Abstract
Membrane-bound O-acyltransferases (MBOATs) are a superfamily of integral transmembrane enzymes that are found in all kingdoms of life1. In bacteria, MBOATs modify protective cell-surface polymers. In vertebrates, some MBOAT enzymes—such as acyl-coenzyme A:cholesterol acyltransferase and diacylglycerol acyltransferase 1—are responsible for lipid biosynthesis or phospholipid remodelling2,3. Other MBOATs, including porcupine, hedgehog acyltransferase and ghrelin acyltransferase, catalyse essential lipid modifications of secreted proteins such as Wnt, hedgehog and ghrelin, respectively4,5,6,7,8,9,10. Although many MBOAT proteins are important drug targets, little is known about their molecular architecture and functional mechanisms. Here we present crystal structures of DltB, an MBOAT responsible for the d-alanylation of cell-wall teichoic acid in Gram-positive bacteria11,12,13,14,15,16, both alone and in complex with the d-alanyl donor protein DltC. DltB contains a ring of 11 peripheral transmembrane helices, which shield a highly conserved extracellular structural funnel extending into the middle of the lipid bilayer. The conserved catalytic histidine residue is located at the bottom of this funnel and is connected to the intracellular DltC through a narrow tunnel. Mutation of either the catalytic histidine or the DltC-binding site of DltB abolishes the d-alanylation of lipoteichoic acid and sensitizes the Gram-positive bacterium Bacillus subtilis to cell-wall stress, which suggests cross-membrane catalysis involving the tunnel. Structure-guided sequence comparison among DltB and vertebrate MBOATs reveals a conserved structural core and suggests that MBOATs from different organisms have similar catalytic mechanisms. Our structures provide a template for understanding structure–function relationships in MBOATs and for developing therapeutic MBOAT inhibitors.
Similar content being viewed by others
Main
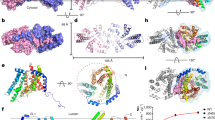
The MBOAT superfamily comprises more than 7,000 proteins (see http://pfam.xfam.org/family/MBOAT). These proteins perform divergent functions with distinct substrate preferences, although many use acyl-coenzyme A (acyl-CoA) as the acyl-group donor (Extended Data Fig. 1). Among bacterial MBOATs, DltB is essential for the d-alanylation of cell-wall teichoic acids11,12,13,14,15,16, which are important for the growth, biofilm formation, adhesion and virulence of Gram-positive bacterial pathogens. To understand the molecular mechanisms of MBOAT proteins, we have determined the crystal structure of full-length DltB from Streptococcus thermophilus at 3.3 Å resolution (Fig. 1, Extended Data Figs. 2, 3, Extended Data Table 1). DltB contains 415 residues arranged into 17 helices, and both the N and the C termini are located in the extracellular space (Fig. 1a). The helices are located mostly within the lipid bilayer, with the exception of the short N- and C-terminal helices. Among them, 11 transmembrane helices form an external ring-shaped ridge, and shield a central basin that is thinner than the lipid bilayer (Fig. 1, Extended Data Fig. 4). The thin central area results from an intracellular concave surface and a more pronounced extracellular structural funnel (Fig. 1d). Because they are more conserved than the peripheral-ring helices among MBOAT proteins and are probably involved in catalysis (see below), we refer to the structural components in this thin central area as the MBOAT central core. The 3D structure of DltB can be approximately divided into three parts: the N-terminal helical ridge (N-ridge), the central core and the C-terminal helical ridge (C-ridge) (Extended Data Fig. 4). A Dali search using our DltB structure did not find any protein with a similar fold.
a, The DltB crystal structure is shown in three orientations with rainbow colours: bottom, front and back (from left to right). b, Cartoon of the transmembrane topology of DltB. DltB contains a ring of 11 peripheral transmembrane helices, which shield a central thin layer (the structural core) highlighted by two red dashed circles. c, The electrostatic surface of DltB. d, A cut-away surface illustration showing the outward funnel connected with the cytosolic side through a tunnel. The histidine residue that is completely conserved among MBOATs (His336) is located at the bottom of the funnel. e, Conservation of the extracellular DltB funnel surface. The surface conservation pattern was generated on the basis of sequence alignment shown in Extended Data Fig. 5. f, Top view of DltB showing the location of His336 and the other three DltB residues (Ser165, Ala209 and Phe250) that, when altered, were found to desensitize S. aureus to the inhibition of LTA d-alanylation by m-AMSA.
The extracellular side of DltB forms a structural funnel, which extends into the middle of the lipid bilayer (Fig. 1d). The surface inside the funnel is formed by residues from several transmembrane helices and loops. Notably, in sharp contrast to the low conservation of residues forming the outer-ridge surfaces, these inner residues are highly conserved among DltB proteins (Fig. 1e, Extended Data Fig. 5). Previous studies have shown that a histidine residue strictly conserved in all confirmed MBOAT proteins is probably involved in catalysis. Mutation of the corresponding histidine residue in all tested MBOATs—porcupine (PORCN), hedgehog acyltransferase (HHAT), ghrelin O-acyltransferase (GOAT), diacylglycerol acyltransferase 1 (DGAT1) and acyl-coenzyme A:cholesterol acyltransferase (ACAT)—either abolished or substantially reduced the acyltransferase activities of the enzymes17,18,19,20,21,22. In our DltB structure, this histidine residue (His336, the last residue of helix H14) is located at the bottom of the extracellular funnel (Fig. 1d, f). Another highly conserved histidine residue (His289) in the MBOAT superfamily1 is also located at the bottom of this funnel and is spatially close to His336 (Extended Data Fig. 3). Our crystal structure and the structural conservation strongly suggest that this extracellular funnel is important for the activity of DltB.
Four Staphylococcus aureus DltB mutations—corresponding to S. thermophilus DltB mutants S165T, A209D, F250L and F250I—have been identified as resistant to the DltB inhibitors m-AMSA (amsacrine) and o-AMSA14. Ser165, Ala209 and Phe250 are spatially located at the surface of the funnel, with Ser165 and Phe250 sitting near the bottom of the funnel and close to His336 (Fig. 1f). We predict that m-AMSA and o-AMSA bind in this DltB funnel, and that the abovementioned four mutations may abolish inhibitor binding. We speculate that this funnel may be involved in extracellular teichoic acid substrate binding or have other key roles in catalysis. Given the biological importance of DltB14 and the marked conservation of the extracellular funnel surface of DltB, inhibitors of DltB that bind to this funnel may act as wide-spectrum antibiotics against Gram-positive bacteria.
