Abstract
The oxidative cleavage of alkenes is an integral process that converts feedstock materials into high-value synthetic intermediates1,2,3. The most viable method to achieve this in one chemical step is with ozone4,5,6,7; however, this poses technical and safety challenges owing to the explosive nature of ozonolysis products8,9. Here we report an alternative approach to achieve oxidative cleavage of alkenes using nitroarenes and purple-light irradiation. We demonstrate that photoexcited nitroarenes are effective ozone surrogates that undergo facile radical [3+2] cycloaddition with alkenes. The resulting ‘N-doped’ ozonides are safe to handle and lead to the corresponding carbonyl products under mild hydrolytic conditions. These features enable the controlled cleavage of all types of alkenes in the presence of a broad array of commonly used organic functionalities. Furthermore, by harnessing electronic, steric and mediated polar effects, the structural and functional diversity of nitroarenes has provided a modular platform to obtain site selectivity in substrates containing more than one alkene.
Similar content being viewed by others
Main
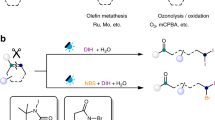
Alkenes are feedstock materials that are obtained on the ton scale from petroleum and vegetable biomass and are exploited by the bulk chemical industry to access oxygen-enriched synthetic intermediates1,2,3. Ozonolysis is a widely adopted method to achieve this and requires specialized apparatus for the conversion of molecular oxygen (O2) into highly reactive ozone (O3)6,7. This species undergoes a [1,3]-dipolar cycloaddition with the alkene, converting a stable chemical into a high-energy 1,2,3-ozonide A from which cycloreversion is immediate. The consequent C–C σ-bond cleavage event generates carbonyl oxide B and carbonyl compound C, which recombine to give 1,2,4-ozonide D. Depending on the reaction solvent and the work-up procedure, B or D can lead to aldehydes or ketones, as well as carboxylic acids or alcohols4,5 (Fig. 1a).
Despite its attractive synthetic versatility, ozone toxicity (lethal at 5 ppm), explosivity and extreme oxidizing power (standard reduction potential E0 = 2.07 V) raise critical safety, technical and chemical concerns8,9. As a result, ozonolytic strategies are often challenging to translate into the fine chemical industry10,11,12, particularly in the discovery sector, which heavily relies on parallel and high-throughput screening platforms13. Consequently, alternative strategies for alkene oxidation based on high-valent heavy-metal oxides (MO4, where M is a metal) have been devised14,15,16. However, these approaches can yield mixtures of products of various oxidation degrees, and cause trace-metal contaminations that are problematic with the stringent pharmaceutical sector regulations17. Oxidative cleavages using O2 and a suitable (photo)catalyst have also been developed, but they are limited to activated alkenes18,19.
Overall, there is no other type of reactivity able to mirror the unique ability of ozone to cleave alkenes. Here we introduce nitroarenes, a class of abundant feedstocks, as photoexcitable and easy-to-dose ozone surrogates. Upon simple purple-light absorption, these species react with alkenes enabling access to ‘N-doped’ ozonides, which can be accumulated until a devised controlled C–C bond cleavage step takes place. This reactivity engages a large class of alkenes, is tolerant of the most used organic functionalities and allows the targeting of specific double bonds in molecules with multiple C–Cπ sites.
In approaching the design of an alternative method to oxidatively cleave alkenes, we considered the possibility of using nitroarenes N as ozone surrogates to access 1,3,2-dioxazolidines E (Fig. 1b). Despite the nitro group being isoelectronic with ozone, nitroarenes do not engage in thermal [1,3]-dipolar cycloadditions with alkenes owing to high kinetic barriers20,21. The pioneering works of refs. 22,23 demonstrated an opportunity to by-pass these challenging pericyclic processes through direct nitroarene photoexcitation. As such, intersystem crossing from the singlet excited nitroarene delivers the long-lived triplet state (T1) *N. In analogy to T1 carbonyls, *N have a (n,π*) configuration, which translates into O-radical-type reactivity. *N can intercept alkenes in radical [3+2]-like fashion and, via the formation of biradical F, deliver N-doped ozonides E. However, this chemistry necessitated high-energy irradiation, utilized the alkene as the solvent, and the mechanism by which E evolves into the C–C cleavage products and defines their subsequent fate was unsolved22,23,24,25. These rather unpractical reactivity requirements and limited understanding have resulted in no synthetic application.
