enriched meta benzene isosteres is that the cyclization occurs with meso diboron substrate 5, a compound that is shelf-stable and readily available on a multigram scale. Reaction of 5 delivers a nortricyclane 6 that retains one boronic ester functional group, apart from the newly appended organic substituent (E). After optimization of the reaction (conditions, ligand structure), it was found that a wide array of enantiomerically enriched boron-containing nortricyclanes can be prepared in excellent yield and enantioselectivity.
Development of the Pd-catalysed reaction
In the presence of catalytic quantities of Pd(OAc)2 and chiral biarylmonophosphine ligand L1 (ref. 30) (Fig. 2), diboron substrate 5 and organic electrophiles engage in catalytic enantioselective ring closure to nortricyclanes. With simple substituted aryl bromides, the reaction occurs in high yield and enantioselectivity. The products from both electron-deficient (11, 12) and electron-rich (13, 15) electrophiles can be readily obtained, although selectivity is diminished with a strong electron-withdrawing nitroarene electrophile (16). Also of note, heterocyclic electrophiles were found to engage in the reaction (products 20–23). With respect to practical features, it merits mention that unprotected anilines and phenols do not seem to interfere with the process and that extension to non-aromatic electrophiles (24, 25) seems promising. Practical application of the nortricyclane synthesis necessitates procedures that operate on a large scale as well as functional group transformations that effectively replace the remaining boronic ester in the catalytic reaction product. With regards to the first aspect, it was found that the bis(boronate) substrate 5 could be prepared on a multigram scale directly from inexpensive commercially available norbornadiene and B2(pin)2 (Fig. 2b, (1)). The reaction of 5 could be conducted on a 5 mmol scale and, so long as the reaction time is extended to 24 h, could be accomplished in good yield with only 0.5 mol% of palladium complex (Fig. 2b, (2)). As the transformations in Fig. 2c indicate, the boronic ester in product 7 can be replaced stereospecifically by Zn-based catalytic cross-coupling31 (26), Cu-catalysed allylation and carboxylation32 (27 and 28), direct amination33 (29), homologation34 (30) or conversion to the derived alcohol (31).
a, Enantioselective coupling of 5 with electrophiles. Yields (y) are isolated yields of the purified products. The enantiomeric ratio (e.r.) was determined by supercritical fluid chromatography. Product 21 required 5 mol% catalyst; product 25 from cinnamyl chloride. Absolute configuration determined by X-ray analysis of compound 20. Bn, benzyl; Ph, phenyl; PMP, para-methoxyphenyl; r.t., room temperature. b, Single-step multigram-scale synthesis of meso bis(boronate) substrate 5 and its conversion to 7. The prices shown are from Ambeed for B2(pin)2 and Sigma-Aldrich for norbornadiene. c, Transformations of boron-containing nortricyclane 7. Conditions: a: t-BuLi then Zn(OAc)2; G3-PdCPhos, Ar-Br. b: t-BuLi, then 20 mol% CuCN, 2,3-dichloropropene. c: t-BuLi, then 20 mol% CuCN, methyl chloroformate. d: MeONH2, n-BuLi; then Boc2O. e: n-BuLi, CH2BrCl. f: NaOH, H2O2.
Study of reaction mechanism
Mechanistic experiments provide insight into a likely pathway that leads to the product from substrate-derived cyclic ate complex 32 (Fig. 3a), a compound that has precedent from previous studies in our lab35,36. The density functional theory calculations were used to determine the free energy profile of reaction paths originating from a palladium–olefin complex (GS0) that would be obtained by oxidative addition between LPd(0) and an electrophile, followed by association with 32. In one route (path A, red), carbopalladation of the alkene (TS1a) delivers Pd(II) intermediate GS1a, which might then undergo an uncommon displacement of palladium(II) (refs. 37,38) by TS2a in which the organoboron serves as a nucleophile to simultaneously expel and reduce the metal. Alternatively (path B, blue), on binding to Pd(II), the alkene may become sufficiently electrophilic that nucleopalladation39 by TS1b may generate GS1b, with direct reductive elimination then delivering the product. Calculations indicate that the carbopalladation pathway is lower in energy, with the olefin migratory insertion to give GS1a occurring by a much lower barrier compared with the nucleopalladation step (0.7 kcal mol−1 versus 18.7 kcal mol−1). From GS1a, dissociation of bromide allows the ring-closing reductive displacement of Pd(II) to occur with an accessible 19.4 kcal mol−1 barrier by TS2a (Fig. 3c). Of note, calculation of the 13C kinetic isotope effects that would arise from TS1a (ref. 40) matches well with those determined experimentally by the Singleton method41 (Fig. 3b). Most notably, palladium-catalysed coupling of 5 exhibits a negligible KIE at C2, which is consistent with the carbopalladation-based mechanism but not the nucleopalladation pathway. Further mechanistic evidence in support of the carbopalladation pathway can be found in the Supplementary Information. According to these experiments, the carbopalladation step is enantio-determining and computational studies were undertaken to elucidate the origin of enantioinduction. As shown in Fig. 3d, the calculated structure of the transition state leading to the minor product stereoisomer places the CH2 bridge of the bicycloheptane motif directly under the sterically encumbered anthracene ring system, whereas the conformation of the transition state for the major isomer avoids this interaction.