In addition to its role in d-alanylation, DltB also has a role in host–pathogen interactions. A missense mutation (T113K) in S. aureus DltB is sufficient to convert an S. aureus strain from a human-specific pathogen to a rabbit-specific pathogen, without any change in the d-alanylation level of lipoteichoic acid (LTA)23. Notably, Thr113—as well as all ten other S. aureus DltB residues that are associated with a change in host specificity—is located at a non-conserved extracellular apex (Extended Data Figs. 4d, 5). This unusual feature strongly suggests that DltB from S. aureus and potentially some other species may interact with one or more unknown host factors using their extracellular ridges.
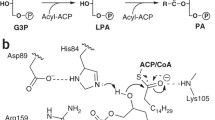
To serve as the d-alanyl donor to teichoic acid in the Dlt-mediated d-alanylation system, DltC first needs to be modified with the 4′-phosphopantetheine (Ppant) group at Ser35, a modification that can be catalysed by acyl carrier protein synthase (AcpS). The Ppant-modified DltC can be further modified with a d-alanyl group by DltA, through a thioester bond (Fig. 2a). To test whether DltB can directly interact with DltC, we co-expressed His-tagged AcpS and GST-tagged DltC. The purified DltC was uniformly modified by Ppant, as confirmed by mass spectrometry (Extended Data Fig. 2b, c). GST pull-down and size-exclusion chromatography experiments showed that DltC-Ppant and DltB form a tight complex (Fig. 2b). Octet binding analysis showed a Kd of 0.26 µM between DltB and DltC-Ppant (Extended Data Fig. 6). In contrast to the tight DltB–DltC interaction, DltB does not form a detectable complex with DltA or the extracellular domain of DltD, and there is no detectable interaction between DltA and DltC on the cytoplasmic side (data not shown).
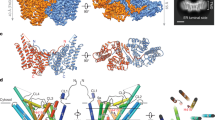
a, Dlt proteins responsible for LTA d-alanylation in Gram-positive bacteria. The magenta dots on glycerophosphate units represent d-Ala moieties. b, Direct stable interaction between DltB and DltC-Ppant, and mutagenesis analysis of the DltB–DltC-Ppant interface, as shown by GST pull-down assays. GST pull-down experiments were performed at least twice with similar results. WT, wild type. c, Overall structure of the DltB–DltC-Ppant complex. DltC-Ppant binds to DltB on the cytosolic side, with the phosphate group of Ppant (which is attached through Ser35 of DltC) pointing towards the DltB tunnel. d, The DltB–DltC-Ppant interface. Side chains corresponding to DltB are shown as green sticks, and side chains of DltC are shown in cyan.
To understand how DltB functions as an MBOAT, we also determined the crystal structure of the DltB–DltC-Ppant complex at 3.15 Å resolution (Fig. 2c). Cytoplasmic DltC contains four helices, with Ppant-bonded Ser35 being the first residue of helix 3 (α3). Residues of DltC α3 and the long loop between α3 and α4 (α3–α4 loop) form the DltB-binding surface. DltC interacts mainly with the C-terminal half of DltB H13 and the N-terminal end of DltB H14. This region is formed by a DltB-specific insertion that is missing in other MBOAT proteins1. The DltB–DltC interface is mostly hydrophobic, formed by DltB residues Met302, Val305, Ile306 and Met309, and DltC residues Met36, Val39, Val43 and Val55. In addition, Arg317—the first residue of helix H14—forms charged hydrogen bonds with DltC Glu40, whereas the phosphate group of Ser35-Ppant is in a position to form a salt bridge with DltB Lys282 in helix H12 (Fig. 2d, Extended Data Figs. 5, 7). The structures of DltB are essentially identical in both the apo state and the DltC-bound state (Extended Data Fig. 7a).
To confirm the structural and functional features of the DltB–DltC interface, we purified DltB mutants V305D and V305D/I306D as well as DltC mutants V39D and V39R, and tested their interactions with their corresponding wild-type partner using GST pull-down and Octet assays (Fig. 2b, Extended Data Fig. 6). Whereas DltB(V305D) showed substantially reduced binding to wild-type DltC, the binding was completely abolished when using DltB(V305D/I306D). Similarly, both DltC(V39D) and DltC(V39R) showed substantially reduced ability to interact with DltB. These mutagenesis analyses demonstrate that Val305 and Ile306 of DltB and Val39 of DltC are critical to the DltB–DltC interaction, and confirm our structural observation that these surface residues are located at the DltB–DltC core interface.
There is an approximately straight tunnel between the bottom of the extracellular funnel and the cytoplasmic side. This tunnel is formed by three DltB helices from the C-ridge (H13–H15) and the small horizontal helix H12 from the central core. DltB residues inside the tunnel are highly conserved among DltB proteins (Fig. 3a, Extended Data Fig. 5), and show a level of conservation in other MBOAT proteins, which suggests that this tunnel is functionally important. It should be noted that in our current structures of DltB and the DltB–DltC complex, the side chain of the conserved Trp285 from helix H12 keeps this tunnel in a closed conformation (Fig. 3a, Extended Data Fig. 7c). We speculate that the conformation we captured is that of the DltB enzyme in a ‘resting’ state.
a, Residues forming the DltB tunnel. b, Cut-away surface illustration of the DltB–DltC-Ppant complex. DltC-Ppant pSer35 is located at the bottom of the tunnel. c, LTA d-alanylation assay. m-AMSA is a DltB inhibitor. The assays were repeated three times. H281, S285, H328 and V297/F298 in B. subtilis correspond to H289, S293, H336 and V305/I306 in S. thermophilus, respectively. d, Lysozyme susceptibility survival assay. Representative images are shown for serial dilutions of cells plated on LB agar (left) and LB agar supplemented with 30 µg ml−1 of lysozyme (right). Dilutions of cells are indicated on the y axis. The survival assay was performed three times. e, A working model for DltB-mediated LTA d-alanylation. Cross-membrane d-alanylation is probably mediated by the DltB tunnel, the opening (activation) of which may be controlled by helix H12. The role of DltD in this reaction is unclear.
