Abstract
The remarkable clinical activity of chimeric antigen receptor (CAR) therapies in B cell and plasma cell malignancies has validated the use of this therapeutic class for liquid cancers, but resistance and limited access remain as barriers to broader application. Here we review the immunobiology and design principles of current prototype CARs and present emerging platforms that are anticipated to drive future clinical advances. The field is witnessing a rapid expansion of next-generation CAR immune cell technologies designed to enhance efficacy, safety and access. Substantial progress has been made in augmenting immune cell fitness, activating endogenous immunity, arming cells to resist suppression via the tumour microenvironment and developing approaches to modulate antigen density thresholds. Increasingly sophisticated multispecific, logic-gated and regulatable CARs display the potential to overcome resistance and increase safety. Early signs of progress with stealth, virus-free and in vivo gene delivery platforms provide potential paths for reduced costs and increased access of cell therapies in the future. The continuing clinical success of CAR T cells in liquid cancers is driving the development of increasingly sophisticated immune cell therapies that are poised to translate to treatments for solid cancers and non-malignant diseases in the coming years.
Similar content being viewed by others
Main
CARs are synthetic modular proteins that redirect immune cell reactivity toward a target of interest. This versatile platform has demonstrated substantial clinical effects in the treatment of B cell and plasma cell malignancies, and the potential to expand its application is driving rapid technological developments and large investments from academia and the biopharmaceutical industry. Six CAR T cell products have been approved by the US Food and Drug Administration (FDA) for 12 indications, including large B cell lymphoma1,2,3,4 (LBCL), B cell acute lymphoblastic leukaemia5,6,7 (B-ALL), mantle cell lymphoma8 and follicular lymphoma9. In pivotal trials, CD19-CAR therapy outperformed standard of care (SOC) as second line therapy for LBCL10,11, and was highly effective as a first line therapy12, paving the way for its application in earlier-stage disease. The generalizability of the CAR platform beyond CD19 targeting is now established, with two BCMA-CAR T cell therapies (BCMA is also known as TNF receptor superfamily member 17) having been approved by the FDA for treatment of multiple myeloma13,14, and high response rates with CD22-CARs in B-ALL15,16 and LBCL17, CD30-CARs in Hodgkin lymphoma18, CD7-CARs in T cell acute lymphoblastic leukaemia19,20,21,22 (T-ALL), CD20-CARs in LBCL23, and GPRC5D-CARs in multiple myeloma24 (Table 1). Standardized toxicity grading and management has resulted in low treatment-related mortality with current commercial CAR T cells1,2,3,4,5,6,7,8,9,10,11.
Despite this progress, many challenges remain. Fewer than 50% of patients treated with commercial CAR T cells for B cell malignancies experience durable disease control1,2,3,4,5,6,7. CAR T cells have shown signs of activity in solid tumours25,26,27,28,29, but high rates of consistent durable responses have not been demonstrated (Table 1). Autologous cell manufacturing is labour-intensive and expensive and commercial scaling is not yet adequate to meet clinical needs. This Perspective synthesizes current understanding of the immunobiology of CAR T cells, emphasizing resistance mechanisms in cancer, design principles and emerging approaches to enhance efficacy. We focus primarily on developing CAR T cells for cancer treatment, but many of the principles are relevant to other immune cell therapies for cancer and to nascent efforts to develop cell therapies for non-malignant diseases. Owing to space constraints, we focus primarily on the most recent literature and on the emerging efforts to enhance efficacy, and refer the reader to recent authoritative reports for additional information on CAR-related toxicities30 and clinical outcomes31.
CAR T immunobiology and mechanisms of resistance
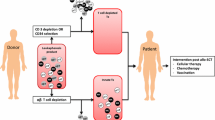
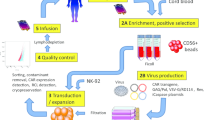
Sustained broad-based advances by many groups focused on developing immune cell therapies for cancer have been essential for the success of CAR T therapy (Fig. 1). CARs were invented by Eshhar and colleagues with the goal of harnessing the expansion, killing and persistence of natural T cells while overcoming major histocompatibility complex (MHC) restriction of the T cell receptor (TCR), to enable broader therapeutic applicability32,33. After iterative optimization by many groups34,35, a receptor incorporating a scFv as the antigen-binding domain, a hinge/transmembrane domain, TCRζ and a CD28 or 4-1BB costimulatory endodomain emerged as the CAR prototype (Fig. 2). This architecture is utilized by five out of the six FDA-approved agents, with the sixth incorporating the same architecture with two nanobody heavy chains (Vhh) as the antigen-binding domains14. Antigen engagement of the prototype 1.5–2.2 kilobase (kb) receptor largely replicates antigen specific activation and killing mediated by the TCR–CD3 complex in natural T cells; however, significant distinctions exist between the biology of CAR T cells and natural T cells that provide opportunities and challenges for application of these therapeutic agents, as discussed below.
Prototype CARs comprise a target-binding domain such as a scFv, a hinge or spacer domain that projects the binder away from the cell surface and provides conformational flexibility, a transmembrane domain that anchors the receptor in the cell surface, and costimulatory and CD3ζ signalling domains that provide activation signals. Small modifications in CAR structure can have profound effects on CAR T cell function, including modulation of the antigen density threshold for activation, persistence, potency, tonic signalling, CAR expression level and the propensity for dimerization237,242,243,244,245,246,247,248,249,249. CDR, complementarity-determining region; FW, framework region.
Resistance due to antigen modulation
A major distinction between CAR and TCR signalling is that CARs require higher antigen density for full T cell activation36,37,38. Despite the higher affinity of single-chain variable fragments (scFvs) compared with TCRs and the generally higher density of CAR expression compared with TCR–CD3 complexes36, TCRs induce full activation in response to less than 100 peptides per antigen presenting cell39,40, whereas CARs require more than 1,000 target molecules per target cell15,41,42,43,44,45. The basis for the difference includes diminished proximal kinase recruitment by CARs38,44,46,47, a less developed immune synapse46, reduced engagement of co-receptors and greater induction of negative downstream regulators36—in part related to tonic signalling, in which CAR aggregation, often driven by the scFv, induces antigen-independent activation48. Modifications to the design of CAR prototypes can tune the antigen density threshold to some extent, with features that modulate signal strength, scFv affinity, CAR expression density, hinge/transmembrane architecture and synapse spacing, having significant effects44,49,50,51,52 (Fig. 2). Because greater signal strength lowers the antigen density threshold, features that enhance T cell fitness independently of CAR design also reduce the CAR antigen density threshold44,53. These insights are foundational for developing safe and effective CAR T cell therapies, since toxicity and efficacy are intimately related to the expression characteristics of the targeted antigen. CARs targeting molecules that are absent from vital tissues, such as B cell lineage antigens, should be engineered for activation at low antigen density to diminish the risk of low antigen recurrence, whereas CARs targeting molecules that are highly expressed in cancer but with low expression in vital tissues should be engineered with higher antigen density thresholds to exploit a therapeutic window on the basis of differential antigen density49.
Antigen modulation is a major cause of CAR T cell resistance in B cell malignancies, and is likely to pose an even greater challenge in solid tumours, where most targetable antigens show significant heterogeneity54,55,56. In B-ALL in children and young adults, approximately 50% of relapses are associated with CD19 loss5,57,58, and in LBCL, approximately 30% of relapses are CD19-negative and an additional 30% express CD19 at levels below the antigen density threshold for commercial CARs45,59. The role of antigen modulation in resistance to BCMA-CAR T therapy in multiple myeloma is less well defined. Baseline BCMA expression levels are heterogeneous among patients and have not been associated with clinical responses60,61,62. BCMA loss—associated with genetic mutation and deletion—is a rare cause of resistance13,63,64 (less than 5% of cases), however, antigen modulation is observed following BCMA-CAR treatment61,62. Antigen density can be modulated through a variety of mechanisms, including genetic mutation63,65,66, alternative RNA splicing65, cell lineage switching67, epigenetic and/or posttranscriptional mechanisms15, trogocytosis50, hyperglycosylation68 and antigen shedding69. Downregulation of some targets is amenable to therapeutic intervention with small molecules, such as CD22 upregulation by bryostatin70, CD70 upregulation by azacitidine71 and BCMA upregulation by γ-secretase inhibitors, which inhibit antigen shedding69,70.
Resistance due to inadequate T cell function
A second major cause of CAR T cell resistance is related to inadequate T cell potency, persistence, functional persistence and/or dysfunction, and is typically associated with disease recurrence in the absence of antigen modulation. Dysfunction often results from T cell exhaustion, characterized by global transcriptional and epigenetic reprogramming that converges on terminal differentiation53,72. T cells in the apheresis and/or manufactured CAR T cell product sometimes manifest exhaustion73,74, and high tumour burdens induce exhaustion following adoptive transfer. CAR-intrinsic factors also contribute to exhaustion, with the costimulatory domain having a major role. CD28-costimulated CARs manifest more rapid and greater expansion, secrete more inflammatory cytokines, and show limited persistence owing to T cell exhaustion when compared with 4-1BB and first-generation CARs, which in some cases, may persist for years5,58,75,76,77. The pro-exhaustion effect of the CD28 costimulatory domain is magnified in CARs with tonic signalling48,53. The effect of tonic signalling depends on the magnitude of the signal and is context-dependent, with some CARs demonstrating enhanced function and persistence in the presence of tonic signalling78. Tuning down the signalling strength of CD28-based CARs through mutations in CD3ζ or CD28 domains can attenuate their propensity for exhaustion and improve persistence79,80, as can mutations that interfere with downregulation and ubiquitination of 4-1BB-costimulated CARs81. Of interest, a recent long-term follow-up study demonstrated that long-lived CARs were CD4-positive, raising the prospect that this subset may be less susceptible to exhaustion and thereby exhibit greater persistence76,82.