a, Calculated ground states and transition states for a carbopalladation-based mechanism (path A, red) and a nucleopalladation-based mechanism (path B, blue) for the Pd-catalysed synthesis of nortricyclanes. Methods: B3PW91(D3)/def2-TZVP/def2-TZVPP(Pd)/smd(THF)//B3LYP(D3BJ)/def2-SVP/def2-TZVP(Pd). b, Selected 13C kinetic isotope effects as determined by the natural abundance isotope method of ref. 41. c, Calculated transition state geometry for TS2a. d, Calculated stereochemistry-determining transition states leading to the major and minor enantiomers of carbopalladation product. ΔG‡, energy difference between the transition state and the reactants.
Biophysical and biochemical properties
To determine whether nortricyclane frameworks are suited for use in bioactive molecules, both enantiomers of 33 were prepared as chiral isosteric analogues of the fatty acid amide hydrolase (FAAH) inhibitor URB597 (refs. 42,43) and subjected to analysis (Fig. 4a). Of note, although the isosteric analogues have similar molecular weight as URB597, they have 10-fold increased solubility in the aqueous buffer while maintaining comparable lipophilicity (α log D). Apart from these positive attributes, the isosteric analogues exhibit measurably increased metabolic stability relative to URB597 as determined by mouse liver microsomal assay. To probe for the effect of chirality on biological activity, compounds (R,S)-33 and (S,R)-33 were compared by quantitative activity-based protein profiling experiments (for complete details, see the Supplementary Information). Mouse-brain homogenates were treated with varying concentrations of each compound before exposure to the serine hydrolase-directed activity-based probe fluorophosphonate (FP)-biotin. FP-labelled proteins were enriched on streptavidin beads and analysed by quantitative tandem mass tagging (TMT)-based mass spectrometry44. Of note, although (R,S)-33 and (S,R)-33 were not as potent as URB597, the isosteric analogues retained the ability to inhibit FAAH and show different potencies: (R,S)-33 exhibits an IC50 of 0.50 μM, whereas that for (S,R)-33 is more than 4 μM. Furthermore, the incorporation of the isosteres did not alter the proteome-wide selectivity of URB597, as both isosteres maintained high selectivity for FAAH over 23 other detected serine hydrolases.
a, Analysis of the FAAH inhibitor URB597 and analogues (R,S)-33 and (S,R)-33. b, Analysis of sonidegib and analogues (S,R)-34 and (R,S)-34. aDetermined in PBS buffer at pH = 7.4 after 24 h. bDefined as log D at pH = 7.4; determined by HPLC analysis. cDetermined at 30 min in the presence of male CD-1 mouse liver microscomes with NADPH. dInhibition of FAAH determined by quantitative mass spectrometry profiling. See the Supplementary Information for a complete dataset. eInhibition of Hedgehog signalling in Shh-LIGHT2 cells. Data are an average of five replicates.