One notable feature of the DltB–DltC complex is that DltC Ser35 is located at the cytoplasmic entrance of the tunnel (Fig. 3b). Whereas the electron density for the Ppant phosphate group is well-defined in our electron density map, the density for the rest of the Ppant chain is too thin for model building; this is consistent with a ‘resting’ state conformation. Consistently, Octet analysis showed that the DltC(S35A) mutant can also interact with DltB with similar affinity (Kd ≈ 0.19 µM) to that of wild-type DltC-Ppant, which indicates that the Ppant group is not essential to the DltB–DltC interaction. The Ppant group can potentially switch between occupying the tunnel and being flexible in the cytoplasmic open space, as the Ser35 phosphate group is positioned between the tunnel entrance and the open cytoplasmic space. While the most conserved residue (His336) is located at the C terminus of the DltB H14 helix, DltC makes contacts with the C-terminal half of the H13 helix and the N terminus of the H14 helix—which suggests that the distance between DltC Ser35 and DltB His336 may be largely fixed during catalysis.
To examine the functional importance of the tunnel, we generated B. subtilis strains that lacked the dlt operon, and then complemented with a heterologous copy of the dlt locus expressed from its native promoter. The LTA d-alanylation level and the viability of dlt-deleted B. subtilis cells complemented with a heterologous copy of the dlt locus, containing either wild-type dltB or various dltB mutations, were evaluated using 14C- d-Ala radiolabelling and lysozyme-sensitivity assays, respectively. Mutation of DltB residues corresponding either to S. thermophilus DltB His336 or to the DltC-binding site completely abolished LTA d-alanylation (Fig. 3c). In addition, both mutations considerably reduced the viability of B. subtilis in the presence of lysozyme, whereas mutations of two other DltB residues did not have a substantial effect in both assays (Fig. 3c, d, Extended Data Fig. 8). Our functional assay data together with the structural features of DltB strongly suggest that the tunnel is important for the catalytic mechanism of DltB (Fig. 3e).
In some other O-acyltransferases, such as carnitine acyltransferase24, a conserved histidine catalyses the acyl-transfer reaction by aligning the carnitine substrate with the acyl-CoA thioester bond. The Ppant- d-Ala chain has a length of around 20 Å between the phosphate group and d-Ala. In our crystal structure, the distance between the Ser35-Ppant phosphate group and His336 is approximately 21 Å. Should the tunnel be open for the Ppant-d-Ala chain binding, this distance would enable His336 to align the substrate that receives the acyl group (probably a glycerol phosphate unit within LTA molecule) with d-alanylated DltC-Ppant. Thus, our structures suggest a model in which d-alanylation of LTA occurs between the LTA bound to the extracellular funnel and the d-alanyl group on DltC-Ppant-d-Ala bound to the cytoplasmic side of the tunnel (Fig. 3e).
Because DltB forms a stable complex with DltC-Ppant even without the d-alanyl group, and the DltC Ser35 is open to the cytosol, we speculate that DltC-Ppant forms a constitutive complex with DltB during catalysis and the Ppant chain can migrate between the tunnel and the cytosol, where loading of the d-alanyl group of DltC-Ppant can be catalysed by DltA. We then asked how the DltB tunnel opens for Ppant binding. The DltB tunnel is formed by the small horizontal helix H12 and the long transmembrane helices (H13–H15) forming the C-ridge of DltB. Compared to the DltB C-ridge helices, helix H12 is more likely to be the mobile structural component. The tunnel opening can be caused by movement or by a conformational change of the short helix H12, the position of which is stabilized through local hydrophobic interactions. H12 may change its position without disturbing the N- and C-ridge structures and lead to the opening of the tunnel. H12 movement may be induced by the presence of an appropriate signal, such as substrate binding with the extracellular funnel and/or binding of intracellular ligands such as DltC-Ppant- d-Ala. It should be noted here that in the Dlt system DltD is required for the d-alanylation of LTA in vivo. It remains unclear how DltD may contribute to this process. A combination of structural and enzymatic analysis is needed to reveal the role of DltD and the detailed catalytic mechanism of DltB.
We next considered the implication of the DltB structure for other MBOAT proteins. Despite a low overall sequence homology, a more conserved region within MBOAT sequences—termed MBOAT2 homology—was identified (http://pfam.xfam.org/family/MBOAT_2). The MBOAT2 homology covers the sequences that correspond to the DltB region from DltB H12 to the N terminus of H15 (Fig. 1b), which forms the majority of the central core that is thinner than the lipid bilayer. Thus, the thin central core and the extracellular (or lumen-facing) funnel are likely to be common structural features in many MBOATs. It has been demonstrated that the most conserved histidine (DltB His336), which is located within this MBOAT2 homology domain, is critical for the enzymatic activities of all tested MBOAT proteins—including PORCN, HHAT, GOAT, ACAT and DGAT17,18,19,20,21,22—which strongly suggests a common or similar catalytic mechanism for the MBOAT superfamily of proteins. The conserved extracellular/lumen structural funnel, the thin central core and the tunnel that we observed in our DltB structure are probably shared by many other MBOATs. Indeed, our crystal structure of DltB is in good agreement with the membrane topology models of HHAT and GOAT that have previously been derived from biochemical data25,26,27 (Extended Data Fig. 9). For example, in each case, the catalytic histidine was predicted to be at the end of an HHAT or a GOAT transmembrane helix facing the lumen side, consistent with our structure of DltB. That the critical horizontal helices H11–H13 in our DltB core structure were predicted to be a cytoplasmic subdomain of HHAT or GOAT is also consistent with models. In addition, a predicted ‘re-entrant helix’ observed in both HHAT and GOAT corresponds to the H7–H8 ‘half-way turn-back’ structure in the DltB central core (Fig. 1b, Extended Data Fig. 9). In contrast to the similarity in the core structure, the N- and C-terminal regions of HHAT and GOAT are much more divergent.