The clinical effect of shorter persistence of CD28- versus 4-1BB-costimulated CARs varies by disease. In LBCL, tumour eradication occurs rapidly, and CD28 and 4-1BB costimulated CAR T cells demonstrate similar efficacies1,3,4,83. By contrast, in B-ALL, persistence of CAR T cells beyond 6 months is associated with increased rates of relapse—thus, CD28 costimulated CAR T cells are less effective unless patients receive a post-CAR bone marrow transplant to consolidate remission57,84. In multiple myeloma, functional persistence of anti-BCMA-CAR T cells is associated with a longer duration of response13. It remains unclear whether CD28 or 4-1BB costimulation is preferred for solid tumours, where both strong signalling strength and persistence are desirable. Prototype CARs incorporating both CD28 and 4-1BB costimulatory domains have not demonstrated superiority, leading investigators to integrate novel85,86 or synthetic costimulatory domains87 with the goal of endowing maximal signalling power alongside durable persistence. Pooled CAR screening has been used to identify optimal CAR signalling domains and designs and elucidate CAR design principles87,88,89. Genome-wide CRISPR screens have identified the CD2–CD58 axis as a mediator of T cell potency90 and IFNγR signalling has been demonstrated to be required for productive CAR T cell adhesion and cytotoxicity in solid but not liquid tumours91. CAR T cell potency is also limited by immunosuppressive molecules (TGFβ, IL-10, IL-6 and checkpoint molecules) in the tumour microenvironment (TME), and work is underway to combine CAR T cell therapies with immunomodulators designed to activate immunity within the TME and/or to arm immune cells to resist specific immunosuppressive mediators (Box 1).
Impaired trafficking and locoregional delivery
Impaired trafficking to the tumour site may also limit CAR T cell efficacy, especially in solid tumours. In preclinical models of central nervous system tumours, intratumoral or intracerebroventricular (ICV) administration has improved therapeutic benefit, with an approximate tenfold lower regional dose being required to achieve the same efficacy as intravenous administration92,93,94. Several clinical trials have demonstrated the safety of locoregional delivery of CAR T cells into the central nervous system27,28,95 and in a patient with glioblastoma multiforme, ICV delivery of IL-13Rα2 CAR T cells induced a complete response, whereas intratumoral administration was not effective27. In a study of patients with diffuse midline gliomas, ICV delivery of GD2-CAR T cells induced antitumour effects and clinical responses, and repeated dosing was associated with sustained benefit, raising the prospect that delivery to the central nervous system may abrogate immune sensitization, which has probably limited the effectiveness of multidose intravenous CAR T cell regimens28,96,97. In patients with lung cancer involving the pleura, regional delivery of mesothelin-CAR T cells in combination with PD-1 blockade mediated stable disease and metabolic responses98. Cell-intrinsic strategies to improve T cell homing to and persistence in the TME, such as secretion of IL-7 and CCL1999, are also being explored.
The next generation of CAR T cells
The various next-generation platforms being used to overcome tumour resistance mechanisms, augment immune cell fitness, improve specificity, tune CAR signalling, enhance safety, and increase antigen sensitivity are discussed in this section.
Platforms to diminish antigen escape
Bispecific CAR targeting may be achieved by administration of a mixed cell product, bicistronic expression of two receptors, two scFvs incorporated into a single receptor100, or co-transduction of multiple CARs, with each approach presenting opportunities and challenges. Co-infusion is financially, labour- and cell-intensive and co-infusion and co-transduction generate heterogeneous products, risking the emergence of a subpopulation that dominates the pool of cells after infusion101,102. Bicistronic vectors may result in reduced protein expression, and in one clinical trial, a bicistronic construct demonstrated limited persistence103. Several trials with bispecific receptors targeting CD19 plus CD20 or CD22 have been reported45,104,105, and in one, the receptor mediated diminished potency toward CD22 and tumour cell variants exhibiting low or no surface expression of CD19 emerged45. Cilta-cel, a BCMA-CAR recently approved by the FDA, incorporates two tandem Vhh binders in one receptor, which binds two different epitopes on BCMA. Clinical results with cilta-cel demonstrate a sCR of 83% and 55% PFS at 27 months, the highest reported so far using CARs for multiple myeloma14,106 (Table 1). In summary, clinical data with multispecific CARs is nascent but demonstrates safety and promise for improved efficacy by diminishing antigen escape.
Novel receptors designed to lower the antigen density threshold are also being developed. Katsarou and colleagues have expressed a chimeric costimulatory receptor (CCR), which lacks a CD3ζ domain, in trans with a prototype CAR, and reported that CCR engagement activated the prototype CAR at very low antigen density, preventing antigen low escape in preclinical models107. Induction of antitumour responses toward non-CAR T cell antigens—as reported following CAR T cell therapy in a patient with rhabdomyosarcoma—could diminish resistance due to antigen modulation54. Several approaches are under development to augment innate and adaptive immunity (Box 1), including CAR-mediated delivery of the immunostimulatory RNA RN7SL1108, coexpression of ligands or cytokines that reshape the TME such as IL-12109,110, IL-18111, CD40L112 or Flt3L113, engineering CAR T cells to secrete bispecific T cell engagers (BiTEs), taking advantage of CAR T cell accumulation within the tumour site and avoiding systemic toxicity of the BiTE114, or using non-traditional immune cells that may mediate more potent endogenous antitumour activity.
Enhancing T cell potency
Extensive work is underway to enhance immune cell fitness (Fig. 3 and Box 1). Significant effort is focused on epigenetic modulation, in part on the basis of an exceptional responder in a clinical trial of CD19-CAR for CLL—in which lentiviral integration disrupted the TET2 gene, a mediator of DNA methylation, resulting in substantial clonal T cell proliferation and a sustained antitumour response115. Similarly, knockout of the DNMT3A gene enhances the antitumour activity of CAR T cells in preclinical models116. Overexpression of transcription factors to prevent exhaustion has also shown promise, including overexpression of the AP-1 factor JUN, which enhances T cell expansion and persistence, diminishes terminal differentiation and lowers the antigen density threshold, presumably owing to increased signal strength53. Similarly, overexpression of BATF transcription factors has been reported to enhance T cell potency117. Manufacturing strategies are being developed to optimize CAR T cell phenotype towards stem-like and central memory subsets, including shorter culture duration118, inhibition of PI3K–mTOR–AKT119, BTK120 or tyrosine kinase121 signalling, and culture in memory-promoting cytokines122.
Investigators have leveraged numerous bioengineering strategies to develop advanced CAR platforms to improve the safety and efficacy of immune cell therapies. Multispecific (bivalent) CARs, which operate as an OR gate, may be able to overcome obstacles related to tumour heterogeneity and antigen loss, whereas combinatorial antigen sensing systems such as SynNotch or LINK CAR increase the specificity of CAR T cells by requiring two antigens for activity. Safety switches such as drug-regulated and adapter CARs could mitigate CAR-induced toxicities and enhance efficacy by tuning signalling. Knockout of TRAC, B2M and CIITA genes ablates TCR, MHC class I and MHC class II expression, respectively, and human leukocyte antigen (HLA)-E and CD47 overexpression shield stealth CAR T cells from natural killer cell- and macrophage-mediated rejection. Deletion of NR4A and TET2 genes or ectopic overexpression of c-Jun results in transcriptional rewiring that renders T cells exhaustion resistant. By fusing a scFv domain to TCR subunit, TRuC and HIT receptors redirect the specificity of the endogenous receptor and exhibit increased antigen sensitivity compared with CARs, while secreting lower levels of cytokine. Integration of a cytokine gene in the CAR vector provides a growth and survival signal to improve the persistence of T cells. Costim, costimulatory domain; TF, transcription factor; TM, transmembrane domain.
CRISPR-mediated gene editing was first applied clinically in the setting of adoptive T cell therapy, in which PD-1 was deleted from cells engineered to express NY-ESO-1, a cancer-specific TCR transgene123. The engineered cells did not demonstrate enhanced persistence or potency, but the study demonstrated the feasibility and safety of the approach, and accelerated efforts to apply gene editing technologies to enhance immune cell therapies. Several genes have been identified as candidates for editing to enhance T cell fitness72,124,125,126,127,128,129,130,131,132,133 (Box 1), and CRISPR-mediated disruption of T cell markers such as CD7 and CD5 has enabled CAR T cell therapies for T cell malignancies, while avoiding CAR T cell lysis134,135 (termed ‘fratricide’). We anticipate increasing clinical trial activity incorporating gene-edited immune cells into adoptive immune cell therapy platforms to enhance their potency, expand the landscape of targetable antigens and avoid immune sensitization.
To enhance persistence, some investigators have sought to integrate cytokine signals into the CAR receptor or express cytokines in trans136,137, including a clinical trial in which CAR-expressing natural killer cells transgenically expressing IL-15 demonstrated prolonged persistence138. Immune rejection may also limit CAR T cell persistence as anti-CAR immune responses—often targeting mouse, humanized or fully human scFvs—can be measured in many patients29,97,139,140. Consistently, clinical experience demonstrates the limited utility of second and subsequent intravenous CAR T cell doses, which can be improved using enhanced lymphodepleting regimens96. These findings raise the prospect that stealth platforms—which are currently being developed to enable off-the-shelf allogeneic products (discussed in ‘Platforms to enhance access and efficacy’)—could enhance CAR T cell efficacy by enhancing persistence or enabling multiple CAR T dosing regimens.
Diverse efforts are underway to address the suppressive TME (Box 1), including genetic ablation or expression of dominant-negative TBGβ141,142, PD-1143,144 or Fas receptors145, and engineering CAR T cells to secrete checkpoint-blocking scFvs146. Some investigators have engineered switch receptors, fusion proteins that convert a suppressive signal within the TME to an activating signal in the CAR T cells147,148. Whether tonic activating signals induced by such receptors result in long-term CAR T cell enhancement or predispose them to exhaustion and terminal differentiation remains to be determined. Biomaterials-based approaches for enhancing the expansion and persistence are also being explored149.
CAR tuning and regulatable platforms
Substantial efforts are underway to enhance safety and potency by tuning or dampening CAR signalling to diminish toxicity and exhaustion. This concept was first proposed by Eyquem and colleagues, who used CRISPR to knock-in CAR receptors into the TRAC locus and observed improved potency and diminished exhaustion due to antigen-induced CAR downregulation mediated by endogenous TRAC regulatory elements150. Weber and colleagues extended this principle using synthetic biology or small molecules to transiently cease CAR signalling, which enhanced CAR T cell potency when used during manufacturing and improved antitumour effects when applied in vivo after adoptive transfer121.
