Abstract
Basal cell carcinoma (BCC) is the most frequent cancer in humans and results from constitutive activation of the Hedgehog pathway1. Several Smoothened inhibitors are used to treat Hedgehog-mediated malignancies, including BCC and medulloblastoma2. Vismodegib, a Smoothened inhibitor, leads to BCC shrinkage in the majority of patients with BCC3, but the mechanism by which it mediates BCC regression is unknown. Here we used two genetically engineered mouse models of BCC4 to investigate the mechanisms by which inhibition of Smoothened mediates tumour regression. We found that vismodegib mediates BCC regression by inhibiting a hair follicle-like fate and promoting the differentiation of tumour cells. However, a small population of tumour cells persists and is responsible for tumour relapse following treatment discontinuation, mimicking the situation found in humans5. In both mouse and human BCC, this persisting, slow-cycling tumour population expresses LGR5 and is characterized by active Wnt signalling. Combining Lgr5 lineage ablation or inhibition of Wnt signalling with vismodegib treatment leads to eradication of BCC. Our results show that vismodegib induces tumour regression by promoting tumour differentiation, and demonstrates that the synergy between Wnt and Smoothened inhibitors is a clinically relevant strategy for overcoming tumour relapse in BCC.
Similar content being viewed by others
Main
Vismodegib (GDC0449) is the first Smoothened inhibitor to be approved for the treatment of locally advanced and metastatic BCC. A small fraction of patients does not respond to vismodegib administration: their tumours continue to grow and do not show inhibition of the Hedgehog (Hh) signalling pathway during vismodegib treatment3. This type of vismodegib resistance is frequently associated with genetic mutations that render vismodegib unable to inhibit the Hh pathway6,7. Most patients treated with vismodegib experience clinical benefits3. However, many patients respond only partially: their tumours initially regress under therapy but relapse after vismodegib discontinuation3,5. The mechanisms by which vismodegib induces tumour regression and that underlie non-genetic resistance to vismodegib therapy are unknown.
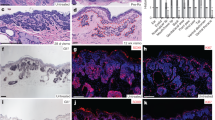
To study the mechanisms by which vismodegib leads to BCC regression, we induced BCC in mice by deleting Ptch1 or overexpressing the constitutive active form of Smo (SmoM2) in the epidermis using Krt14-CreER8,9. BCCs induced by conditional knockout of Ptch1 (Ptch1cKO) arise mainly from the upper hair follicle (infundibulum) whereas those induced by SmoM2 originate from the interfollicular epidermis (IFE)4,8. Eight weeks after deletion of Ptch1 by tamoxifen administration, mice showing fully formed BCCs were treated daily with vismodegib and analysed at different time points (Fig. 1a). A decrease in tumour burden was observed during the first 5 weeks of vismodegib treatment, followed by stabilization of tumour size from 5 to 12 weeks, together with the appearance of vismodegib-persistent lesions (Fig. 1b, c, Extended Data Fig. 1a–d). Vismodegib administration led to the conversion of the BCCs into pre-neoplastic lesions (hyperplasia and dysplasia), which persisted as drug-tolerant lesions (Fig. 1d, Extended Data Fig. 1e). These results show that vismodegib induces tumour shrinkage and the progressive appearance of drug-tolerant lesions.
a, Protocol for tumour induction and vismodegib (vismo) administration. b, Immunostaining for KRT14 and β4-integrin (β4) in ventral skin from Ptch1cKO mice. HF, hair follicle. c, Tumour burden (total area occupied by tumours divided by the length of the analysed epidermis) in untreated and vismodegib-treated mice (n = 3 mice analysed per time point and condition). Squares show data for individual mice, lines show mean. See Source Data. d, Quantification of lesion type (mean ± s.e.m.) upon vismodegib treatment (n = 3 mice, total number of lesions analysed per time point indicated in parentheses). Hyper, hyperplasia; dys, dysplasia. e, Immunostaining for active caspase-3 (AC3) and β4-integrin. f, Percentage of AC3+ tumour cells (mean ± s.e.m.) in untreated and vismodegib-treated mice (n = 30 lesions analysed from 3 mice). Two-sided t-test. g, Immunostaining for Ki67 and β4-integrin. h, Percentage of Ki67+ tumour cells (mean ± s.e.m.) in untreated and vismodegib-treated mice (n = 30 lesions analysed from 3 mice). Two-sided t-test. Hoechst nuclear staining in blue; scale bars, 50 μm. Dashed line in e, g delineates basal lamina. Arrows in b, e, g indicate vismodegib-persistent lesions.
Staining for active caspase-3 two weeks after vismodegib administration showed a similar number of apoptotic cells in treated and untreated mice (Fig. 1e, f, Extended Data Fig. 1f, g), indicating that apoptosis is not the main mechanism by which vismodegib induces BCC regression. As quiescence has been described as a mechanism of cancer resistance to therapy10, we assessed the proportion of Ki67-positive tumour cells and observed a strong decrease in the proportion of proliferative cells in persistent lesions (Fig. 1g, h, Extended Data Fig. 1h, i), suggesting that quiescence contributes to the emergence of drug-tolerant cells.
Lgr5 is expressed by different epithelial stem cells, including hair follicle stem cells (HFSCs)11, and is upregulated during BCC initiation9 (Extended Data Fig. 2a). In situ hybridization (ISH) showed that Lgr5 was highly expressed in untreated BCCs and its expression persisted, albeit at a lower level, in vismodegib-tolerant lesions (Fig. 2a, Extended Data Fig. 2b)
a, ISH for Lgr5 (red) and Gli1 (green) in untreated and treated tumour cells from Krt14CreER;Ptch1cKO mice. b, Percentage of tumour cells (LGR5+ and LGR5−) that express Gli1 (mean ± s.e.m.; n = 3 Krt14CreER;Ptch1cKO;Lgr5DTR-GFP mice, total number of cells analysed indicated in parentheses). c, Distribution of the number of Gli1 mRNA dots per tumour cell with and without treatment (unt) (mean ± s.e.m.; n = 113 and 163 total tumour cells from 3 Krt14CreER;Ptch1cKO;Lgr5DTR-GFP mice per condition and time point). d, Immunostaining for LGR5–GFP and KRT14 in Ptch1cKO ventral skin following vismodegib treatment, discontinuation and vismodegib re-administration. Three independent experiments per condition were analysed and showed similar results. e, Immunostaining for LGR5–GFP and BrdU following BrdU administration and after 5 and 9 weeks of chase in Ptch1cKO-induced BCCs. f, g, Proportions of LGR5+ tumour cells presenting BrdU labelling at T0, after vismodegib treatment and discontinuation (f) and BrdU+EdU+ double-positive tumour cells 5 days after vismodegib discontinuation (g) in Ptch1cKO-induced BCCs (mean ± s.e.m.; n = 30 lesions analysed from 3 mice per condition). Two-sided t-test (f, g). Hoechst nuclear staining in blue; scale bars, 25 μm. Dashed lines delineate basal lamina; white arrows indicate vismodegib-persistent lesions; yellow arrows indicate hair follicle LGR5+ cells. RA, retinoic acid.
ISH for Gli1, a transcription factor that relays Hh signalling and a Hh target gene, demonstrated that Gli1 was co-expressed with Lgr5 before treatment and was strongly downregulated in all tumour cells upon vismodegib treatment (Fig. 2a–c, Extended Data Fig. 2b–d), consistent with the strong inhibition of Hh signalling by vismodegib. Drug-tolerant lesions did not present mutations in Smo, the most frequently mutated gene in vismodegib-resistant BCC6,7 (Extended Data Fig. 2e), reinforcing the notion that the persistence of drug-tolerant lesions is not mediated by mutations that abrogate vismodegib sensitivity, as it occurs in vismodegib-resistant BCCs that continue to grow during treatment6,7.
Relapse of BCC upon vismodegib discontinuation has been reported in human patients5. Discontinuation of vismodegib administration for 4 weeks in Krt14CreER;Ptch1cKO;Lgr5DTR–GFP mice12 bearing drug-persistent lesions led to the re-growth of BCCs to their pre-treatment size. Moreover, re-administration of vismodegib to mice with relapsing BCCs led to tumour regression (Fig. 2d).
To determine whether the quiescent tumour cell population mediates tumour relapse, we performed BrdU pulse-chase label retention studies by administrating BrdU for 3 days in mice with BCC to label proliferative cells, and then monitored the labelling during 5 weeks of vismodegib treatment. We found BrdU label-retaining cells (LRCs) in LGR5+ drug-tolerant lesions, suggesting that persisting tumour cells existed before vismodegib treatment and underwent a phenotype switch from a proliferative to a quiescent state (Fig. 2e, f). Upon discontinuation of vismodegib, relapsed tumours lost the LRCs (Fig. 2e, f), suggesting that quiescent LRCs actively proliferated, diluting the BrdU. To test this possibility directly, we performed BrdU–EdU double-labelling studies. Administration of EdU during vismodegib discontinuation led to EdU incorporation in the majority of the LGR5+BrdU+ LRCs (Fig. 2g, Extended Data Fig. 2f, g), further demonstrating that the quiescent LRCs re-enter cell cycle and proliferate to contribute to tumour relapse.