Among vertebrate MBOATs, PORCN and GOAT are responsible for lipid modifications of secreted Wnts and ghrelin, respectively. They all catalyse reactions across the endoplasmic reticulum membrane, with the acyl-group-accepting proteins located in the endoplasmic reticulum lumen and acyl-CoA in the cytosol6,7. Because DltB also catalyses cross-membrane reactions, we examined the sequence homology among DltB, PORCN and GOAT. It appears that there are four conserved regions: the region covering DltB helices H12–H14 (the MBOAT2 homology region), the DltB H7–H8 region, and two partial helices in the inner circle of the DltB structure (most of helix H6 and the central part of helix H10) (Fig. 4, Extended Data Fig. 9). Therefore, although sequences encoding the N- and C-ridges of DltB are generally not conserved in other MBOATs, the central core of DltB—along with its structural neighbours in the inner circle (for example, parts of helices H6 and H10)—are conserved among vertebrate MBOATs, including PORCN and GOAT. We suggest that the non-conserved nature of the ridges enable recognition of distinct substrates specific to different members within the MBOAT family. It should be noted that the mechanism of acyl-group binding is probably very different between metazoan MBOATs, most of which bind acyl-CoA as a donor, and DltB, which uses DltC-Ppant-d-Ala.
a, Alignment of conserved regions of DltB, PORCN and GOAT. Conserved sequences are highlighted in yellow (and in green under the sequences). The red rectangle indicates the DltB-specific insertion, which is involved in the binding of DltC-Ppant. A red star marks the most conserved histidine residue among MBOATs. St, S. thermophilus; Lc, Lactobacillus casei; Hs, Homo sapiens; Xl, Xenopus laevis; Mm, Mus musculus. b, The conserved MBOAT core. Conserved regions among MBOATs form a central core in the DltB structure (coloured in green), whereas the peripheral helices shielding the core are largely non-conserved (coloured in wheat).
The deep, conserved DltB extracellular structural funnel, as well as the DltB tunnel, may be an excellent target for drug development. Furthermore, many other bacterial and metazoan MBOATs may also be very druggable targets, as many of them are present on the surface of the cell membrane. In addition, the deep extracellular/lumen funnel shape close to the active site is probably a conserved feature of many MBOATs, and may be an excellent drug-binding site. Indeed, even in the absence of a 3D structure and detailed enzymatic analysis using purified PORCN, multiple small-molecule inhibitors with half-maximal inhibitory concentrations in the low-nanomolar range have been found through cell-based screening, and some of them have been used in clinical trials for the treatment of cancer28,29,30. Potent HHAT and GOAT inhibitors have also been reported and examined in several studies5. On the basis of our crystal structures, we predict that many more highly potent MBOAT inhibitors will be discovered in the future.
Three-dimensional structural prediction of MBOAT proteins has been very difficult and unreliable. Our crystal structures of DltB serve as a cornerstone for understanding the structure and function of MBOAT proteins. In addition, our structures reveal an intriguing mechanism for cross-membrane catalysis, and provide a platform for the development of new clinically relevant drugs across species.
Methods
Protein preparation
The cDNA of full-length S. thermophilus DltB was subcloned into pET21b (Novagen). cDNAs of S. thermophilus AcpS, DltA and DltC were subcloned into pQLink vectors (Addgene) with AcpS and DltA bearing an N-terminal 6×His-tag, and DltC bearing a N-terminal GST-tag. Escherichia coli strain C43 (DE3) was used for protein overexpression. Overexpression of the above proteins was induced by 0.4 mM isopropyl β-d-thiogalactoside when cell density reached an optical density at 600 nm (OD600) of 1.0. After induction at 37 °C for 5 h, the cells were collected and homogenized in buffer containing 25 mM Tris-HCl pH 8.0 and 150 mM NaCl.
For DltB purification, after disruption by sonication, cell debris was removed by centrifugation for 10 min at 20,000g. The supernatant was collected and ultracentrifuged for 1.5 h at 100,000g. The membrane fraction was collected and homogenized with buffer containing 25 mM Tris-HCl pH 8.0 and 150 mM NaCl. n-Decyl-β-d-maltopyranoside (Anatrace) was added to the membrane suspension to a final concentration of 1.5% (w/v) and then incubated for 2 h at 4 °C. After another ultracentrifugation step at 100,000g for 30 min, the supernatant was collected and loaded onto Ni-NTA affinity resin (Ni-NTA; Qiagen). After washing with buffer containing 25 mM Tris-HCl pH 8.0, 500 mM NaCl, 25 mM imidazole and 0.2% (w/v) n-decyl-β-d-maltopyranoside, DltB was eluted with a buffer containing 25 mM Tris-HCl pH 8.0, 150 mM NaCl, 400 mM imidazole and various detergents from Anatrace. After being concentrated to 10 mg ml−1, DltB was further purified by gel filtration (Superdex-200 10/30; GE Healthcare). The buffer for gel filtration contained 25 mM Tris-HCl pH 8.0, 150 mM NaCl and various detergents. The peak fractions were collected.
For the purification of DltA and DltC, after sonication the cell debris was removed by centrifugation for 1 h at 35,000g. The supernatants were loaded onto Ni-NTA affinity resin and Glutathione Sepharose 4 resin (GS4B resin, GE Healthcare), respectively. After a wash step, the N-terminal GST-tag was either removed from DltC or maintained, depending on the purpose of the experiment. After elution, DltA and DltC solutions were loaded onto HiTrap Q HP columns (5 ml, GE Healthcare), and protein samples eluted from the Q column were further purified by gel filtration. Peak fractions were collected and concentrated. Finally, DltA and DltC were stored in buffer containing 25 mM Tris-HCl pH 8.0 and 150 mM NaCl.
DltB and DltC mutants were generated with a standard PCR-based strategy and were subcloned, overexpressed and purified in the same way as the wild-type proteins.
Protein crystallization
The hanging-drop vapour-diffusion method was performed at room temperature during crystallization. DltB and DltC proteins were purified as mentioned above, and crystals were obtained from DltB purified with n-nonyl-β-d-glucopyranoside (Anatrace). For crystallization of the DltB–DltC complex, DltB and DltC were purified separately and mixed before crystallization at a molar ratio of 1:2. Crystals belonging to crystal form I (space group P21, Extended Data Table 1) were crystallized in buffer containing 21% PEG400, 100 mM Tris-HCl pH 7.5, 100 mM NaCl and 100 mM MgCl2. Crystals belonging to crystal form II (space group P21, Extended Data Table 1) were crystallized in buffer containing 27% PEG400, 100 mM sodium citrate pH 5.6, 200 mM NH4H2PO4 and 100 mM (NH4)2SO4. Crystals belonging to crystal form III (space group P212121, Extended Data Table 1) were crystallized in buffer containing 27% PEG400, 100 mM HEPES pH 7.5, 200 mM sodium citrate tribasic dihydrate and 3% 1,5-diaminopentane dihydrochloride. For crystals in the different crystal forms above, thin or thick rod-shaped crystals typically grew for 1 to 2 weeks before reaching full crystal size. Gold derivatives were obtained by soaking the crystals in crystal form I for 2 h in mother liquor containing 2 mg ml−1 KAu(CN)2.
