Abstract
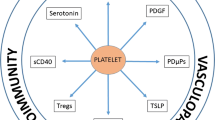
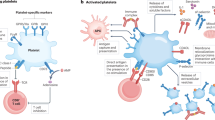
Kawasaki disease, a systemic vasculitis that affects young children and can result in coronary artery aneurysms, is the leading cause of acquired heart disease among children. A hallmark of Kawasaki disease is increased blood platelet counts and platelet activation, which is associated with an increased risk of developing resistance to intravenous immunoglobulin and coronary artery aneurysms. Platelets and their releasate, including granules, microparticles, microRNAs and transcription factors, can influence innate immunity, enhance inflammation and contribute to vascular remodelling. Growing evidence indicates that platelets also interact with immune and non-immune cells to regulate inflammation. Platelets boost NLRP3 inflammasome activation and IL-1β production by human immune cells by releasing soluble mediators. Activated platelets form aggregates with leukocytes, such as monocytes and neutrophils, enhancing numerous functions of these cells and promoting thrombosis and inflammation. Leukocyte–platelet aggregates are increased in children with Kawasaki disease during the acute phase of the disease and can be used as biomarkers for disease severity. Here we review the role of platelets in Kawasaki disease and discuss progress in understanding the immune-effector role of platelets in amplifying inflammation related to Kawasaki disease vasculitis and therapeutic strategies targeting platelets or platelet-derived molecules.
Key points
-
Platelets are essential for haemostasis and thrombosis; however, platelets also have multifaceted roles in regulating immune responses and contributing to the pathogenesis of inflammatory diseases.
-
Thrombocytosis is usually reported in Kawasaki disease, a systemic paediatric vasculitis, and has been associated with increased risk of developing coronary artery aneurysms.
-
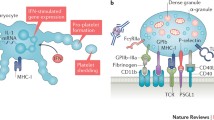
Levels of monocyte–platelet aggregates (MPAs) and neutrophil–platelet aggregates (NPAs) increase during Kawasaki disease; these leukocyte–platelet aggregates (LPAs) might amplify Kawasaki disease pathogenesis through their pro-inflammatory and thrombotic functions and have been related to the development of coronary artery aneurysms in Kawasaki disease.
-
Although aspirin therapy does not reduce MPA formation, studies suggest that targeting the P-selectin–PSGL1 axis or inhibiting the P2Y12 receptor might attenuate platelet-induced monocyte activation.
-
Platelets are active participants and mediators of cardiovascular inflammation during Kawasaki disease vasculitis, not innocent bystanders. Hence, platelets might be promising therapeutic targets for Kawasaki disease vasculitis.



Similar content being viewed by others
References
McCrindle, B. W. et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 135, e927–e999 (2017).
Soni, P. R., Noval Rivas, M. & Arditi, M. A comprehensive update on Kawasaki disease vasculitis and myocarditis. Curr. Rheumatol. Rep. 22, 6 (2020).
Kawasaki, T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi 16, 178–222 (1967).
Orenstein, J. M. et al. Three linked vasculopathic processes characterize Kawasaki disease: a light and transmission electron microscopic study. PLoS ONE 7, e38998 (2012).
Dionne, A. & Dahdah, N. Myocarditis and Kawasaki disease. Int. J. Rheum. Dis. 21, 45–49 (2017).
Dahdah, N. Not just coronary arteritis, Kawasaki disease is a myocarditis, too. J. Am. Coll. Cardiol. 55, 1507 (2010).
Daniels, L. B. et al. Prevalence of Kawasaki disease in young adults with suspected myocardial ischemia. Circulation 125, 2447–2453 (2012).
Daniels, L. B. et al. Long-term health outcomes in young adults after Kawasaki disease. Int. J. Cardiol. Heart Vasc. 40, 101039 (2022).
Gordon, J. B. et al. The spectrum of cardiovascular lesions requiring intervention in adults after Kawasaki disease. JACC Cardiovasc. Interv. 9, 687–696 (2016).
Gordon, J. B., Kahn, A. M. & Burns, J. C. When children with Kawasaki disease grow up: myocardial and vascular complications in adulthood. J. Am. Coll. Cardiol. 54, 1911–1920 (2009).
Scherlinger, M., Richez, C., Tsokos, G. C., Boilard, E. & Blanco, P. The role of platelets in immune-mediated inflammatory diseases. Nat. Rev. Immunol. 23, 409 (2023).