To determine whether quiescence promotes the persistence of the vismodegib-tolerant lesions, we assessed whether increased epidermal proliferation decreased the number of drug-tolerant lesions. Mice bearing LGR5+ persistent lesions were treated for 2 weeks with vismodegib in combination with 12-O-tetradecanoylphorbol-13-acetate (TPA) or retinoic acid, two drugs that promote epidermal proliferation. Combined administration of vismodegib and TPA or retinoic acid promoted proliferation, which led to the elimination of LGR5+ persistent lesions (Extended Data Fig. 2h–j), demonstrating that when persistent slow-cycling cells are forced to proliferate they become sensitive to vismodegib and are eliminated.
We isolated the persistent tumour cells using fluorescence-activated cell sorting (FACS), by combining LGR5–GFP with LRIG1, which does not co-localize with LGR5 in resting hair follicles13 (Extended Data Fig. 2k–m). Upon vismodegib administration, the proportion of LGR5+LRIG1+ cells decreased and there was an increase in the LRG5−LRIG1+ population (Extended Data Fig. 2m, n).
We then characterized the gene signature of FACS-isolated LGR5+LRIG1+ and LRG5−LRIG1+ tumour cell populations from untreated BCCs using microarray analysis. It has been shown that, during BCC initiation, IFE and infundibulum cells targeted by Ptch1cKO or SmoM2 are reprogrammed into fates resembling those of embryonic hair follicle progenitor (EHFP) cells and adult hair follicles in a Wnt-dependent manner9,14. Genes that were upregulated in LGR5+LRIG1+ tumour cells compared to LRG5−LRIG1+ tumour cells (LGR5+ BCC signature) overlapped significantly with the EHFP signature15 (23.3%), resting HFSC signature16 (16.4%) and LGR5+ hair follicle signature17 (44.2%) (Fig. 3a, Extended Data Fig. 3a). The LGR5+ BCC signature included genes downstream of the Hh signalling pathway, such as Ptch1, Ptch2 and Hhip, genes involved in the Wnt signalling pathway, such as Lgr5, Fzd2 and Lef1, transcription factors expressed by EHFPs, such as Runx1 and Lhx2, and genes expressed by HFSCs, such as Tbx1 and Foxc1 (Extended Data Fig. 2b). Immunostaining for LEF1, LHX2, CUX1, TBX1, and ALCAM in Ptch1cKO-induced BCCs confirmed the increased expression of these Wnt signalling, EHFP and HFSC markers in LGR5+ tumour cells (Extended Data Fig. 3c).
a, b, Venn diagrams showing the similarities and the differences from two independent microarray experiments between genes upregulated more than twofold in LGR5+LRIG1+ versus LGR5−LRIG1+ cells (a) or in LGR5+LRIG1+ cells treated with vismodegib versus untreated (b) with the telogen HFSC signature16, hair follicle LGR5-expressing cell signature17 and EHFP signature15. c, mRNA expression of hair follicle genes downregulated in LGR5+LRIG1+ cells after 8 weeks of vismodegib administration (n = 2 independent microarray experiments). d, e, Venn diagrams showing the similarities and differences between genes that were differentially upregulated more than twofold from two independent microarray experiments in untreated LGR5+LRIG1+ versus LGR5−LRIG1+ cells (d) or in untreated versus vimodegib-treated LGR5+LRIG1+ tumour cells (e) compared to IFE16 and LRIG113 signatures. f, mRNA expression of IFE and infundibulum genes that were upregulated in LGR5+LRIG1+ cells after 8 weeks of vismodegib administration (n = 2 independent microarray experiments). g, Immunostaining for LGR5–GFP and SCD1 in untreated and vismodegib-treated Ptch1cKO-induced BCCs. Arrow indicates areas of sebaceous gland differentiation. h, Immunostaining for LGR5–GFP and KRT10 in untreated and vismodegib-treated Ptch1cKO mice. Arrow indicates differentiation of LGR5+ tumour cells into KRT10-expressing cells. Three independent experiments per condition were analysed with similar results (g, h). Hoechst nuclear staining in blue; scale bars, 50 μm. Dashed line delineates basal lamina. P values calculated using hypergeometric test for each intersection of two subsets of genes with phyper function in R software (a, b, d, e).
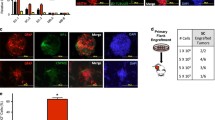
To assess whether the LRG5−LRIG1+ population represents a differentiated part of the BCC, we defined genes that were upregulated in LRG5−LRIG1+ tumour cells compared to LGR5+LRIG1+ tumour cells (LGR5− signature). Notably, the LGR5− signature overlapped significantly with previously reported LRIG113 and IFE16 signatures, including markers of IFE or infundibulum differentiation such as Ovol1, Notch3, Defb6, Krt1 and Krt10 (Extended Data Fig. 3d, e). PCR analysis performed on FACS-isolated LGR5+LRIG1+ and LGR5−LRIG1+ tumour cells confirmed that both populations had Ptch1 deletion, and staining for the proliferation marker Ki67 showed that the LRG5+LRIG1+ population was more proliferative than the LGR5−LRIG1+ population (Extended Data Fig. 3f, g).
To directly assess whether LGR5−LRIG1+ cells were more differentiated than LGR5+LRIG1+ cells, we performed transplantation assays of FACS-isolated tumour cell populations from Krt14CreER;Ptch1cKO;Lgr5DTR–GFP and Krt14CreER;Ptch1cKO;Trp53cKO;Lgr5DTR–GFP mice, which grow faster and form bigger tumours18. Groups of cells resembling early BCC and expressing KRT14, LGR5 and LRIG1 were observed only upon transplantation of LGR5+LRIG1+ cells from Trp53cKO mice (in three out of seven mice). By contrast, no tumour cells were observed following the transplantation of LGR5−LRIG1+ cells from Trp53cKO BCCs or in the absence of Trp53 deletion (Extended Data Fig. 4a, b). Tumours found after transplantation of LGR5+LRIG1+ cells mimicked the different cell types present in BCCs: LGR5+LRIG1+, LGR5−LRIG1+ and cells with a flat differentiated morphology expressing keratin-10 (KRT10) (Extended Data Fig. 4b, c). Together, these results show that BCCs contain a more stem-like or progenitor-like tumour cell population (LGR5+LRIG1+) and a more differentiated population (LGR5−LRIG1+) of tumour cells. Immunostaining for the primary cilia marker ARL13B and the coactivator MKL1 showed that neither loss of primary cilia19 nor serum response factor (SRF)–MKL1 activation20 is involved in the drug-tolerant phenotype described here (Extended Data Fig. 5a–d).
To define the molecular mechanisms by which vismodegib promotes tumour shrinkage and appearance of drug-tolerant lesions, we compared the transcriptional profiles of FACS-isolated LGR5+LRIG1+ and LGR5+LRIG1− tumour cells from untreated BCCs and mice that received vismodegib for 8 weeks. We found that the overlap between the LGR5+LRIG1+ signature and the EHFP15, LGR5+ hair follicle17 and resting HFSC16 signatures was considerably lower in vismodegib-treated cells than in untreated cells (Fig. 3a, b). Vismodegib treatment induced a strong decrease in the expression of Hh target genes such as Gli1, Gli2, Ptch1, Ptch2 and Hhip (Fig. 3c). Only a small part of the reduction in overlap between the vismodegib-treated and EHFP signatures was driven by Hh target genes such as Hhip1, Ptch2 and Gli1, and the reduction in overlap between the HFSC and vismodegib-treated signatures was not mediated by Hh target genes as the HFSC signature was obtained in the resting state, when Hh signalling is not active16. Genes found in the EHFP and HFSC signatures, such as Runx1, Lhx2, Lgr5, Alcam and Tbx1 were also downregulated following vismodegib administration at the mRNA and protein levels (Fig. 3c and Extended Data Fig. 6a).
The overlap between the LGR5+LRIG1+ signature and the infundibulum13 and IFE16 signatures increased considerably upon vismodegib treatment, with genes such as Ovol1, Notch3, Plet1, Defb1, Defb6, Krt1 and Krt10 being strongly upregulated after vismodegib treatment (Fig. 3d–f, Extended Data Fig. 6b), indicating that vismodegib promotes the differentiation of BCC into IFE- and infundibulum-like cells, possibly through a Notch-dependent mechanism21.