We envisaged that by tailoring the nature of the nitroarene, we would have been able to translate this reactivity over the broad spectrum of alkenes, including challenging terminal substrates, and run it in a stoichiometric manner, which is crucial for synthetic purposes. Furthermore, understanding and thereby controlling the decomposition of E would be pivotal to channel its reactivity towards alkene cleavage.
We started our investigation evaluating the initial rates of disappearance of 1 (kobs) in photocycloaddition reactions (purple light-emitting diode irradiation, wavelength λ = 390 nm (refs. 26,27)) with a variety of electronically diverse meta- and/or para-(di)substituted nitroarenes N to give stable bisadamantene-containing24 E (Fig. 2a). The resulting Hammett plot28 showed a strong linear free-energy relationship between the electronic character of the nitroarene and kobs (sensitivity constant ρ = 0.82). This means that the reactivity profile of nitroarenes as photo-responsive oxidants can be easily tuned by correct placement of electron-withdrawing groups on their aromatic core to amplify the electrophilic character of their excited states. Ortho-substituted N were less effective, which suggests that steric hindrance can also influence their reactivity (Supplementary Information).
a, Hammett plot analysis. kX refers to kobs with 3-, 4-, 3,5- or 3,4-(di)substituted nitroarenes, kH refers to the parent unsubstituted nitrobenzene and σ is the Hammett constant. b, Preparation of E1. c, Mechanism for the decomposition of E and the role of H2O. d, 18O-labelling experiments and the role of 6. e, Methods for the removal of H.
We then evaluated the reaction of unactivated 2, which is a challenging type of alkene in this chemistry. Irradiation of 2 with commercial N1 resulted in the high-yielding formation of E1 (Fig. 2b). In contrast to the explosive nature of A, N-doped ozonides can be accumulated in solution at –30 °C and are stable in the solid state (Supplementary Information).
Subsequently, we set out to understand how to convert E into the corresponding carbonyl compounds C and C′ (Fig. 2c). We speculated that two pathways might be operating: an ozonolysis-type cycloreversion would deliver C and carbonyl imine G (path a) or a different cycloreversion-mode could directly lead to C/C′ and nitrene I (path b). To shed light on this, we prepared E2 which features an ortho-3,5-dimethylpyrazole group as a probe for nitrene formation29,30. Simple exposure of E2 to CH3CN–H2O led to the almost quantitative formation of ketone 3 and N-arylhydroxylamine H1. Conversely, in acetonitrile (CH3CN), E2 yielded 3 in 98% yield and 4 in 97% yield, whose structure was confirmed by X-ray analysis. As 4 is indicative of a [1,3]-dipolar cycloaddition between G1 and CH3CN, these experiments rule out the intermediacy of nitrenes and demonstrate that E undergoes an ozonolysis-type cycloreversion generating C and G, which, with water (H2O), is hydrolysed to C′ and H. Although the generation of G was postulated by Huisgen22, its existence has not been demonstrated before. Related dipoles have been engaged in 1,3-dipolar cycloadditions only twice since Huisgen’s initial prediction31,32, but never with nitrile dipolarophiles.
Next, we studied the decomposition of E1 in CH3CN–H218O (Fig. 2d), which gave 5 in 93% yield and 92% 18O-incorporation, along with H2 (20%), azoxy derivative K1 (3%) and nitrone J1 (condensation of H2 with formalin 6, 61%). K1 stems from disproportionation of H2 (Supplementary Information). This experiment demonstrates that the cycloreversion of E1 generates 6 and the more stabilized dipole G, which is then hydrolysed. However, when decomposition of E3 was evaluated in CH3CN–H2O, 7 was obtained in a decreased 71% yield, probably via the in situ formation of nitrone J. Indeed, when decomposition of E3 was run with external formalin, 7 was obtained in 95% yield (Supplementary Information). Despite the decomposition step being very effective, the equilibrium in the condensation between H and C/C′, and subsequent side reactions33, rendered the purification of the aldehydes products challenging. Thus, we developed two simple one-pot work-up procedures to remove H by addition of either dipotassium phosphate (K2HPO4) and urea (conversion of H into K) or N-phenylmaleimide (conversion of nitrones such as J1 into L), which eased the purification of the aldehydes (Fig. 4e and Supplementary Information).