Kill switches such as iCasp9151, HSV tyrosine kinase152 (HSV-tk) and epitope tags153 enable the depletion of engineered cells in the event of severe toxicity, and a transgene-free safety switch that renders T cells auxotrophic for uridine has been developed154. Regulatable platforms can serve as reversible safety switches and also tune CAR signalling, thereby enhancing T cell potency by providing rest periods that prevent T cell exhaustion121. Numerous regulatable platforms have been developed using drug-sensitive promoters155, induced dimerization156,157, disruption of split CARs158, drug-dependent activation of binders159, proteolysis-targeting chimeras160 (PROTACs), chemically-dependent degron domains121,157,161 and drug-regulated CAR proteolysis162,163. These systems represent significant advances in synthetic biology, but remain challenged by leaky activity in the OFF state that risks toxicity, diminished CAR expression or activity in the ON state and the use immunosuppressive drugs as regulators121,155,156,157,158,159,160,161. Labanieh et al. recently developed a protease-regulated grazoprevir-induced ‘drug ON’ platform, signal neutralization by an inhibitable protease (SNIP), which shows no leaky activity and full functional capacity162 (Fig. 3). Similar to synNotch164, SNIP demonstrates superior antitumour efficacy compared with conventional CAR T cells owing to reduced exhaustion, and in an on-target off-tumour ROR1 toxicity model, decreased grazoprevir dosing tuned SNIP CARs to open a therapeutic window in which healthy tissue was spared but ROR1-expressing tumour cells were killed162. Similarly, Hernandez-Lopez et al. iterated the synNotch platform to target very highly expressed tumour antigens while avoiding lower levels of the antigens on normal tissues165. Thus, regulatable CARs show promise for enhancing efficacy and diminishing toxicity.
Enhancing specificity through Boolean logic
B cell and plasma cell malignancies are especially suited to CAR T cell therapy owing to the high, homogenous expression of lineage antigens that are co-expressed predominantly on B cells and plasma cells, the depletion of which is tolerable. However, a recent case report showed the development of parkinsonism in a patient after BCMA-CAR T cell therapy, with postmortem analysis revealing expression of BCMA on subsets of neurons and astrocytes in the patient’s basal ganglia166. In another study, single-cell RNA-sequencing analysis showed the expression of CD19 on brain mural cells, raising the prospect that on-target killing may be responsible for neurotoxicity after CD19-CAR T cell therapy. These results highlight the challenge of identifying targets that are not expressed on vital tissue.
So far, the paucity of tumour-specific surface targets on solid tumours has limited the application of the CAR prototype to solid tumours, with unacceptable off-tumour, on-target toxicity having been observed in trials of CARs targeting CAIX167 and CEACAM5168. However, several clinical trials of CAR T cells and other potent antibody-directed therapies have demonstrated good safety profiles in solid tumours (Table 1). The high CAR antigen density threshold is likely to explain the safe targeting of some antigens with known expression on vital tissues—such as GD2, which is expressed at low levels on neural tissues169,170. A recent trial demonstrated promising clinical activity of claudin-18.2-CARs was associated with significant but non-dose-limiting toxicity, potentially explained by antigen expression restricted to differentiated epithelial cells buried in gastric mucosa that may be less accessible to CAR T cells29. Identifying additional molecules with sufficient differential expression levels for safe targeting, such as oncofetal cell-surface targets is essential for expanding the reach of CAR T cells beyond B cell and plasma cell malignancies. However, the safety of specific targets will need to be continually reassessed as potency and persistence enhancements are deployed, as in recent studies with a PSMA-targeted CAR integrating a dominant-negative TGFβ receptor that was associated with lethal toxicity171,172.
Next-generation receptors incorporating logic gates could allow better discrimination between tumour and healthy tissue through combinatorial antigen sensing, and expand the repertoire of potential antigens (Fig. 3). Roybal et al. developed synNotch, an IF–THEN circuit incorporating a synthetic notch receptor against antigen A, which upon engagement, triggers the transcription of a conventional CAR against antigen B173,174. The synNotch system has not been tested clinically, but in preclinical models it prevented on-target, off-tumour toxicity when tumours and susceptible vital tissues are not colocalized175. Tousley et al. developed an AND gate platform called LINK, which utilizes the proximal TCR signalling molecules LAT and SLP76, each fused to a membrane-bound scFv specific for a unique antigen176. Engagement of both antigens colocalizes LAT and SLP76, leading to T cell activation. In an on-target, off-tumour ROR1 toxicity model, LINK CAR T cells cured mice of tumours expressing both antigens without ROR1-mediated toxicity, whereas mice treated with synNotch T cells succumbed to toxicity175. Other approaches for combinatorial antigen targeting that are under development include SUPRA177 and co-LOCKR178, which redirect CAR T cell specificity through protein switches. Although combinatorial antigen sensing could expand the landscape of targetable tumour antigens, the increased risk of tumour escape owing to loss of either antigen is a potential concern. An alternative approach to enhance specificity is to use an AND NOT gate, in which a prototype activating CAR is expressed in trans with an inhibitory CAR (iCAR) targeting an antigen that is expressed on healthy tissue but not on tumour tissue179,180,181. Limited engineering with NOT gates has been undertaken so far and these applications have not been tested clinically.
TCR-like CARs
With the goal of targeting antigens that are expressed at low levels, HLA-independent TCRs (HIT) are designed with the variable domain of the endogenous TCR being altered to target scFvs by gene editing the endogenous TRAC locus182. When CD80 and 4-1BBL are provided in trans, CD19-directed HITs display superior antigen sensitivity compared with prototype CD19-CARs (Fig. 3). Synthetic TCR and antigen receptors (STARs) have a similar design but are not knocked in to the TRAC locus; thus, the endogenous TCR specificity is retained183. Other approaches for redirecting TCR specificity include the antibody–TCR (AbTCR) platform184, which replaces the variable domains of TCRγδ with a Fab fragment and TCR fusion constructs185 (TRuC), which fuse an scFv to a CD3 subunit. A recent comparison of TCR-like chimeric receptors showed that STAR and HIT receptors reproduce TCR antigen sensitivity, whereas TruCs do not186. One potential drawback of CAR T cells compared with native T cells is the inability to target intracellular antigens, since most aberrant proteins that drive cancer are intracellular. Yarmarkovich et al. overcame this by developing a prototype CAR with specificity for peptides presented by MHC187 (pMHC). Using scFv binders specific for a PHOX2B peptide–MHC overexpressed in neuroblastoma, they targeted pMHCs across several HLA allotypes. This strategy could greatly expand the landscape of CAR targets, including key oncogenic drivers.
Platforms to enhance access and efficacy
Diverse approaches are under exploration to increase access of cell therapies, diminish the high manufacturing costs, create stealth immune cells resistant to rejection, and leverage the unique properties of alternative immune cells.
Distributed manufacturing and allogeneic products
Engineering advances have yielded automated closed-system manufacturing, which is providing opportunities for point-of-care manufacturing to diminish the costs, delays and logistical challenges associated with the centralized manufacturing models that are the industry standard. A recent multicentre trial demonstrated the safety and efficacy of cells manufactured at the point of care188. Defining the regulatory requirements for point-of-care manufacturing is an area of significant current interest, especially for therapies targeting rare indications, such as paediatric cancers189.
Allogeneic CAR T cells manufactured from healthy ‘super donors’ could improve potency by avoiding preexisting T cell dysfunction and decrease the cost and logistical challenges of manufacturing, thereby enhancing access. However, allogeneic T cell therapies must overcome the risk of GVHD mediated by the TCR and rejection of the transferred cells by the host immune system. Gene editing of the endogenous TCR eliminates the risk of GVHD190, but endowing stealth properties to avoid immune rejection remains a significant challenge, since CD8+, CD4+, natural killer and macrophage cells can reject allogeneic cells and each are regulated by distinct axes, necessitating multiple enhancements (Box 1). Knockout of β2-microglobulin can eliminate HLA class I surface expression, but paradoxically increases the risk of rejection by natural killer cells. Additional strategies for inducing allogeneic tolerance include knockout of the CIITA gene to ablate MHC class II expression191, and overexpression of HLA-E192 and CD47193 to ameliorate natural killer cell- and macrophage-mediated cell rejection.
Many allogeneic approaches use CRISPR–Cas9, and the risks of CRISPR-based mutagenic events could be magnified when producing hundreds or thousands of allogeneic products with a singular manufacturing process. Alternative platforms, such as base editing or prime editing may emerge as preferred alternatives to nuclease-based genome editing since they probably involve lower risk owing to an absence of double strand DNA breaks194. CRISPR–Cas systems targeting RNA could also provide opportunities for multiplexed gene knockdowns with greater specificity and efficiency compared with RNA-mediated interference. Although allogeneic donor-derived cells containing multiple gene edits could provide significant advantages, these technologies are nascent and their toxicity profiles remain unknown. Some groups have attempted to prevent immune rejection by augmenting immune suppression of the host using conventional chemotherapy or immunosuppressive antibodies for which the targets are edited from the CAR T cells190. Early response rates with this approach are promising, but long-term safety and efficacy have not been demonstrated and concerns remain regarding infectious risks associated with intensive immune-depleting regimens190.
Alternative immune cells
Several non-T immune cells, including natural killer cells, invariant natural killer T (iNKT) cells, γδ T cells and macrophages exhibit innate antitumour activity and do not induce GVHD, raising the prospect that they could provide an off-the-shelf source of cells with reduced toxicity, enhanced tumour trafficking and/or target antigen-negative variants through innate tumour recognition. However, allogeneic innate immune cells remain susceptible to rejection, raising concerns regarding the durability of their effects if they are not engineered for stealth. Cord blood-derived allogeneic natural killer cells incorporating ectopically expressed IL-15 have shown promise in a phase I trial for NHL and CLL138. iNKT-CAR cells mediated activity in mouse models, in part by cross-priming host CD8 cells towards tumour antigens195, and their safety and feasibility in a phase I trial for neuroblastoma have been demonstrated196. γδ T cells engineered to express a CD20-CAR have also shown impressive activity in early studies197. Expressing CARs in macrophages requires substantial adaptations of vectors and signalling domains, but antitumour effects associated with augmented phagocytosis, modification of the TME and recruitment of T cells198,199 have been demonstrated in preclinical models198, with CD3ζ-based CARs demonstrating equivalent phagocytic activity as Fcγ-based CARs Efforts are also underway to create induced pluripotent stem (iPS) cell-derived CAR T cells200, natural killer cells201 and macrophages202. The differentiation of iPS cells to natural killer cells has been particularly successful, and clinical testing of these off-the-shelf therapies is currently in progress203, whereas iPS cell differentiation to fully functional T cells has been more challenging204. Given their nearly inexhaustible expansion potential, iPS cell-derived products could enable mass production of a homogenous cell product integrating numerous enhancements to endow stealth properties, safety switches and potency, and the long-term safety and efficacy results of these emerging platforms are thus eagerly anticipated.