LRIG1+ stem cells give rise to infundibulum and sebaceous gland under homeostatic conditions13. We performed staining for sebaceous gland markers (SCD1 and adipophilin) and lipids (Oil Red O). Whereas sebaceous cysts were visible in the dermis under untreated conditions, cells expressing sebaceous gland markers were localized within the tumour mass after two weeks of vismodegib treatment and adjacent to the neoplastic lesions after five or eight weeks of treatment (Fig. 3g, Extended Data Fig. 6c, d). We studied the expression of KRT10 and Defensin-β6 (Defb6), which are normally expressed in infundibulum and IFE cells. Upon vismodegib administration, KRT10 and Defb6 were strongly upregulated in tumour cells (Fig. 3h, Extended Data Fig. 6e), consistent with vismodegib inducing tumour differentiation towards a sebaceous gland/infundibulum/IFE-like fate in Ptch1cKO-derived BCCs.
We then assessed whether vismodegib also promotes differentiation of BCC into IFE in SmoM2-induced BCC. Upon vismodegib administration, SmoM2-expressing cells connected to normal differentiating IFE cells expressed high levels of the IFE differentiation marker keratin-1 (KRT1) (Extended Data Fig. 6f). We studied the effect of vismodegib administration on the survival and morphology of the SmoM2 clones during BCC initiation. Two weeks after SmoM2 expression, mice were treated daily with vismodegib for six weeks (Extended Data Fig. 7a). Vismodegib administration led to a progressive loss of SmoM2-expressing clones in comparison to untreated conditions and to the emergence of clones with normal differentiation, with only a small proportion of the clones progressing into hyperplasia and dysplasia (Extended Data Fig. 7b–d). The normally differentiated clones observed during vismodegib treatment were positive for the differentiation marker KRT10 but did not express LHX2, an HFSC marker that is found in hyperplasias and dysplasias (Extended Data Fig. 7e, f), indicating that vismodegib administration inhibits oncogene-induced hair follicle reprograming, promotes differentiation of SmoM2-expressing cells into an IFE-like fate and prevents BCC initiation.
To assess whether LGR5+ tumour cells consist of heterogeneous populations in terms of proliferation and differentiation, we isolated LGR5+LRIG1+ tumour cells on the basis of expression of the proliferation marker CD7110 two weeks after vismodegib administration, when both persistent cells and cells that are responsive to vismodegib co-exist. The CD71+ population expressed higher levels of proliferation (Ki67 and Aurka) and differentiation markers (Krt1, Krt10 and Scd1) (Extended Data Fig. 7g), indicating that the more proliferative tumour cells are more prone to vismodegib-induced differentiation. Immunostaining for the differentiation marker KRT10 in LGR5+ tumour cells after BrdU label-retention followed by two weeks of vismodegib administration showed that the majority of BrdU-labelled cells were negative for KRT10, whereas KRT10 was observed in non-LRCs or in LRCs in which the BrdU signal was lower owing to its dilution following cell division (Extended Data Fig. 7h). These results support the notion that vismodegib induces a higher rate of differentiation in the drug-responsive tumour population that actively cycles.
To determine the relevance of our findings to human patients, we analysed biopsies from four patients with locally advanced BCCs before, during or immediately after discontinuation of vismodegib treatment. Vismodegib did not eradicate all tumour cells in these patients, and small tumorigenic lesions expressing LGR5 persisted despite the administration of vismodegib for months (Extended Data Fig. 8a–c). ISH for GLI1 and quantification of GLI1 mRNA dots per tumour cell before, after or during vismodegib treatment showed that there was almost no GLI1 expression in samples from patients during vismodegib treatment but few more GLI1-expressing cells were found shortly after discontinuation of vismodegib treatment (Extended Data Fig. 8c, d), indicating that vismodegib administration efficiently inhibits Hh signalling in these drug-persistent lesions. Ki67 immunohistochemistry showed that vismodegib-persistent lesions were more quiescent than untreated BCC cells, and vismodegib induced the expression of the differentiation marker KRT10 in human tumour cells (Extended Data Fig. 8e, f). Notably, patients 1 and 2 relapsed 6 and 9 months after treatment discontinuation, respectively, and patient 4 had previously relapsed after vismodegib discontinuation, showing that vismodegib-mediated tumour cell persistence is fully reversible upon drug withdrawal and re-inducible upon a new cycle of vismodegib treatment (Extended Data Fig. 8a). Together, these results show that drug-tolerant lesions exist in human BCC, characterized by the expression of LGR5 and relative quiescence.
To assess whether LGR5+ cells mediate tumour growth, we lineage-ablated LGR5+ tumour cells by administrating diphtheria toxin for 10 days to Krt14CreER;Ptch1cKO;Lgr5DTR–GFP mice and for 15 days to Krt14CreER;RosaSmoM2;Lgr5DTR–GFP mice (Extended Data Fig. 9a). Diptheria toxin treatment could not be extended because LGR5 deletion is toxic to normal liver cells12. Diptheria toxin administration led to a substantial elimination of the tumour mass in both BCC models (80% of the initial tumour mass) and to almost total elimination of LGR5-expressing cells in Ptch1cKO-induced BCC (Extended Data Fig. 9b–g), further demonstrating the importance of LGR5+ tumour cells to sustain BCC growth and maintenance.
To determine whether vismodegib administration together with Lgr5 lineage ablation can eliminate the LGR5-expressing drug-tolerant lesions that are responsible for tumour relapse, we administrated diphtheria toxin for five consecutive days in combination with vismodegib to Krt14CreER;Ptch1cKO;Lgr5DTR–GFP mice bearing persistent lesions (Extended Data Fig. 9h). Lgr5 ablation combined with vismodegib administration led to almost total (99.5%) elimination of the persistent LGR5-expressing tumour cells (Extended Data Fig. 9i–k). We did not observe reappearance of LGR5+ cells from the vast majority (94%) of the initial LGR5+ persistent tumorigenic lesions 15 days after discontinuation of treatment with diphtheria toxin and vismodegib (Extended Data Fig. 9i, k, l), whereas HFSCs were replenished by LGR5-expressing cells as previously reported22, indicating that there is little plasticity within the LGR5−LRIG1+ BCC cells to revert to LGR5+ tumour cells after treatment with diphtheria toxin and vismodegib. The therapeutic benefit of Lgr5 ablation in BCC is reminiscent of the effect of Lgr5 ablation in a mouse model of colorectal cancer, in which Lgr5 ablation prevents metastasis, and in human colorectal cancer organoids, in which Lgr5 ablation promotes tumour regression and synergises with chemotherapy23,24.
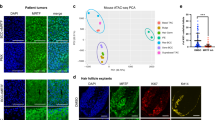
Lgr5 has been identified as a Wnt target gene, and acts as a co-receptor for R-spondin, positively regulating the Wnt signalling pathway11. Administration of vismodegib decreased but did not abolish the expression of different members of the Wnt signalling pathway (Fig. 3c). Immunostaining for LEF1, a transcription factor that relays Wnt signalling and is a Wnt target gene in BCCs9, and ISH for Axin2, another Wnt target gene, showed that both LEF1 and Axin2 were expressed in LGR5+ persistent lesions from mice and humans (Fig. 4a, b, Extended Data Fig. 10a, b), indicating that LGR5+ persistent tumour cells are characterized by active Wnt signalling.
a, Immunostaining for LGR5–GFP and LEF1 in untreated and vismodegib-treated Ptch1cKO mice. b, Immunohistochemistry for LEF1 in biopsies from a patient before and after vismodegib treatment. c, Protocol for dual Hh and Wnt inhibition followed by treatment discontinuation. d, Immunostaining for LGR5–GFP and KRT14 upon vismodegib administration, dual inhibition of Wnt and Hh pathways and following discontinuation in Ptch1cKO-derived BCCs. e, Number of LGR5+ tumorigenic lesions per length of epidermis upon treatment and treatment discontinuation in Ptch1cKO-induced BCCs (mean ± s.e.m.; n = 3 mice, 3 mm of skin analysed per mouse). Two-sided t-test. f, Quantification of the tumour burden upon treatment and treatment discontinuation in mice with Ptch1cKO-induced BCCs (mean ± s.e.m.; n = 3 mice). See Source Data. Two-sided t-test. Three independent experiments per condition were analysed showing similar results (a) and two technical replicates were performed for each sample showing similar results (b). Hoechst nuclear staining in blue; scale bars, 50 μm. Dashed line delineates basal lamina; arrows indicate vismodegib-persistent lesions.