Having devised conditions to accumulate and decompose N-doped ozonides, we decided to benchmark the synthetic utility of the process. Although N1 was able to engage all substrates present in Fig. 3, other nitroarenes (N2–N7) were evaluated to improve the yield depending on the alkene. We believe that this is a powerful aspect of this reactivity as functionalized nitroarenes are readily available and dosable reagents that can be evaluated in screening platforms. Exploration began with linear terminal alkenes 2 and 8–32 equipped with a distal functionality R. Several commonly encountered organic functional groups, such as nitrile (8), aldehyde (9), ketone (10), carboxylic acid (11), halogens (12–15), free (16) and protected (17–20) amines, azide (21), nitro group (22), free (23 also on 5-mmol scale, 27) and protected (24 and 25) alcohols, epoxide (26), thiocyanate (28), thioethers (29 and 30), phosphonate (31) and boronic ester (32), proved compatible, giving the corresponding aldehydes in high yields. In some cases, we found that the use of dichloromethane (CH2Cl2) solvent with hexafluoroisopropanol (HFIP) as the additive to be crucial to ensure good reactivity. As *N can abstract hydridic α-N/O/S C(sp3)–H bonds34,35, the inclusion of HFIP suppressed this unwanted process by hydrogen bonding to the heteroatom36 (Supplementary Information). In the case of amines, simple protonation was required to insulate the substrate from detrimental side oxidations. Disubstituted substrates reacted well, as demonstrated by the cleavage of several industrially relevant oleic acid derivatives 33–38 (Z-), as well as ether E-39 and cyclic systems of different size (40 and 41). Furthermore, both gem-disubstituted (–)-dihydrocarveol 42 and trisubstituted 43 were compatible, as well as diene 44. Electron-rich and -poor styrenes (45–47), (E and Z)-β-Me and α-Me-styrenes (48, 49 and 50), as well as (E and Z)-stilbenes reacted smoothly (51 and 52). Next, we explored the cleavage of structurally complex and densely functionalized derivatives (53–63). Unactivated terminal alkenes of isophytol (53), sclareol (54) and alibendol (55), which also features an electron-rich aromatic core, could all be oxidized. The disubstituted alkenes of caryophyllene oxide (56) and montelukast (57), the trisubstituted alkenes of phytol (58), (–)-α-cedrene (59), triprolidine (60), chlorprothixene (61) and lumefantrine (62), as well as the tetrasubstituted (Z)-tamoxifen 63, were successfully engaged. Another feature of *N is their aptitude to act as triplet sensitizers37, which can isomerize the alkenes during photocycloaddition. Indeed, unreacted 63 was recovered as Z/E mixture.
‘A’ and ‘B’ refer to the reaction conditions and N1–N7 refer to the nitroarenes. If the yield of two cleaved fragments deriving from the same alkene differs, the first yield refers to the carbonyl compound with the higher molecular weight. Cleavage: CH2O (0–6 equiv.) in CH3CN/THF:H2O (3.1:1); work-up: K2HPO4 and urea or N-phenylmaleimide. See Supplementary Information for details. aAlkene with a 10C linear chain. bPerfluoro-tert-butanol (PFTB) used in place of HFIP. cAlkene with a 6C linear chain. d60 h. e5.0-mmol scale; N1 (1.5 equiv.); 48 h. f2,6-Lutidine in place of HFIP. gNMR yield. hAlkene (2 equiv.). iAnother product of S oxidation to sulfoxide, 6% yield. jAminoaldehyde derivative not detected. k40 h. lAlkene (5 equiv.). r.s.m., remaining starting material.