Ribeiro, L. S., Migliari Branco, L. & Franklin, B. S. Regulation of innate immune responses by platelets. Front. Immunol. 10, 1320 (2019).
Noval Rivas, M. & Arditi, M. Kawasaki disease: pathophysiology and insights from mouse models. Nat. Rev. Rheumatol. 16, 391–405 (2020).
Popper, S. J. et al. Gene-expression patterns reveal underlying biological processes in Kawasaki disease. Genome Biol. 8, R261 (2007).
Hoang, L. T. et al. Global gene expression profiling identifies new therapeutic targets in acute Kawasaki disease. Genome Med. 6, 541 (2014).
Jaggi, P. et al. Whole blood transcriptional profiles as a prognostic tool in complete and incomplete Kawasaki disease. PLoS ONE 13, e0197858 (2018).
Wang, Z. et al. Single-cell RNA sequencing of peripheral blood mononuclear cells from acute Kawasaki disease patients. Nat. Commun. 12, 5444 (2021).
Shulman, S. T. & Rowley, A. H. Kawasaki disease: insights into pathogenesis and approaches to treatment. Nat. Rev. Rheumatol. 11, 475–482 (2015).
Sato, W., Yokouchi, Y., Oharaseki, T., Asakawa, N. & Takahashi, K. The pathology of Kawasaki disease aortitis: a study of 37 cases. Cardiovasc. Pathol. 51, 107303 (2021).
Rowley, A. H., Eckerley, C. A., Jack, H. M., Shulman, S. T. & Baker, S. C. IgA plasma cells in vascular tissue of patients with Kawasaki syndrome. J. Immunol. 159, 5946–5955 (1997).
Brown, T. J. et al. CD8 T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J. Infect. Dis. 184, 940–943 (2001).
Yilmaz, A. et al. Activated myeloid dendritic cells accumulate and co-localize with CD3+ T cells in coronary artery lesions in patients with Kawasaki disease. Exp. Mol. Pathol. 83, 93–103 (2007).
Takahashi, K., Oharaseki, T. & Yokouchi, Y. Histopathological aspects of cardiovascular lesions in Kawasaki disease. Int. J. Rheum. Dis. 21, 31–35 (2017).
Furukawa, S. et al. Peripheral blood monocyte/macrophages and serum tumor necrosis factor in Kawasaki disease. Clin. Immunol. Immunopathol. 48, 247–251 (1988).
Leung, D. Y. et al. Endothelial cell activation and high interleukin-1 secretion in the pathogenesis of acute Kawasaki disease. Lancet 2, 1298–1302 (1989).
Leung, D. Y. et al. Two monokines, interleukin 1 and tumor necrosis factor, render cultured vascular endothelial cells susceptible to lysis by antibodies circulating during Kawasaki syndrome. J. Exp. Med. 164, 1958–1972 (1986).
Brodeur, K. E. et al. Elevation of IL-17 cytokines distinguishes Kawasaki disease from other pediatric inflammatory disorders. Arthritis Rheumatol. 76, 285–292 (2024).
Kobayashi, N. et al. Intravenous gamma-globulin therapy improves hypercytokinemia in the acute phase of Kawasaki disease. Mod. Rheumatol. 14, 447–452 (2004).
Takahashi, K., Oharaseki, T., Yokouchi, Y., Naoe, S. & Saji, T. Kawasaki disease: basic and pathological findings. Clin. Exp. Nephrol. 17, 690–693 (2013).
He, M. et al. miR-483 targeting of CTGF suppresses endothelial-to-mesenchymal transition: therapeutic implications in Kawasaki disease. Circ. Res. 120, 354–365 (2017).
Shimizu, C. et al. The role of TGF-β and myofibroblasts in the arteritis of Kawasaki disease. Hum. Pathol. 44, 189–198 (2013).
Kato, H. et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation 94, 1379–1385 (1996).
Suzuki, A. et al. Functional behavior and morphology of the coronary artery wall in patients with Kawasaki disease assessed by intravascular ultrasound. J. Am. Coll. Cardiol. 27, 291–296 (1996).
Shimizu, C. et al. Cardiovascular pathology in 2 young adults with sudden, unexpected death due to coronary aneurysms from Kawasaki disease in childhood. Cardiovasc. Pathol. 24, 310–316 (2015).