To assess whether dual Wnt and Hh inhibition can promote the elimination of LGR5+ persistent tumour cells, we administered LGK-974, a porcupine Wnt inhibitor25, and vismodegib for 10 consecutive days to Ptch1cKO mice bearing LGR5+ persistent lesions (Fig. 4c). Combined Wnt and Hh inhibition resulted in the disappearance of LEF1 expression consistent with efficient Wnt inhibition, the elimination of the vast majority (93%) of initial LGR5+ drug-tolerant lesions and a substantial (87%) decrease in the tumour burden compared to vismodegib treatment alone (Fig. 4d–f, Extended Data Fig. 10c). We found no significant reduction in tumour burden after administration of the Wnt inhibitor alone, showing that although Wnt inhibition can block BCC initiation9,14 it is not efficient as a monotherapy to induce clinically relevant BCC regression (Extended Data Fig. 10d–f), We then investigated whether rare residual tumour cells could lead to tumour relapse upon discontinuation of dual Wnt and Hh inhibition. Four weeks after discontinuation, which corresponds to the time that it takes for drug-tolerant lesions to regrow to their initial size upon vismodegib discontinuation, no tumour relapse was observed, as shown by the stable number of LGR5+ tumour lesions and tumour burden (Fig. 4d–f). Together, these results show that the synergy between Hh and Wnt inhibition in BCC leads to the elimination of the vast majority of LGR5+ persistent tumour cells and thereby prevents tumour relapse.
In summary, we have shown that vismodegib induces BCC regression by promoting tumour differentiation and have identified a quiescent tumour cell population expressing LGR5 that persists after vismodegib treatment in different mouse models and human patients, promoting BCC relapse upon treatment discontinuation (Extended Data Fig. 11). The non-genetic mechanism of drug resistance described here differs from the previously described mutations in Smo or other genes that render cells insensitive to vismodegib treatment6,7,19,20. Administration of vismodegib promotes a switch from a proliferative state that fosters tumour growth to a tumour state characterized by Hh inhibition and slow-cycling properties that is fully reversible upon drug withdrawal and re-inducible upon a new cycle of vismodegib treatment. These persistent LGR5+ tumour cells present residual Wnt signalling activity in both mouse and human BCCs and could be eliminated by dual Wnt and Hh inhibition, leading to tumour eradication in the majority of BCCs (Extended Data Fig. 11). Dual Wnt and Hh inhibition constitutes a clinically relevant strategy to avoid BCC relapse that might also be effective against other cancers, such as medulloblastoma, that are characterized by activation of Hh and Wnt signalling26.
Methods
Ethical compliance
This study complied with all relevant ethical regulations regarding experiments involving mouse and human skin samples. Mouse colonies were maintained in a certified animal facility in accordance with European guidelines. Experiments involving mice presented in this work were approved by Comité d’Ethique du Bien Être Animal (Université Libre de Bruxelles) under protocol numbers 483N and 632N, which state that animals should be euthanized if they present tumours that exceed 1 cm in diameter. The BCCs observed in this study were microscopic and ranged from 1.5 mm to 100 μm in diameter; in none of the experiments performed did the BCCs exceed the limit (1 cm in diameter) described in protocols 483N and 632N.
Experiments involving human samples presented in this study were approved by the ethics committee of Vall d’Hebron Institute of Oncology (VHIO) and by the ethics committee of Hôpital Erasme under protocol number P2012/332. Permission and informed consent were obtained from all the patients in order to use their biopsies in this study.
Mice
Krt14CreER transgenic mice27 were kindly provided by E. Fuchs, Rockefeller University, USA. Ptch1fl/fl mice28 and RosaSmoM2–YFP mice29 were obtained from the JAX repository. Lgr5DTR–GFP mice (knockin mice that contain the diphtheria toxin receptor (DTR) fused to an enhanced green fluorescent protein (GFP) under the control of the Lgr5 regulatory region, allowing us to identify LGR5-expressing cells using the GFP reporter and to selectively ablate Lgr5 tumour cells by diphtheria toxin (DT) administration12) were kindly provided by Genentech (San Francisco, USA). Tp53fl/fl mice30 were obtained from the National Cancer Institute at Frederick.
Female and male animals were used for all experiments and equal gender ratios were respected in the majority of the analysis. Analysis of the different mutant mice was not blind and sample size was calculated to reach statistical significance. The experiments were not randomized.
Tumour induction
For Ptch1cKO deletion, Krt14CreER;Ptch1fl/fl;Lrg5DTR–GFP mice and Krt14CreER;Ptch1fl/fl;Trp53fl/fl;Lrg5DTR–GFP mice (1.5 months old) received one intraperitoneal injection of 2.5 mg tamoxifen on each of three consecutive days. For SmoM2 expression, Krt14CreER;RosaSmoM2;Lgr5DTR–GFP mice (1.5 months old) received one intraperitoneal injection of 1 mg tamoxifen. In the clonal induction experiments, Krt14CreER;RosaSmoM2 mice (1.5 months old) received one intraperitoneal injection of 0.1 mg tamoxifen.
Vismodegib and LGK-974 administration
Vismodegib (GDC-0449) was kindly provided by Genentech (San Francisco, US) and LGK-974 was kindly provided by Novartis (Bâle, Switzerland). During vismodegib treatment, mice received 150 mg/kg vismodegib by oral gavage daily. Vismodegib was administered in two doses (one every 12 h).
During the 10-day LGK-974 treatment mice received: on each of the first six days, 10 mg/kg LGK-974 by oral gavage; and on each of the last four days one topical application of 100 μl of 2 mg/ml LGK-974 diluted in propylene glycol:ethanol (7:3 v/v). For oral gavage, LGK-974 and vismodegib were dissolved in 0.5% methylcellulose solution containing 0.2% Tween-80.
TPA and retinoic acid administration
TPA and retinoic acid (RA) were used to promote epidermal proliferation31,32. TPA (200 μl of 0.02 mg/ml solution in dimethyl sulfoxide) or retinoic acid (200 μl of 0.5 mM all-trans-RA (Sigma) in dimethyl sulfoxide) was administered daily to shaved mouse back skin for 2 weeks.
Diptheria toxin administration
For Lgr5 lineage cell ablation, mice received a daily intraperitoneal injection of 50 μg/kg diphtheria toxin (Sigma).
Immunostaining in sections
The tail for the SmoM2 model and ventral skin or back skin for Ptch1cKO model were embedded in optimal cutting temperature compound (OCT, Sakura) and cut into 5–8 μm frozen sections using a CM3050S Leica cryostat (Leica Microsystems).
Immunostaining was performed on frozen sections. Owing to the fusion of SMOM2 with YFP and DTR with GFP, SMOM2-expressing and LGR5-expressing cells were detected using anti-GFP antibodies. Frozen sections were dried and then fixed with 4% paraformaldehyde/PBS (PFA) for 10 min at room temperature and blocked with blocking buffer for 1 h (PBS, horse serum 5%, BSA 1%, Triton 0.1%). Skin sections were incubated with primary antibodies diluted in blocking buffer overnight at 4 °C, washed with PBS for 3 × 5 min, and then incubated with Hoechst solution and secondary antibodies diluted in blocking buffer for 1h at room temperature. Finally, sections were washed with PBS for 3 × 5 min at room temperature and mounted in DAKO mounting medium supplemented with 2.5% Dabco (Sigma). Primary antibodies used were the following: anti-β4-integrin (rat, 1:200, BD, clone346-11A, ref. 553745, lot 5239648), anti-GFP (chicken, 1:3,000, Abcam, ref. ab13970, lot 236651-23), anti-active Caspase-3 (rabbit, 1:600, R&D, ref. AF835, lot CF23517031), anti-Ki67 (rabbit, 1:1,000, Abcam, ref. ab15580, lot GR3198193-1), anti-LRIG1 (goat, 1:500, R&D, ref. AF3688, lot ZPH0217111), anti-LEF1 (rabbit, 1:100, Cell Signaling, ref. 2230), anti-LHX2 (goat, 1:500, Santa Cruz, sc-19344, lot K1615), anti-CUX1 (rabbit,1:6,000, Santa Cruz, sc-13024), anti-TBX1 (rabbit, 1:100, Invitrogen), anti-ALCAM (goat, 1:1,000, Novus, ref. FAB1172F, lot AASW0111121), anti-KRT10 (rabbit, 1:3,000, Covance, ref. PRB-159P-0100), anti-KRT1 (rabbit, 1:3,000, Covance, ref. PRB-165P-0100), anti-KRT14 (rabbit, 1:3,000, Thermofisher), anti-SCD1 (goat, 1:500, Santa Cruz, ref. sc14719,lot H2610), anti-adipophilin (guinea pig, 1:5,000, Fitzgerald, ref. 20R-AP002, lot P17030911), anti-BrdU (mouse, 1:200, BD, clone 3D4, ref. 560209, lot 4293550), anti-MKL1 (rabbit, 1/200, Sigma, ref. HPA030782, lot C106712) and anti-ARL13B (rabbit, 1:2,000, ref. 17711-1-AP, Proteintech, lot 49885). The following secondary antibodies were used: anti-rabbit, anti-rat, anti-goat, anti-guinea pig and anti-chicken, conjugated to AlexaFluor488 (Molecular Probes) and to rhodamine Red-X and Cy5 (JacksonImmunoResearch). Images of the immunostained sections were acquired using an Axio Imager M2 microscope and Axiovision 4.8.2 software (Carl Zeiss).