Data collection and structure determination
The crystals were directly flash-frozen in liquid nitrogen. Screening and data collection were performed at the Advanced Light Source, beamlines 5.0.1, 8.2.1 and 8.2.2. All diffraction data were processed by HKL200031. The single-wavelength anomalous dispersion (SAD) dataset was collected near the gold L-III absorption edge at a wavelength of 1.02 Å (Extended Data Table 1). The gold derivative sites and the initial phases were determined by PHENIX32. Twenty gold derivative sites were found in one asymmetric unit, and the experimental electron density map clearly showed the presence of four DltB molecules in one asymmetric unit. The B. subtilis DltC crystal structure (PDB ID: 4BPH) was used as the searching model for our DltC molecules33. The complex model was improved using iterative cycles of manual rebuilding with the program Coot34 and refinement with a native dataset of 3.30 Å using Refmac5 of the CCP4 7.0 program suite35. The structures for crystal forms II and III were solved by molecular replacement using the model from crystal form I. All structure model figures in the paper were generated using PyMOL36. The protein conservation surface was generated using the ConSurf server37, based on the alignment of DltB sequences generated using T-Coffee38.
Binding assay
Pull-down assays were performed as described below. Twenty micrograms of wild-type DltB (or DltB mutants), 10 µg of wild-type GST–DltC (or GST–DltC mutants) and 10 µl GS4B resin were mixed in 100 µl of pull-down buffer containing 25 mM HEPES pH 7.5, 150 mM NaCl and 0.15% (w/v) n-decyl-β-d-maltopyranoside. The mixed samples were incubated at 4 °C on a rotisserie for 1 h, followed by washing the resin with pull-down buffer three times. During each wash, 100 µl of pull-down buffer was added to each sample and the solution was incubated at room temperature for 2 min before centrifugation and removal of supernatant. After washing, the resin samples were analysed by SDS–PAGE with Coomassie blue staining.
Binding assays were also performed at room temperature using the Octet system (FortéBio). Free GST, and GST-tagged wild-type DltC or DltC mutants were mobilized on anti-GST biosensors (FortéBio). After quenching with free GST to block free antibody sites on the biosensors, the biosensors were dipped into DltB solutions for binding measurements. The concentration gradient of DltB used in the Octet binding assay is: 0.03 µM, 0.1 µM, 0.3 µM, 1 µM, 3 µM, 10 µM.
Construction of B. subtilis strain for functional assays
The cat gene was amplified by PCR from pGEMcat, and 500 bp upstream and downstream of dlt operon fragments were amplified from the B. subtilis genome. These three pieces were assembled using isothermal assembly and transformed directly into the B. subtilis HM1 strain, resulting in dlt-operon-deleted B. subtilis (∆dlt). The deletion was confirmed by PCR amplification and Sanger sequencing.
The natural dlt locus was amplified and cloned into pMMB752. Mutations of the dltB gene in pMMB752 carrying the dlt operon and Flag-tagged constructs were generated on the basis of a standard PCR method, followed by isothermal assembly to ligate the ends together. The pMMB752 constructs were transformed into B. subtilis with the dlt operon deleted from its native locus to generate strains for use in assays. Cells used here and in the following functional experiments were cultured in the presence of appropriate antibiotics to avoid possible contamination.
Detection of LTA d-alanylation
This assay was established on the basis of a previously reported method14. Wild-type B. subtilis HM1 strain, and dlt-operon-deleted B. subtilis HM1 strain complemented with either empty pMMB752 vector or vectors containing natural dlt-operon-bearing mutations on the dltB gene (untagged or Flag-tagged), were inoculated from fresh colonies on plate into liquid LB medium supplemented with 0.5 µg ml−1 erythromycin. Overnight cultures were diluted into 3 ml of LB at an OD600 of 0.1 and grown to an OD600 of 0.6. Cells were pelleted and resuspended into 1.5 ml of assay medium containing 0.25× LB, 50 mM Bis-Tris pH 6.0, and 200 µg ml−1 d-cycloserine. To test the inhibition of m-AMSA on LTA d-alanylation for wild-type B. subtilis, a final concentration of 150 µM of m-AMSA (Abcam) was supplemented into the assay medium. After incubation in the assay medium for 30 min, 14C-d-alanine (Moravek Biochemicals) was added to a final concentration of 25 μM for an additional incubation of 30 min or 120 min. Cells were pelleted and resuspended with SDS-loading buffer, followed by a freeze–thaw cycle. Samples were vortexed and boiled for 5 min before loading onto 4–20% gradient Tris/glycine gel (Bio-Rad). Gels were dried and exposed to a phosphor storage screen for 3 days before imaging with Typhoon FLA 9000 gel imaging scanner (GE Healthcare).
To compare the expression level of C-terminal Flag-tagged DltB in corresponding B. subtilis strains, each strain was cultured in 1 l LB to OD600 of 0.6. Cells were collected and disrupted by French press, and the cell membrane was isolated by ultracentrifugation after removing cell debris by low-speed centrifugation. The membrane of each strain was resuspended with buffer containing 25 mM Tris-HCl 8.0, 150 mM NaCl into 500 µl, followed by freezing at −80 °C. One microlitre of each membrane sample was run onto SDS–PAGE and the expression of Flag-tagged DltB was detected by western blotting.