Next-generation gene delivery
Viral vector-based gene delivery has been the gold standard in the field, but vector production and qualification is costly and time consuming. Viral-free platforms for gene delivery are under development, with CRISPR-based gene delivery in human T cells demonstrating proof of principle, although DNA templates are toxic to T cells and the efficiency of this approach remains lower than with viral vectors205. Clinical feasibility has been demonstrated with a CD19-CAR site-specifically delivered to the PD-1 locus inducing a high CR rate in NHL, although the manufacturing process did not meet dose requirements for a relatively high proportion of patients206. Modifications to DNA templates and small-molecule inhibitor cocktails are improving knock-in efficiencies and cell yields207. Transposon-based gene delivery has also been used, although malignant transformation of CAR-engineered T cells was reported in two patients associated with high-copy-number integration using a Piggybac transposon platform208. In vivo gene delivery is another emerging approach that could improve accessibility and diminish cost. In this approach, DNA or RNA is delivered systemically using viral vectors209 or nanoparticles210 that preferentially target and transduce immune populations in vivo. Immunogenicity could prohibit repeat administration of viral vectors owing to the induction of neutralizing antibodies. Stable expression of a CD19-CAR has been demonstrated using lipid nanoparticles targeting CD3 in mice210 and T cell-targeted lipid nanoparticles incorporating optimized RNA diminished cardiac fibrosis in a mouse model211.
CAR therapy for non-malignant diseases
The CAR T platform has been optimized for cancer treatment, but the design principles and expansive synthetic biology toolbox used for CAR T cells are providing opportunities to extend this therapeutic approach to non-malignant diseases, including autoimmunity, senescence, fibrosis and infectious diseases. In preclinical studies, CD19-CAR T cells have demonstrated beneficial effects in systemic lupus erythematosus212, and a case study reported sustained activity of CD19-CAR therapy in a patient with refractory lupus nephritis213 . Chimeric autoantibody receptors (CAARs) are prototype CARs that incorporate a scFv targeting the idiotype of an autoreactive B cell clone or use autoantigens as the recognition domain. In preclinical studies, CAARs mediated therapeutic effects against pemphigus vulgaris, and clinical testing is underway. Adoptive transfer of T regulatory (Treg) cells, which mediate suppression rather than cytotoxicity, is an alternative approach for treating autoimmunity. Non-engineered Treg cells have demonstrated activity in mouse models of GVHD, allograft transplantation, type 1 diabetes, systemic lupus erythematosus and multiple sclerosis, and early clinical data demonstrate feasibility of manufacturing and a good safety profile214. Compared with non-engineered cells, Treg cells expressing a CAR targeting antigens expressed on the diseased tissues show enhanced specificity and potency215,216. Recent data have demonstrated that inadvertent expansion of CAR Treg cells limits the efficacy of commercial CAR T cells, providing proof-of-concept for the utility of CAR-engineered Treg cells217,218. Approaches are underway to engineer FOXP3 expression to enforce lineage stability and incorporate safety switches to diminish risk214. Recent promising preclinical data were generated in haemophilic mice, in which Treg cells expressing a factor VIII-targeted CAR and FOXP3 prevented the development of neutralizing anti-factor VIII antibodies219. Senolytic CAR T cells targeting urokinase-type plasminogen activator receptor have been demonstrated to target senescent cells in vitro and restore tissue homeostasis in models of liver fibrosis220. CARs targeting fibroblast activation protein (FAP) have improved cardiac function in a mouse model of cardiac fibrosis221 and in vivo generation of FAP-CARs using CD5-directed lipid nanoparticles loaded with mRNA also demonstrated benefit211. In this model, the non-integrating nature of mRNA ensured that CAR expression was transient, thereby mitigating the risk of toxicity associated with widespread elimination of activated fibroblasts.
Outlook
Adoptive immune cell therapy is established as a transformative therapeutic modality. The past decade has witnessed significant progress in understanding the biology of prototype CAR T cells, identifying antigen modulation and T cell dysfunction as major resistance mechanisms and highlighting the logistical challenges of delivering cell therapies to all patients who could benefit. Modifications to prototype CARs can augment their potency, but increasingly investigators are designing next-generation platforms to create advanced cellular therapies that incorporate a diverse array of enhancements. The fields of immunology, synthetic biology, genetic engineering and cell manufacturing are synergizing to create smarter, safer and more accessible cellular therapies that are poised for increased efficacy and access, diminished risk and cost, and broader utility, for the treatment of cancer as well as non-malignant diseases.
Change history
20 June 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41586-023-06088-3
References
Abramson, J. S. et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396, 839–852 (2020).
Nastoupil, L. J. et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J. Clin. Oncol. 38, 3119–3128 (2020).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Schuster, S. J. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2019).
Maude, S. L., Laetsch, T. W., Buchner, J. & Grupp, S. A. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Pasquini, M. C. et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 4, 5414–5424 (2020).
Schultz, L. M. et al. Disease burden affects outcomes in pediatric and young adult B-cell lymphoblastic leukemia after commercial tisagenlecleucel: a pediatric real-world chimeric antigen receptor consortium report. J. Clin. Oncol. 40, 945–955 (2022).
Wang, M. et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 382, 1331–1342 (2020).
Fowler, N. H. et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat. Med. 28, 325–332 (2022).
Locke, F. L. et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N. Engl. J. Med. 386, 640–654 (2021). This pivotal trial demonstrated CD19-CAR therapy outperforming autologous stem-cell transplant for second line therapy of LBCL.
Kamdar, M. et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysi. Lancet 399, 2294–2308 (2022).
Neelapu, S. S. et al. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat. Med. 28, 735–742 (2022). This study demonstrated that axi-cel is highly effective in the first-line therapy setting for high-risk LBCL.
Munshi, N. C. et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl. J. Med. 384, 705–716 (2021).
Berdeja, J. G. et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 398, 314–324 (2021). This study showed impressive clinical activity of the BCMA-directed therapy ciltacabtagene autoleucel.
Fry, T. J. et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 24, 20–28 (2018).
Shah, N. N. et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase I anti-CD22 CAR T-cell trial. J. Clin. Oncol. 38, 1938–1950 (2020).
Baird, J. H. et al. CD22-directed CAR T-cell therapy induces complete remissions in CD19-directed CAR–refractory large B-cell lymphoma. Blood 137, 2321–2325 (2021).
Ramos, C. A. et al. Anti-CD30 CAR-T cell therapy in relapsed and refractory Hodgkin lymphoma. J. Clin. Oncol. 38, 3794–3804 (2020).
Zhang, M. et al. Autologous nanobody-derived fratricide-resistant CD7-CAR T-cell therapy for patients with relapsed and refractory T-cell acute lymphoblastic leukemia/lymphoma. Clin. Cancer Res. 28, 2830–2843 (2022).
Pan, J. et al. Donor-derived CD7 chimeric antigen receptor T cells for T-cell acute lymphoblastic leukemia: first-in-human, phase I trial. J. Clin. Oncol. 39, 3340–3351 (2021).
Lu, P. et al. Naturally selected CD7 CAR-T therapy without genetic manipulations for T-ALL/LBL: first-in-human phase 1 clinical trial. Blood 140, 321–334 (2022).
Yang, J. et al. High effectiveness and safety of anti-CD7 CAR T-cell therapy in treating relapsed or refractory (R/R) T-cell acute lymphoblastic leukemia (T-ALL). Blood 138, 473 (2021).
Zhang, W. Y. et al. Long-term safety and efficacy of CART-20 cells in patients with refractory or relapsed B-cell non-Hodgkin lymphoma: 5-years follow-up results of the phase I and IIa trials. Signal Transduct. Target. Ther. 2, 17054 (2017).
Mailankody, S. et al. Phase I first-in-class trial of MCARH109, a G protein coupled receptor class C group 5 member D (GPRC5D) targeted CAR T cell therapy in patients with relapsed or refractory multiple myeloma. Blood 138, 827 (2021).
D’angelo, S. P. et al. Antitumor activity associated with prolonged persistence of adoptively transferred NY-ESO-1c259T cells in synovial sarcoma. Cancer Discov. 8, 944–957 (2018).
Robbins, P. F. et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 29, 917–924 (2011).
Brown, C. E. et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 375, 2561–2569 (2016). This case report showed that IL-13Rα2-targeting CAR-T cells administered via intracavitary and intraventricular delivery routes were safe and effective in a patient with glioblastoma.
Majzner, R. G. et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 603, 934–941 (2022). This study demonstrated clinical activity of GD2 CAR-T cells in diffuse midline gliomas.
Qi, C. et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat. Med. 28, 1189–1198 (2022).
Morris, E. C., Neelapu, S. S., Giavridis, T. & Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 22, 85–96 (2022).
Majzner, R. G. & Mackall, C. L. Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med. 25, 1341–1355 (2019).
Gross, G., Waks, T. & Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl Acad. Sci. USA 86, 10024–10028 (1989).
Eshhar, Z., Waks, T., Gross, G. & Schindler, D. G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the γ or ζ subunits of the immunoglobulin and T-cell receptors. Proc. Natl Acad. Sci. USA 90, 720–724 (1993).
Maher, J., Brentjens, R. J., Gunset, G., Rivière, I. & Sadelain, M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nat. Biotechnol. 20, 70–75 (2002).
Imai, C. et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 18, 676–684 (2004).
Harris, D. T. et al. Comparison of T cell activities mediated by human TCRs and CARs that use the same recognition domains. J. Immunol. 200, 1088–1100 (2018).
Anikeeva, N. et al. Efficient killing of tumor cells by CAR-T cells requires greater number of engaged CARs than TCRs. J. Biol. Chem. 297, 101033 (2021).
Gudipati, V. et al. Inefficient CAR-proximal signaling blunts antigen sensitivity. Nat. Immunol. 21, 848–856 (2020).
Sykulev, Y., Joo, M., Vturina, I., Tsomides, T. J. & Eisen, H. N. Evidence that a single peptide–MHC complex on a target cell can elicit a cytolytic T cell response. Immunity 4, 565–571 (1996).