Immunostaining in whole-mounts
Whole-mounts of tail epidermis were performed as previously described33 and used to quantify the proportion of surviving clones. Pieces of tail were incubated for 1 h at 37 °C in EDTA 20 mM in PBS on a rocking plate, then the dermis and epidermis were separated using forceps and the epidermis was fixed for 30 min in paraformaldehyde (PFA) 4% with agitation at room temperature and washed three times with PBS.
For the immunostaining, tail skin pieces were blocked with blocking buffer for 3 h (PBS, horse serum 5%, Triton 0.8%) on a rocking plate at room temperature. Next, the skin pieces were incubated with primary antibodies diluted in blocking buffer overnight at 4 °C. The next day, they were washed with PBS-Tween 0.2% for 3 × 10 min at room temperature, and then incubated with the secondary antibodies diluted in blocking buffer for 3h at room temperature, washed 2 × 10 min with PBS-Tween 0.2% and washed for 10 min in PBS. Finally, they were incubated in Hoechst diluted in PBS for 30 min at room temperature in the rocking plate, washed 3 × 10 min in PBS and mounted in DAKO mounting medium supplemented with 2.5% Dabco (Sigma). Primary antibodies used were the following: anti-GFP (rabbit, 1:100, BD, ref. A11122), anti-β4-integrin (rat, 1:200, BD, ref. 553745) and anti-KRT31 (guinea pig, 1:200, Progen, ref. GP-hHa1). The following secondary antibodies were used: anti-rabbit, anti-rat and anti-guinea pig, conjugated to AlexaFluor488 (Molecular Probes), to rhodamine Red-X (JacksonImmunoResearch) and to Cy5 (1:400, Jackson ImmunoResearch).
BrdU and EdU label retention studies
For the BrdU studies, mice received three daily intraperitoneal injections (150 μl of 10 mg/ml, every 8 h) for three consecutive days. For EdU studies, mice received three daily intraperitoneal injections (150 μl of 1 mg/ml, every 8 h) for three consecutive days. EdU and BrdU stainings were performed as described18.
In situ hybridization and RNA FISH
The tail in the SmoM2 model and ventral skin in the Ptch1cKO model were embedded in OCT and cut into 5–8 μm frozen sections using a CM3050S Leica cryostat (Leica Microsystems). Samples were fixed for 30 min in 4% PFA at 4 °C and the in situ protocol was performed according to the manufacturer’s instructions (Advanced Cell Diagnostics). The following mouse probes were used: Mm-Lgr5 cat. No. 312171, Mm-Gli1 cat. No. 311001-C2, Mm-Axin2 cat no.400331-C3, Mm-Defensinβ6 cat no.430141-C3
Human samples were fixed in 4% formalin and embedded in paraffin. Cut sections were deparaffinized and rehydrated before proceeding to the in situ hybridization, which was performed according to the manufacturer’s instructions. The following probes were used: Hs-Lgr5-C2 cat. no. 310991-C2, Hs-Lgr5 cat. no. 311021 and Hs-Axin2 cat no.400241-C3.
A confocal microscope (LSM-780, Carl Zeiss) and ZEN 2.3 software were used to acquire and analyse the ISH images.
Immunohistochemistry
For KRT14, Ki67, KRT10 and LEF1 immunohistochemistry in human samples, paraffin sections were deparaffinized and rehydrated, followed by antigen unmasking performed for 20 min at 98 °C in citrate buffer (pH 6) using the PT module. Endogenous peroxidase was blocked using 3% H2O2 (Merck) in methanol for 10 min at room temperature. Endogenous avidin and biotin were blocked using the Endogenous Blocking kit (Invitrogen) for 20 min at room temperature. Nonspecific antigen blocking was performed using blocking buffer. Mouse anti-KRT14 (rabbit, 1:2,000, Thermofisher), anti-Ki67 (rabbit, 1:400, Abcam, ab15580), anti-KRT10 (rabbit, 1:200, Biolegend, ref. 90541) and anti-LEF1 (rabbit, 1:100, Cell Signaling, ref. 2230) were incubated overnight at 4 °C. Anti-rabbit biotinylated with blocking buffer, standard ABC kit, and ImmPACT DAB (Vector Laboratories) was used for the detection of horseradish peroxidase (HRP) activity. Slides were then dehydrated and mounted using SafeMount (Labonord).
FACS isolation of tumour cells and microarray analysis
Isolation of tumour cells was performed as previously described34. In brief, Lgr5DTR–GFP and Krt14CreER;Ptch1fl/fl;Lgr5DTR–GFP mice untreated and upon 8 weeks of vismodegib treatment were killed by decapitation. Back skin was placed in a Petri dish and a sterile scalpel was used to remove the adipose tissue and muscle. The skin tissue was incubated with thermolysin (Sigma) for 1 h at 37 °C and then a scalpel was used to separate epidermis from the dermis. The epidermal tissue was chopped into pieces and resuspended in PBS supplemented with 5% chelated fetal calf serum and filtered with 70 μm and 40 μm cell strainers (BD). Cells were stained using anti-LRIG1 (goat polyclonal, R&D Systems, AF3688) followed by the secondary antibody donkey anti-goat-Alexa 647 (Invitrogen).
LRG5+LRIG1+ and LGR5–LRIG1+ cells from untreated or vismodegib-treated (8 weeks) Krt14CreER;Ptch1fl/fl;Lgr5DTR–GFP mice were sorted using LRIG1 staining and native LGR5–GFP. Two thousand sorted cells per sample were collected directly in 45 μl lysis buffer (20 mM DTT, 10 mM Tris–HCl pH 7.4, 0.5% SDS, 0.5 μg μl−1 proteinase K). Samples were then lysed at 65 °C for 15 min and frozen. RNA isolation, amplification and microarray were performed at the IRB Functional Genomics Core, Barcelona. cDNA synthesis, library preparation and amplification were performed as described35. Microarrays using Mouse Genome 430pm strip Affymetrix array were performed and the data were normalized using RMA algorithm. Biological duplicates were performed for all conditions. Genetic signatures were obtained by considering genes presenting a fold change greater or smaller than 2 or −2, respectively, in each replicate.
FACS isolation of CD71+ and CD71– populations of tumour cells, RNA extraction and quantitative PCR
Isolation of tumour cells from mouse skin was performed as described above. Cells were stained using anti-LRIG1 (goat polyclonal, R&D Systems, AF3688) and anti-CD71–PE (rat, BD Biosciences, 553267) followed by the secondary antibody donkey anti-goat-Alexa 647 (Invitrogen). Seven thousand FACS-sorted cells were collected directly in the lysis buffer provided by the manufacturer (RNAeasy Microkit, Quiagen) and RNA extraction was then carried out according to the manufacturer’s protocol. Purified RNA was used to synthesize the first-strand complementary DNA using SuperScript II (Invitrogen) with random hexamers (Roche). Quantitative PCR analyses were carried out with Light Cycler 96 (Roche). Primers used: Ki67-F: CCTGCCTCAGATGGCTCAAA, Ki67-R: GGTTCCCTGTAACTGCTCCC, Aurka-F: AACACAACGCAAGCCAAAGG, Aurka-R: GGCCAGTTGGAGGTTTGGAA, Krt10-F: AACTGACAATGCCAACGTGC, Krt10-R: TAGGTAG GCCAGCTCTTCGT, Krt1-F: ACAACCCGGACCCAAAACTT, Krt1-R: CT CTGCGTTGGTCCTCTTGT, Scd1-F: ACACCATGGCGTTCCAGAAT and Scd1-R: AGCTTCTCGGCTTTCAGGTC. Normalizers: HPRT-F: GCAGTA CAGCCCCAAAATGG, HPRT-R: TCCAACAAAGTCTGGCCTGT, βActin-F: GAAGCTGTGCTATGTTGCTCTA, βActin-R: CAATAGTGATGACCTGGCCGT, β2M-F: TCACCCCCACTGAGACTGAT, β2M-R: TCCCAGTAGACGGTCTTGGG, Gapdh-F: CGTGTTCCTACCCCCAATGT, Gapdh-R: GTGTAGCCCAAGATGCCC TT, Tbox-F: GTACCGCAGCTTCAAAAT ATTGTAT and Tbox-R: AAATCAACGCAGTTGTGCGTG
Sequencing of the Smo gene in vismodegib-persistent lesions
A total of 200,000 LGR5+LRIG1+ tumour cells from three Krt14CreER;Ptch1cKO;Lgr5DTR-GFPmice treated for 8 weeks with vismodegib were FACS sorted following the protocol described above. Exons 3–12 of the mouse Smo gene were amplified using PCR and the products of the PCR were purified using the Monarch DNA Gel Extraction Kit (ref. T1020). The products of the PCR were sequenced following the Sanger standard using chemistry BigDy31.1, the cycle sequencing technology based on dideoxy chain termination/cycle sequencing and performed on a ABI 3730XL sequencer. SnapGene version 4.1.3 was used for the analysis. Information of the amplification primers and sequencing results can be found in Source Data.