In a second comparative analysis, the enantiomeric tricyclanes (S,R)-34 and (R,S)-34 were prepared as analogues of the Hedgehog (Hh) pathway45 inhibitor, sonidegib46 (Fig. 4b). Aberrant Hedgehog signalling has been implicated in certain types of cancer, and sonidegib inhibits activation of this pathway by interacting with the associated signal transducer, Smoothened (SMO). Comparison of stereoisomeric isosteres 34 was conducted by three independent IC50 determinations using a dual luciferase assay in Shh-LIGHT2 cells47, and it was found that the enantiomeric sonidegib analogues inhibit Hh signalling with submicromolar potencies (IC50 = 0.50 μM and 0.20 μM for the (S,R)-34 and (R,S)-34, respectively), and with a modest, at best (P = 0.26), difference in activity for the two stereoisomers. Overall, the preliminary analysis of the nortricyclane isosteres is in line with expectations: replacement of arenes with C(sp3)-based isosteres results in a 10-fold to 50-fold improvement in solubility, probably as a result of increased solvation of the C(sp3) analogues as suggested in refs. 48,49. Of note, the stereochemistry-dependent activity of nortricyclane isosteres (33 and 34) seems to reinforce the notion that shape matters when binding to biological targets, and it is notable that differences in activity are detected even with isosteric analogues that have not been reoptimized to bind their target receptors. We can anticipate that as chiral ligands are fine-tuned for affinity, the absolute stereochemistry of the isostere is likely to become increasingly important.
In summary, catalytic enantioselective nortricyclane synthesis provides an efficient entry into chiral meta benzene bioisosteres, and the chirality of the tricyclic motif plays a part in the shape-dependent binding of the derived ligands with their target receptors. The ease and robustness of the catalytic synthesis, combined with the biophysical properties of nortricyclanes should provide opportunities for pharmaceutical development. Although further exploration of the tricycloheptane skeleton seems warranted, further study of the unique catalytic process that leads to its formation—especially the C–C bond-forming reductive displacement of Pd(II)—may provide new strategies for reaction development.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Crystal structure data for compound 20 have been deposited at the Cambridge Structure Data Centre (CCDC 2325329). Mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium through the PRIDE50 partner repository with the dataset identifier PXD051400. All other data are available in the main text or in the Supplementary Information.
References
Salomen, L. M., Ellermann, M. & Diederich, F. Aromatic rings in chemical and biological recognition: energetics and structures. Angew. Chem. Int. Edn 50, 4808–4842 (2011).
Locke, G. M., Bernhard, S. S. R. & Senge, M. O. Nonconjugated hydrocarbons as rigid‐linear motifs: isosteres for material sciences and bioorganic and medicinal chemistry. Chem. Eur. J. 25, 4590–4647 (2019).
Nishihara, Y. (ed.) Applied Cross-Coupling Reactions Vol. 80 (Springer, 2013).
Dalvie, D., Nair, S., Kang, P. & Loi., C.-M. in Metabolism, Pharmacokinetics and Toxicity of Functional Groups (ed. Smith, D. A.) 275–327 (Royal Society of Chemistry, 2010).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Ritchie, T. J. & Macdonald, S. F. J. The impact of aromatic ring count on compound developability—are too many aromatic rings a liability in drug design? Drug Discov. Today 14, 1011–1020 (2009).
Lovering, F. Escape from Flatland 2: complexity and promiscuity. Med. Chem. Commun. 4, 515–519 (2013).
Mykhailiuk, P. K. Saturated bioisosteres of benzene: where to go next? Org. Biomol. Chem. 17, 2839–2849 (2019).
Stepan, F. F. et al. Application of the bicyclo[1.1.1]pentane motif as a nonclassical phenyl ring bioisostere in the design of a potent and orally active γ-secretase inhibitor. J. Med. Chem. 55, 3414–3424 (2012).
Frank, N. et al. Synthesis of meta-substituted arene bioisosteres from [3.1.1]propellane. Nature 611, 721–726 (2022).
Iida, T. et al. Practical and facile access to bicyclo[3.1.1]heptanes: potent bioisosteres of meta-substituted benzenes. J. Am. Chem. Soc. 144, 21848–21852 (2022).
Wiesenfeldt, M. P. et al. General access to cubanes as benzene bioisosteres. Nature 618, 513–518 (2023).
Kazi, N., Aublette, M. C., Allinson, S. L. & Coote, S. C. A practical synthesis of 1,3-disubstituted cubane derivatives. Chem. Commun. 59, 7971–7973 (2023).
Yang, Y. et al. An intramolecular coupling approach to alkyl bioisosteres for the synthesis of multisubstituted bicycloalkyl boronates. Nat. Chem. 13, 950–955 (2021).
Rigotti, T. & Bach, T. Bicyclo[2.1.1]hexanes by visible light-driven intramolecular crossed [2 + 2] photocycloadditions. Org. Lett. 24, 8821–8825 (2022).