Irvine, D. J., Purbhoo, M. A., Krogsgaard, M. & Davis, M. M. Direct observation of ligand recognition by T cells. Nature 419, 845–849 (2002).
Watanabe, K. et al. Target antigen density governs the efficacy of anti-CD20–CD28–CD3 ζ chimeric antigen receptor-modified effector CD8+ T cells. J. Immunol. 194, 911–920 (2015).
Walker, A. J. et al. Tumor antigen and receptor densities regulate efficacy of a chimeric antigen receptor targeting anaplastic lymphoma kinase. Mol. Ther. 25, 2189–2201 (2017).
Majzner, R. G. et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin. Cancer Res. 25, 2560–2574 (2019).
Majzner, R. G. et al. Tuning the antigen density requirement for car T-cell activity. Cancer Discov. 10, 702–723 (2020). This study demonstrated that modifications to the CAR prototype structure modulate antigen density threshold.
Spiegel, J. Y. et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat. Med. 27, 1419–1431 (2021).
Dong, R. et al. Rewired signaling network in T cells expressing the chimeric antigen receptor (CAR). EMBO J. 39, e104730 (2020).
Salter, A. I. et al. Comparative analysis of TCR and CAR signaling informs CAR designs with superior antigen sensitivity and in vivo function. Sci. Signal. 14, eabe2606 (2021). This study demonstrated diminished recruitment of proximal signaling molecules in CARs compared to TCRs.
Long, A. H. et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 21, 581–90 (2015).
Heitzeneder, S. et al. GPC2-CAR Tcells tuned for low antigen density mediate potent activity against neuroblastoma without toxicity. Cancer Cell 40, 53–69.e9 (2022).
Hamieh, M. et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature 568, 112–116 (2019). This study showed that CAR-T trogocytosis could lower the antigen density of target tumor cells and provide an alternative mechanism of resistance to therapy.
James, S. E. et al. Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane. J. Immunol. 180, 7028–7038 (2008).
Haso, W. et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 121, 1165–1171 (2013).
Lynn, R. C. et al. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature 576, 293–300 (2019). This study showed that overexpression of c-Jun improves the fitness of CAR-T cells.
Hegde, M. et al. Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma. Nat. Commun. 11, 3549 (2020).
Krenciute, G. et al. Transgenic expression of IL15 improves antiglioma activity of IL13Rα2-CAR T cells but results in antigen loss variants. Cancer Immunol. Res. 5, 571–581 (2017).
O’Rourke, D. M. et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 9, eaaa0984 (2017).
Gardner, R. A. et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 129, 3322–3331 (2017).
Lee, D. W. et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385, 517–528 (2015).
Plaks, V. et al. CD19 target evasion as a mechanism of relapse in large B-cell lymphoma treated with axicabtagene ciloleucel. Blood 138, 1081–1085 (2021).
Raje, N. et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N. Engl. J. Med. 380, 1726–1737 (2019).
Cohen, A. D. et al. B cell maturation antigen–specific CAR T cells are clinically active in multiple myeloma. J. Clin. Invest. 129, 2210–2221 (2019).
Brudno, J. N. et al. T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J. Clin. Oncol. 36, 2267–2280 (2018).
Da Vià, M. C. et al. Homozygous BCMA gene deletion in response to anti-BCMA CAR T cells in a patient with multiple myeloma. Nat. Med. 27, 616–619 (2021).
Samur, M. K. et al. Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nat. Commun. 12, 868 (2021).
Sotillo, E. et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 5, 1282–1295 (2015).
Orlando, E. J. et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat. Med. 24, 1504–1506 (2018).
Jacoby, E. et al. CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat. Commun. 7, 12320 (2016).
Heard, A. et al. Antigen glycosylation regulates efficacy of CAR T cells targeting CD19. Nat. Commun. 13, 3367 (2022).
Pont, M. J. et al. γ-Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood 134, 1585–1597 (2019).
Ramakrishna, S. et al. Modulation of target antigen density improves CAR T-cell functionality and persistence. Clin. Cancer Res. 25, 5329–5341 (2019).
Leick, M. B. et al. Non-cleavable hinge enhances avidity and expansion of CAR-T cells for acute myeloid leukemia. Cancer Cell 40, 494–508.e5 (2022).
Good, C. R. et al. An NK-like CAR Tcell transition in CAR Tcell dysfunction. Cell 184, 6081–6100.e26 (2021). This study used continuous antigen exposure to identify gene signatures associated with CAR dysfunction.
Finney, O. C. et al. CD19 CAR T cell product and disease attributes predict leukemia remission durability. J. Clin. Invest. 129, 2123–2132 (2019).
Fraietta, J. A. et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 24, 563–571 (2018). This study identified features of CAR-T immunobiology by comparing responding and non-responding patients with CLL treated with CD19-CAR T cells.
Salter, A. I. et al. Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci. Signal 11, eaat6753 (2018).
Melenhorst, J. J. et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 602, 503–509 (2022). This study identified a subset of CD4+ CD19 CAR-T cells that exhibited extended persistence in CLL patients.
Scholler, J. et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 4, 132ra53 (2012).
Singh, N. et al. Antigen-independent activation enhances the efficacy of 4-1BB-costimulated CD22 CAR T cells. Nat. Med. 27, 842–850 (2021).
Feucht, J. et al. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat. Med. 25, 82–88 (2019).
Guedan, S. et al. Single residue in CD28-costimulated CAR-T cells limits long-term persistence and antitumor durability. J. Clin. Invest. 130, 3087–3097 (2020).
Li, W. et al. Chimeric antigen receptor designed to prevent ubiquitination and downregulation showed durable antitumor efficacy. Immunity 53, 456–470.e6 (2020).
Yang, Y. et al. TCR engagement negatively affects CD8 but not CD4 CAR T cell expansion and leukemic clearance. Sci. Transl. Med. 9, eaag1209 (2017).
Frank, M. J. et al. Monitoring of circulating tumor DNA improves early relapse detection after axicabtagene ciloleucel infusion in large B-cell lymphoma: results of a prospective multi-institutional trial. J. Clin. Oncol. 39, 3034–3043 (2021).
Shah, N. N. et al. Long-term follow-up of CD19-CAR T-cell therapy in children and young adults with B-ALL. J. Clin. Oncol. 39, 1650–1659 (2021).
Lange, S. et al. A chimeric GM-CSF/IL18 receptor to sustain CAR T-cell function. Cancer Discov. 11, 1661–1671 (2021).
Mata, M. et al. Inducible activation of myD88 and CD40 in CAR T cells results in controllable and potent antitumor activity in preclinical solid tumor models. Cancer Discov. 7, 1306–1319 (2017).
Daniels, K. G. et al. Decoding CAR T cell phenotype using combinatorial signaling motif libraries and machine learning. Science 378, eabq0225 (2022).
Goodman, D. B. et al. Pooled screening of CAR T cells identifies diverse immune signaling domains for next-generation immunotherapies. Sci. Transl. Med. 14, eabm1463 (2022).
Gordon, K. S. et al. Screening for CD19-specific chimaeric antigen receptors with enhanced signalling via a barcoded library of intracellular domains. Nat. Biomed. Eng. 6, 855–866 (2022).
Yan, X. et al. CD58 loss in tumor cells confers functional impairment of CAR T cells. Blood Adv. 6, 5844–5856 (2022).
Larson, R. C. et al. CAR T cell killing requires the IFNγR pathway in solid but not liquid tumours. Nature 604, 563–570 (2022). This study showed that the IFNγR pathway was required for CAR-T activity against solid but not liquid tumors.
Donovan, L. K. et al. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat. Med. 26, 720–731 (2020).
Theruvath, J. et al. Locoregionally administered B7-H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nat. Med. 26, 712–719 (2020).
Priceman, S. J. et al. Regional delivery of chimeric antigen receptor-engineered T cells effectively targets HER2+ breast cancer metastasis to the brain. Clin. Cancer Res. 24, 95–105 (2018).
Vitanza, N. A. et al. Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: an interim analysis. Nat. Med. 27, 1544–1552 (2021).
Gauthier, J. et al. Factors associated with outcomes after a second CD19-targeted CAR T-cell infusion for refractory B-cell malignancies. Blood 137, 323–335 (2021).
Turtle, C. J. et al. CD19 CAR− T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 1, 2123–2138 (2016).
Adusumilli, P. S. et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti–PD-1 agent pembrolizumab. Gynecol. Oncol. 11, 2748–2763 (2020).
Adachi, K. et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 36, 346–351 (2018).
Hegde, M. et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J. Clin. Invest. 126, 3036–3052 (2016).
Annesley, C. et al. SCRI-CAR19x22v2 T cell product demonstrates bispecific activity in B-ALL. Blood 138, 470 (2021).
Gardner, R. et al. Early clinical experience of CD19 × CD22 dual specific CAR T cells for enhanced anti-leukemic targeting of acute lymphoblastic leukemia. Blood 132, 278 (2018).
Cordoba, S. et al. CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: a phase 1 trial. Nat. Med. 27, 1797–1805 (2021).
Shah, N. N. et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat. Med. 26, 1569–1575 (2020).
Zhang, Y. et al. Long-term activity of tandem CD19/CD20 CAR therapy in refractory/relapsed B-cell lymphoma: a single-arm, phase 1–2 trial. Leukemia 36, 189–196 (2022).
Martin, T. et al. Ciltacabtagene autoleucel, an anti–B-cell maturation antigen chimeric antigen receptor T-Cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J. Clin. Oncol. https://doi.org/10.1200/JCO.22.00842 (2022).
Katsarou, A. et al. Combining a CAR and a chimeric costimulatory receptor enhances T cell sensitivity to low antigen density and promotes persistence. Sci. Transl. Med. 13, 1962 (2021).
Johnson, L. R. et al. The immunostimulatory RNA RN7SL1 enables CAR-T cells to enhance autonomous and endogenous immune function. Cell 184, 4981–4995.e14 (2021).
Agliardi, G. et al. Intratumoral IL-12 delivery empowers CAR-T cell immunotherapy in a pre-clinical model of glioblastoma. Nat. Commun. 12, 444 (2021).
Alsaieedi, A., Holler, A., Velica, P., Bendle, G. & Stauss, H. J. Safety and efficacy of Tet-regulated IL-12 expression in cancer-specific T cells. Oncoimmunology 8, 1542917 (2018).