Grafting experiments
For transplantation experiments, 100,000 cells that had been FACS-sorted to obtain pure populations of LGR5+LRIG1+ and LGR5–LRIG1+ cells were transplanted into the interscapular fat pad of NOD-SCID immunodeficient mice. The 100,000 LGR5+LRIG1+ and LGR5–LRIG1+ cells were mixed in a proportion of 1/40 with tumour-associated fibroblasts from the same tumours (FACS-sorted using CD140a marker (clone APA5; eBiosciences)). The tumour cells and fibroblasts were embedded in 50 μl matrigel containing ROCK inhibitor (3.3 μg/ml) and transplanted into the fat pad.
GSEA analysis
The GSEA program was downloaded from the BROAD institute website (http://www.broadinstitute.org/gsea/). We used the GSEA preranked option with standard parameters of weighted enrichment score calculation to run the GSEA against a user-supplied fold-change-ranked list of genes. The results of the enrichment analysis were plotted using R software
Ptch1 deletion
To determine the deletion of the two Ptch1 alleles in the LGR5+LRIG1+ and LGR5–LRIG1+ populations, 200,000 cells were FACS-sorted and DNA was extracted using the QiAmp DNA Mini-Kit (Qiagen). The following primers were used to determine the presence of the floxed/floxed or deleted alleles: Forward: AAAGAGATCTTGTGGGCAAGG; Reverse: CTACTTCCATTTGTCACGTCC.
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this paper.
Data availability
Data associated with this study have been deposited in the NCBI Gene Expression Omnibus under accession number GSE117458 (microarray).
References
Epstein, E. H. Basal cell carcinomas: attack of the hedgehog. Nat. Rev. Cancer 8, 743–754 (2008).
Basset-Seguin, N., Sharpe, H. J. & de Sauvage, F. J. Efficacy of Hedgehog pathway inhibitors in basal cell carcinoma. Mol. Cancer Ther. 14, 633–641 (2015).
Sekulic, A. et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 366, 2171–2179 (2012).
Kasper, M., Jaks, V., Hohl, D. & Toftgård, R. Basal cell carcinoma - molecular biology and potential new therapies. J. Clin. Invest. 122, 455–463 (2012).
Tang, J. Y. et al. Inhibition of the hedgehog pathway in patients with basal-cell nevus syndrome: final results from the multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 17, 1720–1731 (2016).
Atwood, S. X. et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell 27, 342–353 (2015).
Sharpe, H. J. et al. Genomic analysis of smoothened inhibitor resistance in basal cell carcinoma. Cancer Cell 27, 327–341 (2015).
Youssef, K. K. et al. Identification of the cell lineage at the origin of basal cell carcinoma. Nat. Cell Biol. 12, 299–305 (2010).
Youssef, K. K. et al. Adult interfollicular tumour-initiating cells are reprogrammed into an embryonic hair follicle progenitor-like fate during basal cell carcinoma initiation. Nat. Cell Biol. 14, 1282–1294 (2012).
Brown, J. A. et al. TGF-β-induced quiescence mediates chemoresistance of tumor-propagating cells in squamous cell carcinoma. Cell Stem Cell 21, 650–664.e8 (2017).
Barker, N., Tan, S. & Clevers, H. Lgr proteins in epithelial stem cell biology. Development 140, 2484–2494 (2013).
Tian, H. et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478, 255–259 (2011).
Page, M. E., Lombard, P., Ng, F., Göttgens, B. & Jensen, K. B. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell 13, 471–482 (2013).
Yang, S. H. et al. Pathological responses to oncogenic Hedgehog signaling in skin are dependent on canonical Wnt/β3-catenin signaling. Nat. Genet. 40, 1130–1135 (2008).
Rhee, H., Polak, L. & Fuchs, E. Lhx2 maintains stem cell character in hair follicles. Science 312, 1946–1949 (2006).
Blanpain, C., Lowry, W. E., Geoghegan, A., Polak, L. & Fuchs, E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118, 635–648 (2004).
Latil, M. et al. Cell-type-specific chromatin states differentially prime squamous cell carcinoma tumor-initiating cells for epithelial to mesenchymal transition. Cell Stem Cell 20, 191–204.e5 (2017).
Sánchez-Danés, A. et al. Defining the clonal dynamics leading to mouse skin tumour initiation. Nature 536, 298–303 (2016).
Zhao, X. et al. A transposon screen identifies loss of primary cilia as a mechanism of resistance to SMO inhibitors. Cancer Discov. 7, 1436–1449 (2017).
Whitson, R. J. et al. Noncanonical hedgehog pathway activation through SRF-MKL1 promotes drug resistance in basal cell carcinomas. Nat. Med. 24, 271–281 (2018).
Eberl, M. et al. Tumor architecture and notch signaling modulate drug response in basal cell carcinoma. Cancer Cell 33, 229–243.e4 (2018).
Hoeck, J. D. et al. Stem cell plasticity enables hair regeneration following Lgr5+ cell loss. Nat. Cell Biol. 19, 666–676 (2017).
de Sousa e Melo, F. et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 543, 676–680 (2017).
Shimokawa, M. et al. Visualization and targeting of LGR5+ human colon cancer stem cells. Nature 545, 187–192 (2017).
Liu, J. et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc. Natl Acad. Sci. USA 110, 20224–20229 (2013).
Northcott, P. A. et al. Medulloblastomics: the end of the beginning. Nat. Rev. Cancer 12, 818–834 (2012).
Vasioukhin, V., Degenstein, L., Wise, B. & Fuchs, E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc. Natl Acad. Sci. USA 96, 8551–8556 (1999).
Uhmann, A. et al. The Hedgehog receptor Patched controls lymphoid lineage commitment. Blood 110, 1814–1823 (2007).
Mao, J. et al. A novel somatic mouse model to survey tumorigenic potential applied to the Hedgehog pathway. Cancer Res. 66, 10171–10178 (2006).
Jonkers, J. et al. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat. Genet. 29, 418–425 (2001).
Aldaz, C. M., Conti, C. J., Gimenez, I. B., Slaga, T. J. & Klein-Szanto, A. J. Cutaneous changes during prolonged application of 12-O-tetradecanoylphorbol-13-acetate on mouse skin and residual effects after cessation of treatment. Cancer Res. 45, 2753–2759 (1985).
Collins, C. A. & Watt, F. M. Dynamic regulation of retinoic acid-binding proteins in developing, adult and neoplastic skin reveals roles for β-catenin and Notch signalling. Dev. Biol. 324, 55–67 (2008).
Braun, K. M. et al. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development 130, 5241–5255 (2003).
Jensen, K. B., Driskell, R. R. & Watt, F. M. Assaying proliferation and differentiation capacity of stem cells using disaggregated adult mouse epidermis. Nat. Protoc. 5, 898–911 (2010).
Gonzalez-Roca, E. et al. Accurate expression profiling of very small cell populations. PLoS One 5, e14418 (2010).
Acknowledgements
We thank J.-M. Vanderwinden and M. Martens for help with confocal microscopy. C.B. is an investigator of WELBIO. A.S.-D. and J.-C.L. are supported by fellowships from the FNRS and FRIA, respectively. This work was supported by the FNRS, the Marian Family, the ULB fondation, the foundation Baillet Latour, and a consolidator grant from the European Research Council.
Author contributions
A.S.-D. and C.B. designed the experiments, performed data analysis and wrote the manuscript; A.S.-D. performed most of the biological experiments; J.-C.L, G.L. and V.S. helped with Lgr5 ablation experiments; M.L. performed immunostaining; C.D. performed FACS; E.M.-C., M.S., V.d.M. and J.T. provided patient samples; and A.B. performed GSEA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
C.B. is a consultant at Genentech (San Francisco, USA).