Levterov, V. V., Panasyuk, Y., Pivnytska, V. O. & Mykhailiuk, P. K. Water‐soluble non‐classical benzene mimetics. Angew. Chem. Int. Edn 59, 7161–7167 (2020).
Levterov, V. V. et al. 2‐Oxabicyclo[2.1.1]hexanes: synthesis, properties, and validation as bioisosteres of ortho‐ and meta‐benzenes. Angew. Chem. Int. Edn 63, e202319831 (2024).
Zhao, J.-X. et al. 1,2-Difunctionalized bicyclo[1.1.1]pentanes: long–sought-after mimetics for ortho/meta-substituted arenes. Proc. Natl Acad. Sci. USA 118, e2108881118 (2021).
Epplin, R. C. et al. [2]-Ladderanes as isosteres for meta-substituted aromatic rings and rigidified cyclohexanes. Nat. Commun. 13, 6056 (2022).
Smith, E. et al. Silver(I)-catalyzed synthesis of cuneanes from cubanes and their investigation as isosteres. J. Am. Chem. Soc. 145, 16365–16373 (2023).
Son, J.-Y. et al. Exploring cuneanes as potential benzene isosteres and energetic materials: scope and mechanistic investigations into regioselective rearrangements from cubanes. J. Am. Chem. Soc. 145, 16355–16364 (2023).
Fujiwara, K. et al. Biological evaluation of isosteric applicability of 1,3-substituted cuneanes as m-substituted benzenes enabled by selective isomerization of 1,4-substituted cubanes. Chem. Eur. J. 30, e202303548 (2023).
Nguyen, L. A., He, H. & Pham-Huy, C. Chiral drugs: an overview. Int. J. Biomed. Sci. 2, 85–100 (2006).
de Groot, C. O. et al. A cell biologist’s field guide to aurora kinase inhibitors. Front. Oncol. 5, 285 (2015).
Lake, E. W. et al. Quantitative conformational profiling of kinase inhibitors reveals origins of selectivity for Aurora kinase activation states. Proc. Natl Acad. Sci. USA 115, E11894–E11903 (2018).
Martin, H.-D. & Mayer, B. Proximity effects in organic chemistry—the photoelectron spectroscopic investigation of non-bonding and transannular interactions. Angew. Chem. Int. Edn 22, 283–314 (1983).
Houk, K. N. et al. Ionization potentials, electron affinities, and molecular orbitals of 2-substituted norbornadienes. Theory of 1,2 and homo-1,4 carbene cycloaddition selectivities. J. Am. Chem. Soc. 105, 5563–5569 (1983).
Winstein, S. & Shatavsky, M. 2,6-Homoconjugate addition to bicycloheptadiene. Chem. Ind. 1956, 56–57 (1956).
Matteson, D. S. & Waldbillig, J. O. A preferred inversion in an electrophilic displacement: mercurideboronation of exo- and endo-5-norbornene-2-boronic acids. J. Am. Chem. Soc. 85, 1019–1020 (1963).
Tang, W. et al. Efficient monophosphorus ligands for palladium-catalyzed Miyaura borylation. Org. Lett. 13, 1366–1369 (2011).
Liang, H. & Morken, J. P. Stereospecific transformations of alkylboronic esters enabled by direct boron-to-zinc transmetalation. J. Am. Chem. Soc. 145, 9976–9981 (2023).
Xu, N., Liang, H. & Morken, J. P. Copper-catalyzed stereospecific transformations of alkylboronic esters. J. Am. Chem. Soc. 144, 11546–11552 (2022).
Mlynarski, S. N., Karns, A. S. & Morken, J. P. Direct stereospecific amination of alkyl and aryl pinacol boronates. J. Am. Chem. Soc. 134, 16449–16451 (2012).
Sadhu, K. M. & Matteson, D. S. (Chloromethyl)lithium: efficient generation and capture by boronic esters and a simple preparation of diisopropyl (chloromethyl)boronate. Organometallics 4, 1687–1689 (1985).
Xu, N., Kong, Z., Wang, J. Z., Lovinger, G. J. & Morken, J. P. Copper-catalyzed coupling of alkyl vicinal bis(boronic esters) to an array of electrophiles. J. Am. Chem. Soc. 144, 17815–17823 (2022).