Avanzi, M. P. et al. Engineered tumor-targeted T cells mediate enhanced anti-tumor efficacy both directly and through activation of the endogenous immune system. Cell Rep. 23, 2130–2141 (2018).
Curran, K. J. et al. Enhancing antitumor efficacy of chimeric antigen receptor T cells through constitutive CD40L expression. Mol. Ther. 23, 769–778 (2015).
Lai, J. et al. Adoptive cellular therapy with T cells expressing the dendritic cell growth factor Flt3L drives epitope spreading and antitumor immunity. Nat. Immunol. 21, 914–926 (2020).
Choi, B. D. et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol. 37, 1049–1058 (2019).
Fraietta, J. A. et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 558, 307–312 (2018). This study showed that random integration of the CAR into the TET2 locus disrupted the gene and resulted in improved CAR-T function and persistence in a patient with CLL.
Prinzing, B. et al. Deleting DNMT3A in CAR T cells prevents exhaustion and enhances antitumor activity. Sci. Transl. Med. 13, eabh0272 (2021).
Seo, H. et al. BATF and IRF4 cooperate to counter exhaustion in tumor-infiltrating CAR T cells. Nat. Immunol. 22, 983–995 (2021).
Ghassemi, S. et al. Reducing ex vivo culture improves the antileukemic activity of chimeric antigen receptor (CAR) T cells. Cancer Immunol. Res. 6, 1100–1109 (2018).
Zheng, W. et al. PI3K orchestration of the in vivo persistence of chimeric antigen receptor-modified T cells. Leukemia 32, 1157–1167 (2018).
Good, Z. et al. Proliferation tracing with single-cell mass cytometry optimizes generation of stem cell memory-like T cells. Nat. Biotechnol. 37, 259–266 (2019).
Weber, E. W. et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science 372, eaba1786 (2021). This study showed that CAR-T functionality could be restored and features of exhaustion could be diminished by providing CAR-T cells with periods of rest.
Xu, Y. et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood 123, 3750–3759 (2014).
Stadtmauer, E. A. et al. CRISPR-engineered T cells in patients with refractory cancer. Science 367, eaba7365 (2020).
Wang, D. et al. Crispr screening of CAR T cells and cancer stem cells reveals critical dependencies for cell-based therapies. Cancer Discov. 11, 1192–1211 (2021).
Freitas, K. A. et al. Enhanced T cell effector activity by targeting the Mediator kinase module. Science 378, eabn5647 (2022).
Chen, J. et al. NR4A transcription factors limit CAR T cell function in solid tumours. Nature 567, 530–534 (2019). This study demonstrated enhanced T cell fitness by deletion of exhaustion-associated transcription factors.
Shifrut, E. et al. Genome-wide CRISPR screens in primary human T cells reveal key regulators of immune function. Cell 175, 1958–1971.e15 (2018).
Wei, J. et al. Targeting REGNASE-1 programs long-lived effector T cells for cancer therapy. Nature 576, 471–476 (2019).
Dong, M. B. et al. Systematic immunotherapy target discovery using genome-scale in vivo CRISPR screens in CD8 T cells. Cell 178, 1189–1204.e23 (2019).
LaFleur, M. W. et al. PTPN2 regulates the generation of exhausted CD8+ T cell subpopulations and restrains tumor immunity. Nat. Immunol. 20, 1335–1347 (2019).
Carnevale, J. et al. RASA2 ablation in T cells boosts antigen sensitivity and long-term function. Nature 609, 174–182 (2022).
Si, J. et al. Hematopoietic progenitor kinase 1 (HPK1) mediates T cell dysfunction and is a druggable target for T cell-based immunotherapies. Cancer Cell 38, 551–566.e11 (2020).
Beavis, P. A. et al. Targeting the adenosine 2A receptor enhances chimeric antigen receptor T cell efficacy. J. Clin. Invest. 127, 929–941 (2017).
Gomes-Silva, D. et al. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood 130, 285–296 (2017).
Chun, I. et al. CRISPR–Cas9 knock out of CD5 enhances the anti-tumor activity of chimeric antigen receptor T cells. Blood 136, 51–52 (2020).
Shum, T. et al. Constitutive signaling from an engineered IL7 receptor promotes durable tumor elimination by tumor-redirected T cells. Cancer Discov. 7, 1238–1247 (2017).
Kagoya, Y. et al. A novel chimeric antigen receptor containing a JAK–STAT signaling domain mediates superior antitumor effects. Nat. Med. 24, 352–359 (2018).
Liu, E. et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 382, 545–553 (2020). This study showed that HLA-mismatched, cord blood-derived anti-CD19-CAR natural killer cells were safe and effective against CD19-positive cancers.
Hege, K. M. et al. Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer. J. Immunother. Cancer 5, 22 (2017).
Brudno, J. N. et al. Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma. Nat. Med. 26, 270–280 (2020).
Shaim, H. et al. Targeting the αv integrin/TGF-β axis improves natural killer cell function against glioblastoma stem cells. J. Clin. Invest. 131, e142116 (2021).
Tang, N. et al. TGF-β inhibition via CRISPR promotes the long-term efficacy of CAR T cells against solid tumors. JCI Insight 5, e133977 (2020).
Cherkassky, L. et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 126, 3130–3144 (2016).
Ren, J. et al. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 8, 17002–17011 (2017).
Yamamoto, T. N. et al. T cells genetically engineered to overcome death signaling enhance adoptive cancer immunotherapy. J. Clin. Invest. 129, 1551–1565 (2019).
Rafiq, S. et al. Targeted delivery of a PD-1-blocking scFV by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 36, 847–858 (2018).
Mohammed, S. et al. Improving chimeric antigen receptor-modified T cell function by reversing the immunosuppressive tumor microenvironment of pancreatic cancer. Mol. Ther. 25, 249–258 (2017).
Oda, S. K. et al. A Fas-4-1BB fusion protein converts a death to a pro-survival signal and enhances T cell therapy. J. Exp. Med. 217, e20191166 (2020).
Liao, Z. et al. Leveraging biomaterials for enhancing T cell immunotherapy. J. Control. Release 344, 272–288 (2022).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017). This study demonstrated that genetic engineering of a CAR into the TRAC locus improved T cell fitness compared to viral overexpression.
Straathof, K. C. et al. An inducible caspase 9 safety switch for T-cell therapy. Blood 105, 4247–4254 (2005).
Bordignon, C. et al. Transfer of the HSV-tk gene into donor peripheral blood lymphocytes for in vivo modulation of donor anti tumor immunity after allogeneic bone marrow transplantation. Hum. Gene Ther. 6, 813–819 (1995).
Wang, X. et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 118, 1255–1263 (2011).
Wiebking, V. et al. Metabolic engineering generates a transgene-free safety switch for cell therapy. Nat. Biotechnol. 38, 1441–1450 (2020).
Sakemura, R. et al. A Tet-On inducible system for controlling CD19-chimeric antigen receptor expression upon drug administration. Cancer Immunol. Res. 4, 658–668 (2016).
Wu, C.-Y., Roybal, K. T., Puchner, E. M., Onuffer, J. & Lim, W. A. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science 350, aab4077 (2015).
Jan, M. et al. Reversible ON- and OFF-switch chimeric antigen receptors controlled by lenalidomide. Sci. Transl. Med. 13, eabb6295 (2021).
Giordano-Attianese, G. et al. A computationally designed chimeric antigen receptor provides a small-molecule safety switch for T-cell therapy. Nat. Biotechnol. 38, 426–432 (2020).
Zajc, C. U. et al. A conformation-specific ON-switch for controlling CAR T cells with an orally available drug. Proc. Natl Acad. Sci. USA 117, 14926–14935 (2020).
Lee, S. M. et al. A chemical switch system to modulate chimeric antigen receptor T cell activity through proteolysis-targeting chimaera technology. ACS Synth. Biol. 9, 987–992 (2020).
Richman, S. A. et al. Ligand-induced degradation of a CAR permits reversible remote control of CAR T cell activity in vitro and in vivo. Mol. Ther. 28, 1932 (2020).
Labanieh, L. et al. Enhanced safety and efficacy of protease-regulated CAR-T cell receptors. Cell 185, 1745–1763.e22 (2022). The SNIP drug-regulated CAR system manifested a full dynamic range of activity devoid of leakiness, and in a ROR1 toxicity model could be tuned to selectively target tumour cells expressing high levels of ROR1 while sparing healthy cells expressing low levels of ROR1.
Li, H.-S. et al. High-performance multiplex drug-gated CAR circuits. Cancer Cell 40, 1294–1305 (2022).
Hyrenius-Wittsten, A. et al. SynNotch CAR circuits enhance solid tumor recognition and promote persistent antitumor activity in mouse models. Sci. Transl. Med. 13, eabd8836 (2021). This study demonstrated that SynNotch-inducible CAR-T cells have lower tonic signalling, greater stemness and enhanced potency compared with conventional CAR-T cells.
Hernandez-Lopez, R. A. et al. T cell circuits that sense antigen density with an ultrasensitive threshold. Science 371, 1166–1171 (2021).
Van Oekelen, O. et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat. Med. 27, 2099–2103 (2021).
Lamers, C. H. J. et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: Clinical evaluation and management of on-target toxicity. Mol. Ther. 21, 904–912 (2013).
Thistlethwaite, F. C. et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol. Immunother. 66, 1425–1436 (2017).
Straathof, K. et al. Antitumor activity without on-target off-tumor toxicity of GD2-chimeric antigen receptor T cells in patients with neuroblastoma. Sci. Transl. Med. 12, eabd6169 (2020).
Mount, C. W. et al. Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M+ diffuse midline gliomas letter. Nat. Med. 24, 572–579 (2018).
Kloss, C. C. et al. Dominant-negative TGF-β receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol. Ther. 26, 1855–1866 (2018).
Narayan, V. et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat. Med. 28, 724–734 (2022).
Roybal, K. T. et al. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell 164, 770–779 (2016).
Zhu, I. et al. Modular design of synthetic receptors for programmed gene regulation in cell therapies. Cell 185, 1431–1443.e16 (2022).
Srivastava, S. et al. Logic-gated ROR1 chimeric antigen receptor expression rescues T cell-mediated toxicity to normal tissues and enables selective tumor targeting. Cancer Cell 35, 489–503.e8 (2019).