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Vismodegib leads to tumour shrinkage and emergence of vismodegib-persistent lesions in mice.
a, Immunostaining for SMOM2, KRT14 and β4-integrin in tail from SmoM2 mice after different durations of vismodegib administration. b, Tumour burden in micrometres (total area occupied by tumours divided by the length of the analysed epidermis) in untreated and vismodegib-treated SmoM2 mice (n = 3 mice analysed per time point and condition). Centre values define the mean. See Source Data for description of the skin length and tumour area analysed per mouse. c, Immunostaining for KRT14 and β4-integrin in ventral skin from Ptch1cKO mice. d, Immunostaining for SMOM2, KRT14 and β4-integrin in tail skin from SmoM2 mice. e, Quantification (mean ± s.e.m.) of lesion type upon vismodegib treatment in SmoM2 mice (n = 3 mice, total number of lesions analysed per time point indicated in parentheses). f, Immunostaining for active caspase-3 (AC3) and SMOM2. g, Percentage of AC3+ tumour cells (mean ± s.e.m.) in untreated and vismodegib-treated SmoM2 mice (n = 30 lesions analysed from 3 mice). Two-sided t-test. h, Immunostaining for Ki67 and SMOM2. i, Percentage of Ki67+ tumour cells (mean ± s.e.m.) in untreated and vismodegib-treated SmoM2 mice (n = 30 lesions analysed from 3 mice). Two-sided t-test. Three independent experiments per condition were analysed showing similar results (a, c, d, f, h). Hoechst nuclear staining in blue; scale bars, 100 μm (c, d), 50 μm (a, f, h). Dashed line delineates basal lamina. Arrows indicate vismodegib-persistent lesions.
Extended Data Fig. 2 Vismodegib-persistent lesions express LGR5 in mice.
a, Immunostaining for LGR5–GFP and β4-integrin at different time points after tamoxifen administration in the Ptch1cKO model. b, In situ hybridization for Lgr5 and Gli1 in untreated and treated tumour cells in SmoM2 mice. c, Percentage (mean ± s.e.m.) of tumour cells (LGR5+ and LGR5–) that express Gli1 in SmoM2 mice (n = 3 mice, total number of cells analysed indicated in parentheses). d, Distribution (mean ± s.e.m.) of the number of Gli1 mRNA dots per tumour cell with and without treatment in SmoM2 mice (n = 104 and 111 total tumour cells from 3 mice per condition and time point). e, Representation of the mouse Smo gene, showing in red the exons (E) in which genetic mutations have been described6,7 (top). Results from the sequencing of exon 7 from vismodegib-persistent lesions obtained by pooling drug-persistent cells from three Krt14CreER;Ptch1cKO;Lgr5DTR-GFP mice, showing absence of genetic mutations in the exon analysed (bottom). See Source Data for the results of the sequencing of exons 3–12. f, Protocol for BrdU and EdU double-labelling studies in Ptch1cKO-induced BCCs followed by vismodegib administration and discontinuation. g, Immunostaining for LGR5–GFP, BrdU and EdU in Ptch1cKO-derived BCCs following 5 days of vismodegib discontinuation. h, Protocol for treatment with vismodegib and retinoic acid (RA) or TPA. i, Immunostaining for LGR5–GFP, Ki67 and β4 in the back skin of Ptch1cKO mice treated with vismodegib and RA or TPA. j, Quantification of LGR5+ tumorigenic lesions per length of skin upon treatment with vismodegib or vismodegib with RA or TPA (n = 3 mice, 3 mm of skin analysed per mouse). Two-sided t-test. k, Immunostaining for LGR5–GFP and LRIG1 in untreated and treated Ptch1cKO mice. l, Immunostaining for LGR5–GFP and LRIG1 in untreated and vismodegib-treated (8 weeks) mice before and after enzymatic and physical separation of epidermis from dermis in Krt14CreER;Ptch1cKO;Lgr5DTR-GFP mice. Note that hair follicles co-expressing LGR5 and LRIG1 and sebaceous cysts remained in the dermal fraction whereas the BCCs were isolated with the epidermal fraction, indicating that normal hair follicles did not significantly contaminate the FACS-isolated tumour cells. m, Cell sorting strategy to isolate LGR5+LRIG1–, LGR5+LRIG1+ and LGR5–LRIG1+ in normal skin and in Ptch1cKO-derived BCCs before and after vismodegib administration. Forward scatter (FSC) and side scatter (SSC) were performed to exclude cell debris and doublets. Living cells were selected by Hoechst dye exclusion. Finally, the different LGR5 and LRIG1 cell populations were isolated by FACS sorting. A, area; W, width. n, Proportion of cells (mean ± s.e.m.) expressing LGR5–GFP and LRIG1 determined by FACS (n = 3 independent experiments per condition). These experiments indicate that LRIG1 can be used to discriminate between LGR5+ cells from the HFSC or lower hair follicle (LGR5+LRIG1–) and BCC cells (LGR5+LRIG1+). Three independent experiments per condition were analysed with similar results (a, k, l). Hoechst nuclear staining in blue; scale bars, 50 μm (a, i, k, l) and 25 μm (b). Dashed line delineates basal lamina separating IFE from the dermis. Dotted line delineates BCC. Arrows indicate vismodegib-persistent lesions.
Extended Data Fig. 3 Characterization of LGR5+LRIG1+ and LGR5–LRIG1+ tumour cells.
a, GSEA showing the enrichment of genes upregulated in the LGR5+LRIG1+ population compared to the LRG5–LRIG1+ population from two independent microarray experiments with the EHFP15 (left) in telogen HFSCs16 (middle) and hair follicle Lgr5-expressing cell signatures17 (right), showing that LGR5-expressing BCC cells express many genes of the embryonic and adult hair follicle signatures. The normalized enrichment score (NES) and P value (one-sided test) were calculated using the GSEA program. b, mRNA expression of genes upregulated in LGR5+LRIG1+ tumour cells compared to LGR5–LRIG1+ tumour cells in untreated conditions (n = 2 independent microarray experiments). c, Immunostaining for LGR5–GFP with LEF1, LHX2, CUX1, TBX1 and ALCAM in untreated Ptch1cKO-derived BCCs. d, Venn diagram showing the similarities and differences between genes that were upregulated more than twofold from two independent microarray experiments in LGR5–LRIG1+ versus LGR5+LRIG1+ cells compared to IFE16 and LRIG113 signatures. P value calculated using the hypergeometric test for each intersection of two subsets of genes with phyper function in R software. The high overlap indicates that LGR5–LRIG1+ cells expressed IFE and infundibulum differentiation markers. e, mRNA expression of genes upregulated in LGR5–LRIG1+ tumour cells compared to LGR5+LRIG1+ cells in untreated conditions (n = 2 independent microarray experiments). f, PCR analysis of the recombination of the floxed Ptch1 alleles in control samples and in FACS-isolated tumour-derived LGR5+LRIG1+ and LGR5–LRIG1+ populations from Ptch1cKO-induced BCCs. Two technical replicates were analysed for each sample with similar results. g, Immunostaining for LGR5–GFP, LRIG1 and Ki67 in Ptch1cKO-derived BCCs shows higher proliferation rate in LGR5+LRIG1+ than in LGR5–LRIG1+ tumour cells. Three independent experiments per condition were analysed with similar results (c, g). Hoechst nuclear staining in blue; scale bars, 50 μm.
Extended Data Fig. 4 Transplantation of LGR5+LRIG1+ Ptch1;Trp53 double conditional knockout BCC cells leads to the formation of BCC-like structures.
a, Table summarizing the number of grafted mice that presented KRT14+ BCC-like structures upon transplantation of FACS-isolated LGR5+LRIG1+ and LGR5–LRIG1+ cells from BCCs arising in Krt14CreER;Ptch1cKO;Lgr5DTR–GFP and Krt14CreER;Ptch1cKO;Trp53cKO;Lgr5DTR–GFP mice. b, c, Immunostaining for LGR5–GFP, KRT14 and LRIG1 (b) and for LGR5–GFP and KRT10 (c) in the BCC-like structures obtained upon transplantation of LGR5+LRIG1+ cells from Ptch1;Trp53 double conditional knockout BCCs in the dorsal fat pads of NOD/SCID mice. Three independent experiments per condition were analysed with similar results (b, c). Hoechst nuclear staining in blue; scale bars, 50 μm.
Extended Data Fig. 5 Vismodegib-persistent lesions do not show decreased primary cilia numbers or nuclear localization of MKL1.
a, b, Immunostaining for ARL13B and LGR5–GFP in Ptch1cKO model (a) and for ARL13B and SMOM2 in SmoM2 model (b) in untreated and vismodegib-treated lesions. c, d, Immunostaining for MKL1 and LGR5–GFP in vismodegib-persistent lesions in Ptch1cKO mice (c) and for MKL1 and SMOM2 in vismodegib-persistent lesions in SmoM2 mice (d) treated for 8 weeks with vismodegib. White boxes are expanded on right. Three independent experiments per condition were analysed with similar results. Scale bars, 25 μm.