Survival assays
Dlt knockout strains of the Gram-positive bacterium B. subtilis are sensitive to the cell-wall-degrading enzyme lysozyme39. B. subtilis strains were struck on LB plates (supplemented with the appropriate antibiotic when needed) from freezer stocks and incubated overnight at 37 °C. The resulting growth on plates was used to inoculate 2-ml LB broth cultures in glass tubes. The cultures were grown at 37 °C with shaking (260 r.p.m.) to an OD600 of 1.0–2.0. All of the cultures were adjusted to an OD600 of 0.3 and then serially diluted in LB broth with tenfold dilutions. For each strain, 5 µl of each dilution was plated onto LB plates and LB plates supplemented with 30 µg ml−1 of lysozyme (Fisher) and incubated at 30 °C overnight. After incubation, colonies were enumerated and plates were imaged with a Bio-Rad Gel Doc XR+ Molecular Imager.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Data availability
References
Hofmann, K. A superfamily of membrane-bound O-acyltransferases with implications for Wnt signaling. Trends Biochem. Sci. 25, 111–112 (2000).
Chang, T. Y., Chang, C. C., Ohgami, N. & Yamauchi, Y. Cholesterol sensing, trafficking, and esterification. Annu. Rev. Cell Dev. Biol. 22, 129–157 (2006).
Liu, Q., Siloto, R. M., Lehner, R., Stone, S. J. & Weselake, R. J. Acyl-CoA:diacylglycerol acyltransferase: molecular biology, biochemistry and biotechnology. Prog. Lipid Res. 51, 350–377 (2012).
Chang, S. C. & Magee, A. I. Acyltransferases for secreted signalling proteins (review). Mol. Membr. Biol. 26, 104–113 (2009).
Masumoto, N. et al. Membrane bound O-acyltransferases and their inhibitors. Biochem. Soc. Trans. 43, 246–252 (2015).
Resh, M. D. Fatty acylation of proteins: the long and the short of it. Prog. Lipid Res. 63, 120–131 (2016).
Tuladhar, R. & Lum, L. Fatty acyl donor selectivity in membrane bound O-acyltransferases and communal cell fate decision-making. Biochem. Soc. Trans. 43, 235–239 (2015).
Resh, M. D. Palmitoylation of proteins in cancer. Biochem. Soc. Trans. 45, 409–416 (2017).
Lanyon-Hogg, T., Faronato, M., Serwa, R. A. & Tate, E. W. Dynamic protein acylation: new substrates, mechanisms, and drug targets. Trends Biochem. Sci. 42, 566–581 (2017).
Madan, B. & Virshup, D. M. Targeting Wnts at the source—new mechanisms, new biomarkers, new drugs. Mol. Cancer Ther. 14, 1087–1094 (2015).
Perego, M. et al. Incorporation of d-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis. Identification of genes and regulation. J. Biol. Chem. 270, 15598–15606 (1995).
Neuhaus, F. C., Heaton, M. P., Debabov, D. V. & Zhang, Q. The dlt operon in the biosynthesis of d-alanyl-lipoteichoic acid in Lactobacillus casei. Microb. Drug Resist. 2, 77–84 (1996).
Reichmann, N. T., Cassona, C. P. & Gründling, A. Revised mechanism of d-alanine incorporation into cell wall polymers in Gram-positive bacteria. Microbiology 159, 1868–1877 (2013).
Pasquina, L. et al. A synthetic lethal approach for compound and target identification in Staphylococcus aureus. Nat. Chem. Biol. 12, 40–45 (2016).
Neuhaus, F. C. & Baddiley, J. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in Gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67, 686–723 (2003).
Peschel, A. et al. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274, 8405–8410 (1999).
Rios-Esteves, J., Haugen, B. & Resh, M. D. Identification of key residues and regions important for porcupine-mediated Wnt acylation. J. Biol. Chem. 289, 17009–17019 (2014).
Yang, J., Brown, M. S., Liang, G., Grishin, N. V. & Goldstein, J. L. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132, 387–396 (2008).
Buglino, J. A. & Resh, M. D. Identification of conserved regions and residues within hedgehog acyltransferase critical for palmitoylation of sonic hedgehog. PLoS ONE 5, e11195 (2010).
Das, A., Davis, M. A. & Rudel, L. L. Identification of putative active site residues of ACAT enzymes. J. Lipid Res. 49, 1770–1781 (2008).
Lin, S., Lu, X., Chang, C. C. & Chang, T. Y. Human acyl-coenzyme A:cholesterol acyltransferase expressed in chinese hamster ovary cells: membrane topology and active site location. Mol. Biol. Cell 14, 2447–2460 (2003).
McFie, P. J., Stone, S. L., Banman, S. L. & Stone, S. J. Topological orientation of acyl-CoA:diacylglycerol acyltransferase-1 (DGAT1) and identification of a putative active site histidine and the role of the N terminus in dimer/tetramer formation. J. Biol. Chem. 285, 37377–37387 (2010).
Viana, D. et al. A single natural nucleotide mutation alters bacterial pathogen host tropism. Nat. Genet. 47, 361–366 (2015).
Jogl, G., Hsiao, Y. S. & Tong, L. Structure and function of carnitine acyltransferases. Ann. NY Acad. Sci. 1033, 17–29 (2004).
Taylor, M. S. et al. Architectural organization of the metabolic regulatory enzyme ghrelin O-acyltransferase. J. Biol. Chem. 288, 32211–32228 (2013).
Matevossian, A. & Resh, M. D. Membrane topology of hedgehog acyltransferase. J. Biol. Chem. 290, 2235–2243 (2015).
Konitsiotis, A. D. et al. Topological analysis of hedgehog acyltransferase, a multipalmitoylated transmembrane protein. J. Biol. Chem. 290, 3293–3307 (2015).
Barnett, B. P. et al. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science 330, 1689–1692 (2010).
Ho, S. Y. & Keller, T. H. The use of porcupine inhibitors to target Wnt-driven cancers. Bioorg. Med. Chem. Lett. 25, 5472–5476 (2015).
Chen, B. et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 5, 100–107 (2009).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Zimmermann, S. et al. High-resolution structures of the d-alanyl carrier protein (Dcp) DltC from Bacillus subtilis reveal equivalent conformations of apo- and holo-forms. FEBS Lett. 589, 2283–2289 (2015).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994).
Delano, W. L. & Brünger, A. T. Helix packing in proteins: prediction and energetic analysis of dimeric, trimeric, and tetrameric GCN4 coiled coil structures. Proteins 20, 105–123 (1994).
Ashkenazy, H. et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 44, W344–W350 (2016).