An often-encountered challenge in oxidative cleavage chemistry is achieving regiocontrol in substrates containing more than one C–Cπ site. We speculated that the inherent modularity of our approach would enable chemoselective differentiation through the interplay of electronic effects. To test this hypothesis, we prepared substrates 64–69 that contain two different alkenes linked by an identical alkyl spacer and evaluated them in stoichiometric reactions with N1, N2, N4 and N8 providing the heat map shown in Fig. 4a. These results show that site selectivity depends on the electronic nature of the nitroarene and that of the two alkenes. Specifically, the selectivity increases when using less electrophilic nitroarenes, and when the two alkenes have substituents that make one C–Cπ bond increasingly more electron-rich than the other. This means that the reactivity of *N (ref. 23) parallels that of ozone and Huisgen type-III dipolar cycloadditions in general38. Consequently, modulation of the nitroarene electronics can be used to amplify narrow reactivity differences when substrate control is difficult to implement. Indeed, the use of N8 and N4 enabled the fully selective cleavage of trisubstituted alkene (64) and styrene (65) in the presence of monosubstituted alkenes. Furthermore, striking discrimination was achieved between internal and terminal double bonds (66, 97%), tri-alkyl versus di-alkyl substituted C–Cπ sites (67, 87%), styrene versus an internal alkene (68, 85%), as well as the two highly activated alkenes of 69 (69%). Moreover, a complete selectivity for the terminal alkene of 70 was obtained even with N1, yielding the corresponding alkyne-containing product in 82% yield.
a, Competition experiments. N1, N2, N4 and N8 refer to the nitroarenes used. Cleavage: CH2O (0–6 equiv.) in CH3CN/THF:H2O (3.1:1). Selectivity determined considering bis gas chromatography-flame ionization detection (GC-FID) yield. See Supplementary Information for details. aAlkene (2.5 equiv.). bN1 (2 equiv.); CH2Cl2 with HFIP (1 equiv.). b, Complex examples. N1–N4, N7, N9 and N10 refer to the nitroarenes used. aCH2Cl2 with HFIP (0.5–6 equiv.). bAlkene (2 equiv.). cNMR yield. dN (2 equiv.). eEtOAc. fAlkene (3 equiv.). gBis-cleaved product 3% yield. hBis-cleaved product 2% yield. iCH2Cl2 without HFIP. jBis-cleaved product 6% yield. kAlkene (5 equiv.).
To demonstrate reactivity control through electronic, steric and mediated polar effects, we evaluated several complex and bio-active molecules containing multiple C–Cπ sites (Fig. 4b and Supplementary Information). (–)-Carvone 71 and fusidic acid 72 showed regiocontrol based on electronics as alkene conjugation with carbonyl functionalities directed the reactivity towards the other alkenes. In the case of polyunsaturated steroids exemestane 73 and megestrol acetate 74, oxidative cleavage occurred at the distal, hence less deactivated, C–Cπ sites. Lynestrol 75 showcased alkene oxidation in the presence of alkynes, whereas allylestrenol acetate 76, linalool 77 and trans-caryophyllene 78 showed the selective cleavage of trisubstituted alkenes over terminal and gem-disubstituted ones. Geranyl acetate 79 and perillyl acetate 80 contain trisubstituted alkenes with an allylic OAc group that provides weak inductive deactivation. Although this enables the preferential cleavage of the other C–Cπ sites, higher selectivity was obtained adding HFIP (hydrogen bonding with the OAc group). Analogously, the presence of the electron-withdrawing ammonium group in cyclobenzaprine 81 allowed the disubstituted stilbene-type alkene to react over the trisubstituted one. We propose that steric control might be the main factor determining the selectivity in the oxidative cleavage of bisabolol acetate 82 and valencene 83 where the least hindered acyclic alkenes were oxidized despite their degree of substitution.
Owing to the striking functional group compatibility and levels of site selectivity achievable, our findings demonstrate that nitroarenes are tunable and easy-to-dose photo-responsive ozone surrogates, which have the premise to become a powerful and reliable tool to oxidatively cleave alkenes.
Data availability
The data that support the findings of this study are available from the corresponding author (D.L.) upon reasonable request.
References
Baumann, H. et al. Natural fats and oils—renewable raw materials for the chemical industry. Angew. Chem. Int. Ed. 27, 41–62 (1988).
Corma, A., Iborra, S. & Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 107, 2411–2502 (2007).
Köckritz, A. & Martin, A. Oxidation of unsaturated fatty acid derivatives and vegetable oils. Eur. J. Lipid Sci. Technol. 110, 812–824 (2008).