Tousley, A. M. et al. Coopting T cell proximal signaling molecules enables Boolean logic-gated CAR T cell control. Preprint at bioRxiv https://doi.org/10.1101/2022.06.17.496457 (2022). LINK CAR utilizes proximal signalling molecules fused to membrane-bound scFvs to create an AND-gated CAR system.
Cho, J. H., Collins, J. J. & Wong, W. W. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell 173, 1426–1438.e11 (2018).
Lajoie, M. J. et al. Designed protein logic to target cells with precise combinations of surface antigens. Science 369, 1637–1643 (2020).
Fedorov, V. D., Themeli, M. & Sadelain, M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci. Transl. Med. 5, 215ra172 (2013).
Richards, R. M. et al. NOT-gated CD93 CAR T cells effectively target AML with minimized endothelial cross-reactivity. Blood Cancer Discov. 2, 648–665 (2021).
Tokatlian, T. et al. Mesothelin-specific CAR-T cell therapy that incorporates an HLA-gated safety mechanism selectively kills tumor cells. J. Immunother. Cancer 10, e003826 (2022).
Mansilla-Soto, J. et al. HLA-independent T cell receptors for targeting tumors with low antigen density. Nat. Med. 28, 345–352 (2022). This study showed that T cells expressing HIT receptors targeted to the TRAC locus are able to recognize and kill tumour cells with low antigen density.
Liu, Y. et al. Chimeric STAR receptors using TCR machinery mediate robust responses against solid tumors. Sci. Transl. Med. 13, eabb5191 (2021).
Xu, Y. et al. A novel antibody–TCR (AbTCR) platform combines Fab-based antigen recognition with gamma/delta-TCR signaling to facilitate T-cell cytotoxicity with low cytokine release. Cell Discov. 4, 62 (2018).
Baeuerle, P. A. et al. Synthetic TRuC receptors engaging the complete T cell receptor for potent anti-tumor response. Nat. Commun. 10, 2087 (2019).
Burton, J. et al. Inefficient exploitation of accessory receptors reduces the sensitivity of chimeric antigen receptors. Preprint at bioRxiv https://doi.org/10.1101/2021.10.26.465853 (2022).
Yarmarkovich, M. et al. Cross-HLA targeting of intracellular oncoproteins with peptide-centric CARs. Nature 599, 477–484 (2021). The authors developed a peptide-centric CAR targeting a peptide derived from an intracellular protein that is overexpressed in neuroblastoma.
Maschan, M. et al. Multiple site place-of-care manufactured anti-CD19 CAR-T cells induce high remission rates in B-cell malignancy patients. Nat. Commun. 12, 7200 (2021).
Pearson, A. D. et al. Paediatric Strategy Forum for medicinal product development of chimeric antigen receptor T-cells in children and adolescents with cancer. Eur. J. Cancer 160, 112–133 (2022).
Benjamin, R. et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: results of two phase 1 studies. Lancet 396, 1885–1894 (2020). This study showed the feasibility of using allogeneic, genome-edited CAR T cells.
Kagoya, Y. et al. Genetic ablation of HLA class I, class II, and the T-cell receptor enables allogeneic T cells to be used for adoptive T-cell therapy. Cancer Immunol. Res. 8, 926–936 (2020).
Wang, B. et al. Generation of hypoimmunogenic T cells from genetically engineered allogeneic human induced pluripotent stem cells. Nat. Biomed. Eng. 5, 429–440 (2021).
Hu, X. et al. Engineered hypoimmune allogeneic CAR T cells exhibit innate and adaptive immune evasion even after sensitization in humanized mice and retain potent anti-tumor activity. Blood 138, 1690 (2021).
Anzalone, A. V., Koblan, L. W. & Liu, D. R. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38, 824–844 (2020).
Simonetta, F. et al. Allogeneic CAR invariant natural killer T cells exert potent antitumor effects through host CD8 T-cell cross-priming. Clin. Cancer Res. 27, 6054–6064 (2021).
Heczey, A. et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat. Med. 26, 1686–1690 (2020).
Neelapu, S. S. et al. A phase 1 study of ADI-001: anti-CD20 CAR-engineered allogeneic gamma delta (γδ) T cells in adults with B-cell malignancies. J. Clin. Oncol. 40, 7509 (2022).
Klichinsky, M. et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 38, 947–953 (2020). The authors adapted vectors for efficient viral gene delivery of a CAR to macrophages.
Morrissey, M. A. et al. Chimeric antigen receptors that trigger phagocytosis. eLife 7, e36688 (2018).
Themeli, M. et al. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat. Biotechnol. 31, 928–933 (2013).
Zhu, H. et al. Metabolic reprograming via deletion of CISH in human iPSC-derived NK cells promotes in vivo persistence and enhances anti-tumor activity. Cell Stem Cell 27, 224–237.e6 (2020).
Zhang, L. et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J. Hematol. Oncol. 13, 153 (2020).
Bachanova, V. et al. Safety and efficacy of FT596, a first-in-class, multi-antigen targeted, off-the-shelf, iPSC-derived CD19 CAR NK cell therapy in relapsed/refractory B-cell lymphoma. Blood 138, 823 (2021).
Wang, Z. et al. 3D-organoid culture supports differentiation of human CAR+ iPSCs into highly functional CAR Tcells. Cell Stem Cell 29, 515–527.e8 (2022).
Roth, T. L. et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature 559, 405–409 (2018). The authors developed a non-viral approach to engineer T cells with genomic precision.
Zhang, J. et al. Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL. Nature 609, 369–374 (2022).
Shy, B. R. et al. High-yield genome engineering in primary cells using a hybrid ssDNA repair template and small-molecule cocktails. Nat. Biotechnol. https://doi.org/10.1038/S41587-022-01418-8 (2022).
Micklethwaite, K. P. et al. Investigation of product-derived lymphoma following infusion of piggyBac-modified CD19 chimeric antigen receptor T cells. Blood 138, 1391–1405 (2021).
Weidner, T. et al. Genetic in vivo engineering of human T lymphocytes in mouse models. Nat. Protoc. 16, 3210–3240 (2021).
Smith, T. T. et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotechnol. 12, 1–21 (2017).
Rurik, J. G. et al. CAR T cells produced in vivo to treat cardiac injury. Science 375, 91–96 (2022). This study utilized CD5-targeting nanoparticles for in vivo gene engineering of T cells to treat cardiac fibrosis.
Kansal, R. et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci. Transl. Med. 11, eaav1648 (2019).
Mougiakakos, D. et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N. Engl. J. Med. 385, 567–569 (2021). This case report showed a strong clinical benefit for treatment of systemic lupus erythematosus with CD19-CAR T cells.
Raffin, C., Vo, L. T. & Bluestone, J. A. Treg cell-based therapies: challenges and perspectives. Nat. Rev. Immunol. 20, 158–172 (2020).
MacDonald, K. G. et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J. Clin. Invest. 126, 1413–1424 (2016).
Noyan, F. et al. Prevention of allograft rejection by use of regulatory T cells with an MHC-specific chimeric antigen receptor. Am. J. Transplant. 17, 917–930 (2017).
Good, Z. et al. Post-infusion CAR Treg cells identify patients resistant to CD19-CAR therapy. Nat. Med. 28, 1860–1871 (2022).
Haradhvala, N. J. et al. Distinct cellular dynamics associated with response to CAR-T therapy for refractory B cell lymphoma. Nat. Med. 28, 1848–1859 (2022).
Fu, R. Y. et al. CD4+ T cells engineered with FVIII-CAR and murine Foxp3 suppress anti-factor VIII immune responses in hemophilia a mice. Cell. Immunol. 358, 104216 (2020).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132 (2020). This study showed that CAR T cells directed against the urokinase-type plasminogen activator receptor could be used as senolytic agents.
Aghajanian, H. et al. Targeting cardiac fibrosis with engineered T cells. Nature 573, 430–433 (2019).
Jacobson, C. A. et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 23, 91–103 (2022).
Shah, B. D. et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet 398, 491–502 (2021).
Frank, M. J. et al. CD22-CAR T-cell therapy mediates high durable remission rates in adults with large B-cell lymphoma who have relapsed after CD19-CAR T-cell therapy. Blood 138, 741 (2021).
Cui, Q. et al. CD38-directed CAR-T cell therapy: a novel immunotherapy strategy for relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. J. Hematol. Oncol. 14, 82 (2021).
Ramos, C. A. et al. Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J. Clin. Invest. 126, 2588–2596 (2016).
Louis, C. U. et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 118, 6050–6056 (2011).
Majzner, R. G. et al. Major tumor regressions in H3K27M-mutated diffuse midline glioma (DMG) following sequential intravenous (IV) and intracerebroventricular (ICV) delivery of GD2-CAR T cells. Cancer Res. 82, CT001 (2022).
Navai, S. A. et al. Administration of HER2-CAR T cells after lymphodepletion safely improves T cell expansion and induces clinical responses in patients with advanced sarcomas. Cancer Res. 79, LB147 (2019).
Guo, Y. et al. Phase I study of chimeric antigen receptor–modified T cells in patients with EGFR-positive advanced biliary tract cancers. Clin. Cancer Res. 24, 1277–1286 (2018).
Irving, B. A. & Weiss, A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell 64, 891–901 (1991).
Levine, B. L. et al. Large-scale production of CD4+ T cells from HIV-1-infected donors after CD3/CD28 costimulation. J. Hematotherapy Stem Cell Res. 7, 437–448 (1998).
Haynes, N. M. et al. Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors. Blood 100, 3155–3163 (2002).
Fry, T. J. et al. A potential role for interleukin-7 in T-cell homeostasis. Blood 97, 2983–2990 (2001).
Dudley, M. E. et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298, 850–854 (2002).
Pule, M. A. et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 14, 1264–1270 (2008).
Milone, M. C. et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 17, 1453–1464 (2009).
Kochenderfer, J. N. et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010).
Brentjens, R. J. et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 118, 4817–4828 (2011).
Savoldo, B. et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J. Clin. Invest. 121, 1822–1826 (2011).
Porter, D. L., Levine, B. L., Kalos, M., Bagg, A. & June, C. H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–33 (2011).
Hudecek, M. et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin. Cancer Res. 19, 3153–3164 (2013).