Taly, J. F. et al. Using the T-Coffee package to build multiple sequence alignments of protein, RNA, DNA sequences and 3D structures. Nat. Protoc. 6, 1669–1682 (2011).
Guariglia-Oropeza, V. & Helmann, J. D. Bacillus subtilis σV confers lysozyme resistance by activation of two cell wall modification pathways, peptidoglycan O-acetylation and d-alanylation of teichoic acids. J. Bacteriol. 193, 6223–6232 (2011).
Acknowledgements
We are grateful to the staff at Advanced Light Source beamlines 5.0.1, 8.2.1 and 8.2.2 for assistance with synchrotron data collection. We thank N. Zheng and P. Hsu for comments on this manuscript, T. Hinds for discussion and advice on assays, S. Ovchinnikov for computational modelling, L. Kruse for use of the radioactive gel scanner and M. Ragheb for construction of the dlt operon deletion strain. This work was supported by National Institutes of Health grant R01 GM127316 to W.X. and a Jane Coffin Childs postdoctoral fellowship to D.M. This work was also supported by Chinese Academy of Sciences grant XDB08010303 to Z.R. and W.X., the National Institute of Health grant DP2GM110773 to H.M. and the Bacterial Pathogenesis Training Grant 5T32AI055396-13 to K.S.L.
Reviewer information
Nature thanks E. Tate and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
D.M. carried out protein purification, crystallization, and related binding and enzymatic analysis. D.M. and Z.W. collected diffraction data and determined the crystal structure. Z.W. performed structural refinement. C.N.M. constructed the B. subtilis strains, and C.N.M., K.S.L. and H.M. performed the cell survival assays. P.L. contributed to molecular cloning and sample preparation. X.L. and Z.R. contributed to screening of other MBOAT proteins. D.M., Z.W. and W.X. analysed structural data and wrote the paper. All authors participated in manuscript revision and analysis of biochemical data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 MBOAT-catalysed reactions and chemical structures of MBOAT substrates.
a, General reaction catalysed by MBOATs. b, Structure of CoA and acyl-CoA. The red rectangle highlights the Ppant prosthetic group within the CoA structure. For known acyl-group donors of MBOATs, the acyl groups are covalently linked with a sulfhydryl group (for example, that of Ppant in acyl-CoA or DltC-Ppant). c, Comparison of acyl-group donors and acceptors of PORCN, GOAT, DGAT1, ACAT and DltB. In the acyl-group donor column, the red dashed lines indicate the bonds that are broken during acyl-transfer reactions. In the acyl-group acceptor column, the hydroxyl groups that accept acyl groups are highlighted in red. ACAT1, ACAT2 and DGAT1 use saturated and unsaturated long-chain acyl-CoA. d, The reaction catalysed by DltB. DltB catalyses d-alanylation of both wall teichoic acid and LTA. Because the d-alanylation of wall teichoic acid is at least partially dependent on LTA d-alanylation, here we discuss only the d-alanylation of LTA. DltB transfers d-alanyl groups onto hydroxyl groups of the polyglycerolphosphate chain of the LTA molecule. For simplicity, only the type I LTA structure is shown here. The fatty-acid chains are responsible for the anchoring of LTA to the membrane of Gram-positive bacteria.
Extended Data Fig. 2 Purification of DltB, DltC-Ppant and DltB mutants.
a, SEC profile of DltB. DltB can be purified to homogeneity in most detergents and is well-behaved during SEC. b, SDS–PAGE and SEC profile of DltC. c, Mass spectrometry analysis of DltC species. This indicates that purified DltC has a molecular mass of 9,590 Da, which is equal to the calculated molecular mass of Ppant-modified DltC, referred to as DltC-Ppant. d, SEC profile of wild-type and mutant DltB proteins. DltB mutants including V305D/I306D, S293A, H289A and H336A are properly folded, as they migrate predominantly as a monomeric peak, similar to wild-type DltB.
Extended Data Fig. 3 Electron density map of DltB.
a, Stereo experimental electron density map, using phases derived from an Au-SAD phasing (Extended Data Table 1). This 2Fo–Fc map is contoured at 1.0σ. DltB backbone tracing is shown in red. b, The final 2Fo–Fc electron density map of the crystal form II (Extended Data Table 1). This map is contoured at 1.0σ, shown in stereo and in an orientation approximately looking down the funnel. The catalytic His336 as well as His289 (another conserved residue (either His or Asn) among MBOAT proteins) are labelled. Both His336 and His289 are located at the bottom of the extracellular funnel, and sandwich the top opening of the transmembrane tunnel.
Extended Data Fig. 4 Stereo view of DltB structure, and an extracellular ‘ring’ of DltB residues associated with a switch of pathogen host.
a, The ‘front’ side view of DltB (stereo view is provided). b, The ‘top’ view of DltB, looking from the extracellular space (stereo view is provided). The His336 side chain is shown as sticks. The extracellular funnel is clear at this angle. c, Cartoon illustration of the N- and C-ridges of DltB in two orthogonal views. d, Locations of pathogen-host-sensitive sites in S. aureus DltB (I2, V61, T113, H121, I227, Q231, Y247, Y250, Y346, G401 and K402) are labelled with red balls in corresponding residues of the S. thermophilus DltB structure. It is clear that all 11 sites are located at the apex of the extracellular ridge of DltB. S. aureus DltB T113 is not conserved and does not have a corresponding residue in other DltBs (see Extended Data Fig. 5): here, the position of its closest residue is labelled. The intracellular DltC is shown in magenta. The DltB structure in these two panels are related with a 45° rotation.
Extended Data Fig. 5 DltB sequence alignment.
DltB sequences of representatives from 10 different genera of Gram-positive bacteria were chosen for sequence alignment using the T-Coffee server. Secondary structural elements of DltB are indicated above the alignment. Residues that form the funnel are identified by purple squares, and residues that form the tunnel are identified with dark red dots. DltB residues involved in direct interaction with DltC are indicated with orange inverted triangles. Residues corresponding to the three sites for which single-point mutations desensitize S. aureus to inhibition by m-AMSA are indicated with blue triangles. Residues of S. aureus DltB, the mutation of which alter the host preference from being human-specific to being capable of infecting rabbits, are indicated with green diamonds. A red star highlights the histidine residue that is completely conserved among MBOATs. ST, S. thermophilus; BS, B. subtilis; LC, L. casei; SA, S. aureus; Lm, Listeria monocytogenes; EF, Enterococcus faecalis; CD, Clostridioides difficile; LM, Leuconostoc mesenteroides; LS, Lysinibacillus sphaericus; BT, Brochothrix thermosphacta.