Zhang, M., Lee, P. S., Allais, C., Singer, R. A. & Morken, J. P. Desymmetrization of vicinal bis(boronic) esters by enantioselective Suzuki–Miyaura cross-coupling reaction. J. Am. Chem. Soc. 145, 8308–8313 (2023).
Chen, C., Hou, L., Cheng, M., Su, J. & Tong, X. Palladium(0)‐catalyzed iminohalogenation of alkenes: synthesis of 2‐halomethyl dihydropyrroles and mechanistic insights into the alkyl halide bond formation. Angew. Chem. Int. Edn 54, 3092–3096 (2015).
Chen, X. et al. Pd(0)-catalyzed asymmetric carbohalogenation: H-bonding-driven C(sp3)–halogen reductive elimination under mild conditions. J. Am. Chem. Soc. 143, 1924–1931 (2021).
McDonald, R. I., Liu, G. & Stahl, S. S. Palladium(II)-catalyzed alkene functionalization via nucleopalladation: stereochemical pathways and enantioselective catalytic applications. Chem. Rev. 111, 2981–3019 (2011).
Saunders, M., Laidig, K. E. & Wolfsberg, M. Theoretical calculation of equilibrium isotope effects using ab initio force constants: application to NMR isotope perturbation studies. J. Am. Chem. Soc. 111, 8989–8994 (1989).
Franz, D. E., Singleton, D. A. & Snyder, J. P. 13C kinetic isotope effects for the addition of lithium dibutylcuprate to cyclohexenone. Reductive elimination is rate-determining. J. Am. Chem. Soc. 119, 3383–3384 (1997).
Kathuria, S. et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 9, 76–81 (2003).
Piomelli, D. et al. Pharmacological profile of the selective FAAH inhibitor KDS‐4103 (URB597). CNS Drug Rev. 12, 21–38 (2006).
Van Esbroeck, A. C. M. et al. Activity-based protein profiling reveals off-target proteins of the FAAH inhibitor BIA 10-2474. Science 356, 1084–1087 (2017).
Chen, J. K. I only have eye for ewe: the discovery of cyclopamine and development of Hedgehog pathway-targeting drugs. Nat. Prod. Rep. 33, 595–601 (2016).
Pan, S. et al. Discovery of NVP-LDE225, a potent and selective smoothened antagonist. ACS Med. Chem. Lett. 1, 130–134 (2010).
Taipale, J. et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature 406, 1005–1009 (2000).
Jain, N. & Yalkowsky, S. H. Estimation of the aqueous solubility I: application to organic nonelectrolytes. J. Pharm. Sci. 90, 234–252 (2001).
Nicolaou, K. C. et al. Synthesis and biopharmaceutical evaluation of imatinib analogues featuring unusual structural motifs. ChemMedChem 11, 31–37 (2016).
Perez-Riverol, Y. et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 50, D543–D552 (2022).
Acknowledgements
We thank B. Li and T. Jayasundera of Boston College for assistance with X-ray structure analysis and NMR spectroscopy, respectively. Funding from the National Institutes of Health R35GM127140 (J.P.M.), R35GM127030 (J.K.C.), R35GM134694 (E.W.), S10OD026910 (Boston College), National Science Foundation MRI Award CHE2117276 (Boston College) and a Lamattina Fellowship (H.L.).
Author information
Authors and Affiliations
Contributions
M.Z. and J.P.M. conceptualized the study; M.Z. and M.C. conducted the synthetic investigation; H.L. performed the density functional theory calculations; B.X. and E.W. studied the hydrolase inhibition; B.R.S. and J.K.C. investigated the Hh inhibition; J.P.M. assisted with the writing of the initial draft; and all authors contributed to the review and editing of the paper.
Corresponding authors
Ethics declarations
Competing interests
J.P.M., M.Z. and H.L. declare that provisional patent applications have been filed on boron-containing cyclic molecules (US provisional application 63/509,173 and international patent application PCT/US24/34873). All other authors have no competing interests.
Peer review
Peer review information
Nature thanks Antonia Stepan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains experimental data. See the Contents page for details.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, M., Chapman, M., Sarode, B.R. et al. Catalytic asymmetric synthesis of meta benzene isosteres. Nature 633, 90–95 (2024). https://doi.org/10.1038/s41586-024-07865-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07865-4
- Springer Nature Limited