Landoni, E. et al. Modifications to the framework regions eliminate chimeric antigen receptor tonic signaling. Cancer Immunol. Res. 9, 441–453 (2021).
Awasthi, R. et al. Tisagenlecleucel cellular kinetics, dose, and immunogenicity in relation to clinical factors in relapsed/refractory DLBCL. Blood Adv. 4, 560–572 (2020).
Leddon, S. A. et al. The CD28 transmembrane domain contains an essential dimerization motif. Front. Immunol. 11, 1519 (2020).
Bridgeman, J. S. et al. The optimal antigen response of chimeric antigen receptors harboring the CD3 transmembrane domain is dependent upon incorporation of the receptor into the endogenous TCR/CD3 complex. J. Immunol. 184, 6938–6949 (2010).
Fujiwara, K. et al. Hinge and transmembrane domains of chimeric antigen receptor regulate receptor expression and signaling threshold. Cells 9, 1182 (2020).
Brentjens, R. J. et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat. Med. 9, 279–86 (2003).
Kawalekar, O. U. et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 44, 380–390 (2016).
Yan, Z. et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: a single-arm, phase 2 trial. Lancet Haematol. 6, e521–e529 (2019).
Schneider, D. et al. Trispecific CD19–CD20–CD22-targeting duoCAR-T cells eliminate antigen-heterogeneous B cell tumors in preclinical models. Sci. Transl. Med. 13, eabc6401 (2021).
Kloss, C. C., Condomines, M., Cartellieri, M., Bachmann, M. & Sadelain, M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 31, 71–75 (2013).
Han, X. et al. Masked chimeric antigen receptor for tumor-specific activation. Mol. Ther. 25, 274–284 (2017).
Kosti, P. et al. Hypoxia-sensing CAR T cells provide safety and efficacy in treating solid tumors. Cell Rep. Med. 2, 100227 (2021).
Ho, P., Ede, C. & Chen, Y. Y. Modularly constructed synthetic granzyme B molecule enables interrogation of intracellular proteases for targeted cytotoxicity. ACS Synth. Biol. 6, 1484–1495 (2017).
Scharping, N. E. et al. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity 45, 374–388 (2016).
Seo, H. et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion. Proc. Natl Acad. Sci. USA 116, 12410–12415 (2019).
Yang, W. et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature 531, 651–655 (2016).
Jung, I. Y. et al. CRISPR/Cas9-mediated knockout of DGK improves antitumor activities of human T cells. Cancer Res. 78, 4692–4703 (2018).
Zhang, Y. et al. CRISPR–Cas9 mediated LAG-3 disruption in CAR-T cells. Front. Med. 11, 554–562 (2017).
Sterner, R. M. et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood 133, 697–709 (2019).
Qasim, W. et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 9, eaaj2013 (2017).
Johnson, A. J. et al. Rationally designed transgene-encoded cell-surface polypeptide tag for multiplexed programming of CAR T-cell synthetic outputs. Cancer Immunol. Res. 9, 1047–1060 (2021).
Becerra, C. R. et al. Ligand-inducible, prostate stem cell antigen (PSCA)-directed GoCAR-T cells in advanced solid tumors: preliminary results from a dose escalation. J. Clin. Oncol. 37, 282–283 (2019).
Munoz, J. et al. A phase 1 study of ACTR087 in combination with rituximab, in subjects with relapsed or refractory CD20-positive B-cell lymphoma. Blood 134, 244 (2019).
Ma, J. S. Y. et al. Versatile strategy for controlling the specificity and activity of engineered T cells. Proc. Natl Acad. Sci. USA 113, E450–E458 (2016).
Nikolaenko, L. et al. First in human study of an on/off switchable CAR-T cell platform targeting CD19 for B cell malignancies (CLBR001 + SWI019). Blood 138, 2822 (2021).
Frigault, M. J. et al. Phase 1 study of CART-ddBCMA in relapsed or refractory multiple myeloma. J. Clin. Oncol. 40, 8003–8003 (2022).
Wu, Y. et al. Control of the activity of CAR-T cells within tumours via focused ultrasound. Nat. Biomed. Eng. 5, 1336–1347 (2021).
Allen, M. E. et al. An AND-gated drug and photoactivatable Cre- loxP system for spatiotemporal control in cell-based therapeutics. ACS Synth. Biol. 8, 2359–2371 (2019).
O’Cearbhaill, R. E. et al. A phase I clinical trial of autologous chimeric antigen receptor (CAR) T cells genetically engineered to secrete IL-12 and to target the MUC16ecto antigen in patients (pts) with MUC16ecto+ recurrent high-grade serous ovarian cancer (HGSOC). Gynecol. Oncol. 159, 42 (2020).
Hu, B. et al. Augmentation of antitumor immunity by human and mouse CAR T cells secreting IL-18. Cell Rep. 20, 3025–3033 (2017).
Svoboda, J. et al. Interleukin-18 secreting autologous anti-CD19 CAR T-cells (huCART19-IL18) in patients with non-Hodgkin lymphomas relapsed or refractory to prior CAR T-cell therapy. Blood 140, 4612–4614 (2022).
Zhang, L. et al. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol. Ther. 19, 751–759 (2011).
Batra, S. A. et al. Glypican-3-specific CAR T cells coexpressing IL15 and IL21 have superior expansion and antitumor activity against hepatocellular carcinoma. Cancer Immunol. Res. 8, 309–320 (2020).
Liu, X. et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res. 76, 1578–1590 (2016).
Papa, S. et al. A phase I trial of T4 CAR T-cell immunotherapy in head and neck squamous cancer (HNSCC). J. Clin. Oncol. 36, 3046–3046 (2018).
Kakarla, S. et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol. Ther. 21, 1611–1620 (2013).
Caruana, I. et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med. 21, 524–9 (2015).
Ligtenberg, M. A. et al. Coexpressed catalase protects chimeric antigen receptor-redirected T cells as well as bystander cells from oxidative stress-induced loss of antitumor activity. J. Immunol. 196, 759–66 (2016).
Boice, M. et al. Loss of the HVEM tumor suppressor in lymphoma and restoration by modified CAR-T Cells. Cell 167, 405–418.e13 (2016).
Newick, K. et al. Augmentation of CAR T-cell trafficking and antitumor efficacy by blocking protein kinase a localization. Cancer Immunol. Res. 4, 541–551 (2016).
Li, G. et al. CXCR5 guides migration and tumor eradication of anti-EGFR chimeric antigen receptor T cells. Mol. Ther. 22, 507–517 (2021).
Di Stasi, A. et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood 113, 6392–6402 (2009).
Craddock, J. A. et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J. Immunother. 33, 780–788 (2010).
Siddiqui, I., Erreni, M., van Brakel, M., Debets, R. & Allavena, P. Enhanced recruitment of genetically modified CX3CR1-positive human T cells into Fractalkine/CX3CL1 expressing tumors: importance of the chemokine gradient. J. Immunother. Cancer 4, 21 (2016).
Feng, J. et al. Treatment of aggressive T cell lymphoblastic lymphoma/leukemia using anti-CD5 CAR T cells. Stem Cell Rev. Rep. 17, 652–661 (2021).
Hurton, L. V. et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc. Natl Acad. Sci. USA 113, E7788–E7797 (2016).
Hunter, M. R. et al. Chimeric γc cytokine receptors confer cytokine independent engraftment of human T lymphocytes. Mol. Immunol. 56, 1–11 (2013).
Zhang, Q. et al. A human orthogonal IL-2 and IL-2Rβ system enhances CAR T cell expansion and antitumor activity in a murine model of leukemia. Sci. Transl. Med. 13, eabg6986 (2021).
Kalbasi, A. et al. Potentiating adoptive cell therapy using synthetic IL-9 receptors. Nature 607, 360–365 (2022).
Krause, A. et al. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. J. Exp. Med. 188, 619–626 (1998).
Ren, J. et al. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin. Cancer Res. 23, 2255–2266 (2017).
Kamiya, T., Wong, D., Png, Y. T. & Campana, D. A novel method to generate T-cell receptor-deficient chimeric antigen receptor T cells. Blood Adv. 2, 517–528 (2018).
Acknowledgements
C.L.M. and L.L are members of the Parker Institute for Cancer Immunotherapy, which supports the Stanford University Cancer Immunotherapy Program. This work was supported by the St Baldrick’s Foundation Empowering Pediatric Immunotherapies for Childhood Cancer (EPICC) Team and NCI 5P30CA124435 (C.L.M.). L.L. was supported by a Siebel Scholars Fellowship, Stanford Graduate Fellowship, National Science Foundation Graduate Research Fellowship (DGE-1656518), and Discovery Innovation Award philanthropically supported through the Biomedical Innovation Initiative.
Author information
Authors and Affiliations
Contributions
L.L. and C.L.M. conceptualized, wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.L. and C.L.M. are inventors on several patents related to CAR T cell therapies. C.L.M. is a cofounder of Lyell Immunopharma, CARGO Therapeutics and Link Cell Therapies, which are developing CAR-based therapies, and consults for Lyell, Syncopation, Link, Apricity, Nektar, Immatics, Ensoma, Mammoth, Glaxo Smith Kline and Bristol Myers Squibb. L.L. is a cofounder of, consults for, and holds equity in CARGO Therapeutics. L.L. is a consultant for and holds equity in Lyell Immunopharma.
Peer review
Peer review information
Nature thanks Justin Eyquem, Kristen Hege, Hans Stauss and Jie Sun for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Labanieh, L., Mackall, C.L. CAR immune cells: design principles, resistance and the next generation. Nature 614, 635–648 (2023). https://doi.org/10.1038/s41586-023-05707-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-05707-3
- Springer Nature Limited
This article is cited by
-
A new era of cancer immunotherapy: combining revolutionary technologies for enhanced CAR-M therapy
Molecular Cancer (2024)
-
CD19/CD20 dual-targeted chimeric antigen receptor-engineered natural killer cells exhibit improved cytotoxicity against acute lymphoblastic leukemia
Journal of Translational Medicine (2024)
-
Gene therapy for polygenic or complex diseases
Biomarker Research (2024)
-
Rapid identification of early infections in febrile patients after CD19 target CAR-T cell therapy for B-cell malignancies
Journal of Translational Medicine (2024)
-
Role of Exosomes in Cancer and Aptamer-Modified Exosomes as a Promising Platform for Cancer Targeted Therapy
Biological Procedures Online (2024)