Extended Data Fig. 6 Vismodegib promotes BCC differentiation.
a, Immunostaining for LGR5–GFP, LHX2 and ALCAM in untreated and vismodegib-treated Ptch1cKO-derived BCCs. b, GSEA showing enrichment of genes upregulated in LGR5+LRIG1+ vismodegib-treated tumours compared to untreated BCCs with IFE16 and LRIG113 signatures, showing that vismodegib treatment promotes the expression of the IFE and infundibulum signatures. The normalized enrichment score (NES) and P value (one-sided test) were calculated using the GSEA program. c, Oil Red O and haematoxylin and eosin staining in ventral skin of untreated and vismodegib-treated Ptch1cKO mice. Arrows indicate areas of sebaceous differentiation. d, Immunostaining for LGR5–GFP and adipophilin in untreated and vismodegib-treated Ptch1cKO-derived BCCs. Arrows indicate areas of sebaceous differentiation. e, In situ hybridization for Lgr5 and Defb6 in untreated and vismodegib-treated Ptch1cKO-derived BCCs. f, Immunostaining for KRT1 and SMOM2 in untreated and vismodegib-treated SmoM2 mice. Three independent experiments per condition were analysed with similar results (a, c–f). Hoechst nuclear staining in blue; scale bars, 50 μm.
Extended Data Fig. 7 Vismodegib promotes differentiation of SMOM2-expressing cells during BCC initiation and in Ptch1cKO tumour cells.
a, Protocol for tumour induction and timing of vismodegib administration to Krt14CreER;RosaSmoM2 mice. b, Quantification of surviving SMOM2 clones in the interscale (tail epidermis) in untreated mice and after different durations of vismodegib treatment (n = 3 mice per time point and condition). Centre values show mean. See Source Data for description of total number of clones counted per time point and condition. c, Immunostaining for KRT31 and SMOM2 in whole-mount tail skin (left) and orthogonal views of the clones highlighted in the left panel stained for β4-integrin and SMOM2 (right). d, Quantification (mean ± s.e.m.) of the type of SMOM2-expressing clones after different durations of vismodegib treatment (n = 3 or 4 mice as indicated in the graph, total number of lesions quantified indicated in parentheses). e, f, Immunostaining for KRT10 and SMOM2 (e) and for LHX2 and SMOM2 (f) in untreated and vismodegib-treated mice. Three independent experiments per condition were analysed with similar results. g, mRNA expression of genes upregulated in the LGR5+LRIG1+CD71+ population compared to the LGR5+LRIG1+CD71– population obtained by quantitative PCR (n = 3 mice). Bars represent the average fold change over LGR5+LRIG1+CD71– cells and error bars the s.e.m. h, Immunostaining for LGR5–GFP, BrdU and KRT10 in mice that received three injections of BrdU followed by two weeks of vismodegib administration. Three independent experiments per condition were analysed with similar results. Hoechst nuclear staining in blue; scale bars, 100 μm (c) and 50 μm (e, f, h).
Extended Data Fig. 8 LGR5 expression in vismodegib-persistent lesions in human BCCs.
a, Tables summarizing the BCC and treatment characteristics in the patients analysed. b, Immunohistochemistry for KRT14 in biopsies before, after and during vismodegib treatment. c, In situ hybridization for LGR5 and GLI1 in biopsies from patients before, during and after vismodegib treatment. d, Percentage (mean ± s.e.m.) of tumour cells (LGR5+ and LGR5–) that express GLI1 in biopsies from patients, during or after vismodegib treatment (n = 3 samples from different patients (Patients 1–3) or body locations (Patient 4), total number of cells analysed indicated in parentheses). e, f, Immunohistochemistry for Ki67 (e) and KRT10 (f) in biopsies before, during and after vismodegib treatment. Hoechst nuclear staining in blue; scale bars, 25 μm.
Extended Data Fig. 9 Lgr5 lineage ablation leads to BCC shrinkage and elimination of vismodegib-persistent lesions.
a, Protocol for tamoxifen and diphtheria toxin (DT) administration. b, c, Immunostaining for KRT14 and LGR5–GFP in the Ptch1cKO model (b) and for KRT14 and SMOM2 in the SmoM2 model (c) after different durations of DT administration. d, Quantification of tumour burden in untreated mice and after DT administration (n = 3 mice per time point and condition). Centre values define the mean. See Source Data for description of the skin length and tumour area analysed per mouse. e, Number of LGR5–GFP+ tumour cells in untreated conditions and following DT administration (n = 3 mice per time point and condition, 1 mm of skin analysed per mouse). Centre values define the mean. f, Quantification of tumour burden (SMOM2-expressing cells) in untreated conditions and following DT treatment (n = 3 mice per time point and condition). Centre values define the mean. See Source Data for description of the skin length and tumour area analysed per mouse. g, Immunostaining for active caspase-3 (AC3) and LGR5–GFP (top) and for active caspase-3 and SMOM2 (bottom) after five administrations of DT. Three independent experiments per condition were analysed with similar results. h, Experimental strategy for combination of vismodegib treatment and Lgr5 ablation in Krt14CreER;Ptch1cKO;Lgr5DTR–GFP mice. i, Immunostaining for LGR5–GFP and KRT14 in Krt14CreER;Ptch1cKO;Lgr5DTR–GFP mice upon treatment, Lgr5 ablation and discontinuation. j, Immunostaining for active caspase-3 and LGR5–GFP following administration of vismodegib and DT. k, Quantification of the number of LGR5–GFP+ cells (mean ± s.e.m.) in the different experimental conditions upon treatment and discontinuation (n = 3 mice, 3 mm of skin analysed per mouse). Two-sided t-test. l, Quantification of the number of LGR5+ lesions (mean ± s.e.m.) per length of epidermis (mm) in mice treated with vismodegib and upon discontinuation of treatment with vismodegib and DT (n = 3 mice, 3 mm of skin analysed per mouse). Two-sided t-test. Hoechst nuclear staining in blue; scale bars, 50 μm. Dashed line delineates basal lamina separating IFE from the dermis. Dotted line delineates BCC. Arrows indicate tumorigenic lesions in b, c and indicate vismodegib-persistent lesions in i.
Extended Data Fig. 10 Wnt signalling is active in vismodegib-persistent lesions in mouse and human BCCs.
a, ISH for Lgr5 and Axin2 in untreated and vismodegib-treated lesions from Ptch1cKO and SmoM2 mice. b, ISH for LGR5 and AXIN2 in biopsies from patients before, during and after vismodegib treatment. c, Immunostaining for LEF1 and LGR5–GFP in Ptch1cKO-derived tumorigenic lesion following treatment with vismodegib and LGK-974. d, Protocol used for LGK-974 treatment in Ptch1cKO mice. e, Immunostaining for LGR5–GFP and KRT14 in BCC treated with LGK-974 for 9 days from the Ptch1cKO model. f, Quantification of the tumour burden (mean ± s.e.m.) in mice treated with LGK-974 for 9 days or untreated (n = 3 mice). See Source Data for description of skin length and tumour area analysed per mouse. Two-sided t-test. Hoechst nuclear staining in blue; scale bars, 25 μm. Dashed line delineates basal lamina separating IFE from the dermis. Dotted line delineates BCC. Three independent experiments per condition were analysed with similar results (a, c, e) and two technical replicates were performed for each sample with similar results (b).
Extended Data Fig. 11 Model.
Vismodegib administration promotes tumour cell differentiation leading to BCC regression. However, upon vismodegib treatment a small proportion of LGR5+ BCC cells persists, forming vismodegib-tolerant lesions that are slow cycling and characterized by Wnt signalling activation. Discontinuation of vismodegib treatment results in proliferation of LGR5-persistent lesions that lead to BCC relapse. Vismodegib treatment in combination with Lgr5 lineage ablation or Wnt signalling inhibition results in eradication of BCCs.
Supplementary information
Rights and permissions
About this article
Cite this article
Sánchez-Danés, A., Larsimont, JC., Liagre, M. et al. A slow-cycling LGR5 tumour population mediates basal cell carcinoma relapse after therapy. Nature 562, 434–438 (2018). https://doi.org/10.1038/s41586-018-0603-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0603-3
- Springer Nature Limited
Keywords
This article is cited by
-
Cancer cell plasticity, stem cell factors, and therapy resistance: how are they linked?
Cancer and Metastasis Reviews (2024)
-
Cancer cell plasticity: from cellular, molecular, and genetic mechanisms to tumor heterogeneity and drug resistance
Cancer and Metastasis Reviews (2024)
-
Modeling stress-induced responses: plasticity in continuous state space and gradual clonal evolution
Theory in Biosciences (2024)
-
Cancer drug-tolerant persister cells: from biological questions to clinical opportunities
Nature Reviews Cancer (2024)
-
Rational combinations of targeted cancer therapies: background, advances and challenges
Nature Reviews Drug Discovery (2023)