Extended Data Fig. 6 GST pull-down and Octet assays for analysis of the interaction between DltB and DltC-Ppant.
a, Results of using wild-type GST–DltC to pull-down either wild-type or mutant DltB, with GST to pull-down wild-type DltB as a negative control. Lanes 1–5 show inputs in this experiment. Pull-down results demonstrate that DltB and DltC can form a stable complex at an almost 1:1 molar ratio. DltB(V305D) loses most of its capacity to bind to wild-type GST–DltC, whereas the binding between DltB and DltC was completely abolished with the double mutant DltB(V305D/I306D). b, Results of using wild-type or mutant GST–DltC to pull-down wild-type DltB. Lanes 1–5 show inputs in this experiment. The mutant GST–DltC(V39D) runs slightly slower than wild-type GST–DltC and GST–DltC(V39R) on SDS–PAGE. Both GST–DltC(V39D) and GST–DltC(V39R) lost most of their capacity to bind with wild-type DltB. Pull-down experiments were performed at least twice technically, with the same results. c. Binding-affinity measurements for DltB and DltC using the Octet technique. Wild-type GST–DltC-Ppant and GST–DltC(S35A) show similar binding affinities with wild-type DltB. Data are shown in blue, with the corresponding fits in red. The DltB concentration gradient used here is: 0.03 µM, 0.1 µM, 0.3 µM, 1 µM, 3 µM, 10 µM. Octet assays were performed twice technically. d, Summary of Octet binding assay. Wild-type DltC and GST–DltC(S35A) show similar binding affinities to wild-type DltB. Mean Kd values and s.d. are shown for each assay. Mutation of residues on the binding surface of either DltB or DltC can reduce or abolish their binding.
Extended Data Fig. 7 Structural details of the DltB–DltC interface and the DltB tunnel.
a, Superposition of crystal structures of DltB and the DltB–DltC complex. There is no significant conformational change in DltB upon the binding of DltC-Ppant. b, Cylinder illustration of the DltB–DltC-Ppant complex, viewed from the bottom of the DltB tunnel. DltB is coloured in rainbow, with DltC in purple. c, Conservation of the DltB tunnel region. Residues involved in tunnel formation are also highly conserved among DltB proteins from different species (Extended Data Fig. 5). d, Stereo view of the DltB tunnel and residues forming this tunnel. The tunnel is formed by three helices from the C-ridge (H13, H14 and H15) and the short H12 helix. Residues involved in tunnel formation in our structures are: Lys282, Trp285, Asn286, Ser293, Phe294, Phe296, Arg297, Phe301, Met302, Tyr325, Asn328, Met329, Met332, Leu353, and His336 (which is also involved in the formation of extracellular funnel).
Extended Data Fig. 8 Survival and LTA d-alanylation assays for wild-type and mutant DltB.
a, Lysozyme susceptibility survival assay. For DltB residues used in both LTA d-alanylation and survival assays, corresponding DltB residue numbers in two species are listed. The endogenous dlt operon was deleted in the B. subtilis strain and complemented with an ectopic copy of the wild-type dlt operon without tag on DltB. Representative images of serial dilutions of cells plated on LB agar (left) and LB agar supplemented with 30 µg ml−1 of lysozyme (right). The genotype of the dltB gene is indicated above the corresponding column of serial dilutions. Dilutions of cells are indicated on the y axis. Mutation of the critical histidine (His328) and residues of DltB involved in binding with DltC(V297/F298) increase the susceptibility to lysozyme of B. subtilis. b, Per cent survival of B. bacillus variants towards lysozyme treatment. This was calculated by dividing the colony-forming units (CFUs) from lysozyme plates by the CFUs from LB-only plates. Data are mean ± s.d. of three biological replicates. The genotype of dltB is indicated at the bottom. B. subtilis strains containing untagged DltB show a similar lysozyme susceptibility pattern to those containing Flag-tagged DltB. c, LTA d-alanylation assay. In experiment 1, the assay time was 120 min after 14C-d-alanine was added, whereas for experiments 2 and 3, the assay time was 30 min. Experiments 2 and 3 are two parallel assays for LTA d-alanylation detection. AMSA represents m-AMSA, a DltB inhibitor.
Extended Data Fig. 9 Comparison and rationalization of topological data.
a, Comparison of HHAT topology data with the DltB structure. b, Comparison of GOAT topology data with the DltB structure. In both panels, secondary structures above DltB sequences are generated from our DltB crystal structure. Reported topology assignments of HHAT and GOAT were achieved using human proteins. Here we highlighted the predicted HHAT or GOAT transmembrane helices for each protein with yellow background within sequences. Residues for human HHAT and GOAT that were experimentally verified to be located on the cytoplasmic side are coloured in red, and residues which are on the lumenal side are coloured in green. Helices and/or loops that are predicted to be associated with the membrane surface or buried halfway within the membrane on the cytoplasmic side are indicated with red and magenta rectangles, respectively. It is clear that the regions corresponding to DltB H7–H14 are topologically more conserved than those forming the DltB N- and C-ridges.
Supplementary information
Rights and permissions
About this article
Cite this article
Ma, D., Wang, Z., Merrikh, C.N. et al. Crystal structure of a membrane-bound O-acyltransferase. Nature 562, 286–290 (2018). https://doi.org/10.1038/s41586-018-0568-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0568-2
- Springer Nature Limited
Keywords
This article is cited by
-
Structure and mechanism of lysosome transmembrane acetylation by HGSNAT
Nature Structural & Molecular Biology (2024)
-
Unlocking ferroptosis in prostate cancer — the road to novel therapies and imaging markers
Nature Reviews Urology (2024)
-
Structural insights into the transporting and catalyzing mechanism of DltB in LTA D-alanylation
Nature Communications (2024)
-
The structure of phosphatidylinositol remodeling MBOAT7 reveals its catalytic mechanism and enables inhibitor identification
Nature Communications (2023)
-
Mechanisms and inhibition of Porcupine-mediated Wnt acylation
Nature (2022)