Bailey P. S. Ozonization in Organic Chemistry Vol. 1. (Academic Press, 1978).
Fisher, T. J. & Dussault, P. H. Alkene ozonolysis. Tetrahedron 73, 4233–4258 (2017).
Surburg, H. & Panten, J. Common Fragrance and Flavor Materials (Wiley-VHC, 2006).
Caron, S., Dugger, R. W., Ruggeri, S. G., Ragan, J. A. & Ripin, D. H. B. Large-scale oxidations in the pharmaceutical industry. Chem. Rev. 106, 2943–2989 (2006).
Allian, A. in Managing Hazardous Reactions and Compounds in Process Chemistry (eds Pesti, J. A. & Abdel-Magid, A. F.) 353–382 (ACS, 2014).
Kula, J. Safer ozonolysis reactions: a compilation of laboratory experience. Chem. Health Saf. 6, 21–22 (1999).
Van Ornum, S. G., Champeau, R. M. & Pariza, R. Ozonolysis applications in drug synthesis. Chem. Rev. 106, 2990–3001 (2006).
Gabric, A., Hodnik, Z. & Pajk, S. Oxidation of drugs during drug product development: problems and solutions. Pharamaceutics 14, 325 (2000).
Hoelderich, W. F. & Kollmer, F. Oxidation reactions in the synthesis of fine and intermediate chemicals using environmentally benign oxidants and the right reactor system. Pure Appl.Chem. 72, 1273–1287 (2000).
High-Throughput Screening in Drug Discovery (ed. Hüser, J.) Vol. 35 (Wiley-VCH, 2006).
Spannring, P., Bruijnincx, P. C. A., Weckhuysen, B. M. & Klein Gebbink, R. J. M. Transition metal-catalyzed oxidative double bond cleavage of simple and bio-derived alkenes and unsaturated fatty acids. Catal. Sci. Technol. 4, 2182–2209 (2014).
Yang, D. & Zhang, C. Ruthenium-catalyzed oxidative cleavage of alkenes to aldehydes. J. Org. Chem. 14, 4814–4818 (2001).
Yu, W., Mei, Y., Kang, Y., Hua, Z. & Jin, Z. Improved procedure for the oxidative cleavage of alkenes by OsO4–NaIO4. Org. Lett. 6, 3217–3219 (2004).
Maithani, M., Raturi, R., Sharma, P., Gupta, V. & Bansal, P. Elemental impurities in pharmaceutical products adding fuel to the fire. Regul. Toxicol. Pharmacol. 108, 104435 (2019).
Urgoitia, G., SanMartin, R., Herrero, M. T. & Domínguez, E. Aerobic cleavage of alkenes and alkynes into carbonyl and carboxyl compounds. ACS Catal. 7, 3050–3060 (2017).
Huang, Z. et al. Oxidative cleavage of alkenes by O2 with a non-heme manganese catalyst. J. Am. Chem. Soc. 143, 10005–10013 (2021).
Ranganathan, S., Ranganathan, D., Ramachandran, P. V., Mahanty, M. K. & Bamezai, S. A chemical and thermochemical study of non-observed symmetry allowed reactions. Tetrahedron 37, 4171–4184 (1981).
Leitich, J. 1,3,2-Dioxazolidines by thermal 1,3-cycloaddition of nitro groups to strained alkenes. Angew. Chem. Int. Ed. 15, 372–373 (1976).
Buchi, G. & Ayer, D. E. Light catalyzed organic reactions. IV. The oxidation of alkenes with nitrobenzene. J. Am. Chem. Soc. 78, 689–690 (1956).
De Mayo, P., Charlton, J. L. & Liao, C. C. Photochemical synthesis. XXXV. Addition of aromatic nitro compounds to alkenes. J. Am. Chem. Soc. 93, 2463–2471 (1971).
Okada, K., Saito, Y. & Oda, M. Photochemical reaction of polynitrobenzenes with adamantylideneadamantane: the X-ray structure analysis and chemical properties of the dispiro N-(2,4,6-trinitrophenyl)-1,3,2-dioxazolidine product. J. Chem. Soc. Chem. Commun. 1731–1732 (1992).
D’Auria, M., Esposito, V. & Mauriello, G. Photochemical reactivity of aromatic and heteroaromatic nitroderivatives in the presence of arylalkenes. Tetrahedron 52, 14253–14272 (1996).
Lu, C. et al. Intramolecular reductive cyclization of o-nitroarenes via biradical recombination. Org. Lett. 21, 1438–1443 (2019).
Gang, L. et al. Light-promoted C–N coupling of aryl halides with nitroarenes. Angew. Chem. Int. Ed. 133, 5290–5294 (2021).
Hansch, C., Leo, A. & Taft, R. W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991).
Albini, A., Bettinetti, G. & Minola, G. Photodecomposition of some para-substituted 2-pyrazolylphenyl azides. substituents affect the phenylnitrene S-T gap more than the barrier to ring expansion. J. Am. Chem. Soc. 121, 3104–3113 (1999).
Carra, C., Bally, T. & Albini, A. Role of conformation and electronic structure in the chemistry of ground and excited state o-pyrazolylphenylnitrenes. J. Am. Chem. Soc. 127, 5552–5562 (2005).
Partridge, K. M., Guzei, I. A. & Yoon, T. P. Carbonyl imines from oxaziridines: generation and cycloaddition of N–O=C dipoles. Angew. Chem. Int. Ed. 49, 930–934 (2010).
Zhao, E., Zhou, F. & Zhao, Y. Lewis acids promoted 3 + 2 cycloaddition of oxaziridines and cyclic allylic alcohols through carbonyl imine intermediates. J. Org. Chem. 84, 4282–4293 (2019).
Murahashi, S.-I. & Imada, Y. Synthesis and transformations of nitrones for organic synthesis. Chem. Rev. 119, 4684–4716 (2019).
Hurley, R. & Testa, A. C. Photochemical n → π* excitation of nitrobenzene. J. Am. Chem. Soc. 88, 4330–4332 (1966).
Hurley, R. & Testa, A. C. Nitrobenzene photochemistry. II. Protonation in the excited state. J. Am. Chem. Soc. 89, 6917–6919 (1967).
Bietti, M. Activation and deactivation strategies promoted by medium effects for selective aliphatic C–H bond functionalization. Angew. Chem. Int. Ed. 57, 16618–16637 (2018).
Strieth-Kalthoff, F. & Glorius, F. Triplet energy transfer photocatalysis: unlocking the next level. Chem 6, 1888–1903 (2020).
Huisgen, R. 1,3-Dipolar cycloadditions. 76. Concerted nature of 1,3-dipolar cycloadditions and the question of diradical intermediates. J. Org. Chem. 41, 403–419 (1976).
Acknowledgements
D.L. thanks EPSRC for a Fellowship (EP/P004997/1) and a grant (EP/V046799/1), the European Research Council for a research grant (758427), the Leverhulme Trust for additional support (Philip Leverhulme Prize to D.L.). We acknowledge I. J. Vitorica-Yrezabal (University of Manchester) for solving the X-ray crystal structure of 4, and N. S. Sheikh for discussions.
Author information
Authors and Affiliations
Contributions
M.S. and D.L. designed the project; M.S. directed the work; M.S., A.R. and C.H. performed all synthetic and mechanistic experiments. M.S. and D.L. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Martins Oderinde and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Sections 1–26—see Contents page for details.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ruffoni, A., Hampton, C., Simonetti, M. et al. Photoexcited nitroarenes for the oxidative cleavage of alkenes. Nature 610, 81–86 (2022). https://doi.org/10.1038/s41586-022-05211-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05211-0
- Springer Nature Limited
This article is cited by
-
Persistent organonickel complexes as general platforms for Csp2–Csp3 coupling reactions
Nature Chemistry (2024)
-
Water mediated redox-neutral cleavage of arylalkenes via photoredox catalysis
Nature Communications (2024)
-
Synthesis of polysubstituted azepanes by dearomative ring expansion of nitroarenes
Nature Chemistry (2024)
-
Multiple photofluorochromic luminogens via catalyst-free alkene oxidative cleavage photoreaction for dynamic 4D codes encryption
Nature Communications (2024)
-
Characterization of A π–π stacking cocrystal of 4-nitrophthalonitrile directed toward application in photocatalysis
Nature Communications (2024)