Wagner, D. D. & Burger, P. C. Platelets in inflammation and thrombosis. Arterioscler. Thromb. Vasc. Biol. 23, 2131–2137 (2003).
Machlus, K. R. & Italiano, J. E. Jr The incredible journey: from megakaryocyte development to platelet formation. J. Cell Biol. 201, 785–796 (2013).
Semple, J. W., Italiano, J. E. Jr. & Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 11, 264–274 (2011).
Patel, S. R., Hartwig, J. H. & Italiano, J. E. Jr The biogenesis of platelets from megakaryocyte proplatelets. J. Clin. Invest. 115, 3348–3354 (2005).
Miura, N., Terai, M., Meng, Y. G., Sato, T. & Niimi, H. Serum thrombopoietin levels in Kawasaki disease. Br. J. Haematol. 100, 387–388 (1998).
Ishiguro, A. et al. Elevation of serum thrombopoietin precedes thrombocytosis in Kawasaki disease. Thromb. Haemost. 79, 1096–1100 (1998).
Kaushansky, K. Thrombopoiesis. Semin. Hematol. 52, 4–11 (2015).
Wu, Y. et al. Interleukin-6 is prone to be a candidate biomarker for predicting incomplete and IVIG nonresponsive Kawasaki disease rather than coronary artery aneurysm. Clin. Exp. Med. 19, 173–181 (2019).
Ueno, Y. et al. The acute phase nature of interleukin 6: studies in Kawasaki disease and other febrile illnesses. Clin. Exp. Immunol. 76, 337–342 (1989).
Matsubara, T. Interleukin 6 activities and tumor necrosis factor-alpha levels in serum of patients with Kawasaki disease. Arerugi 40, 147–154 (1991).
Maury, C. P., Salo, E. & Pelkonen, P. Circulating interleukin-1 β in patients with Kawasaki disease. N. Engl. J. Med. 319, 1670–1671 (1988).
Alphonse, M. P. et al. Inositol-triphosphate 3-kinase c mediates inflammasome activation and treatment response in Kawasaki disease. J. Immunol. 197, 3481–3489 (2016).
Lefrançais, E. et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 544, 105–109 (2017).
Pariser, D. N. et al. Lung megakaryocytes are immune modulatory cells. J. Clin. Invest. 131, e137377 (2021).
Livada, A. C., Pariser, D. N. & Morrell, C. N. Megakaryocytes in the lung: history and future perspectives. Res. Pract. Thromb. Haemost. 7, 100053 (2023).
Sharma, G. K. & Talbot, I. C. Pulmonary megakaryocytes: “missing link” between cardiovascular and respiratory disease? J. Clin. Pathol. 39, 969–976 (1986).
Kaufman, R. M., Airo, R., Pollack, S. & Crosby, W. H. Circulating megakaryocytes and platelet release in the lung. Blood 26, 720–731 (1965).
Nakai, S., Aihara, K. & Hirai, Y. Interleukin-1 potentiates granulopoiesis and thrombopoiesis by producing hematopoietic factors in vivo. Life Sci. 45, 585–591 (1989).
Kimura, H. et al. Interleukin-1 β (IL-1 β) induces thrombocytosis in mice: possible implication of IL-6. Blood 76, 2493–2500 (1990).
Chuen, C. K. et al. Interleukin-1β up-regulates the expression of thrombopoietin and transcription factors c-Jun, c-Fos, GATA-1, and NF-E2 in megakaryocytic cells. J. Lab. Clin. Med. 143, 75–88 (2004).
Beaulieu, L. M. et al. Interleukin 1 receptor 1 and interleukin 1β regulate megakaryocyte maturation, platelet activation, and transcript profile during inflammation in mice and humans. Arterioscler. Thromb. Vasc. Biol. 34, 552–564 (2014).
Cognasse, F. et al. Platelets as key factors in inflammation: focus on CD40L/CD40. Front. Immunol. 13, 825892 (2022).
Kraemer, B. F. et al. Novel anti-bacterial activities of β-defensin 1 in human platelets: suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog. 7, e1002355 (2011).
Ambrosio, A. L. & Di Pietro, S. M. Storage pool diseases illuminate platelet dense granule biogenesis. Platelets 28, 138–146 (2017).
Morrell, C. N., Pariser, D. N., Hilt, Z. T. & Vega Ocasio, D. The platelet Napoleon complex — small cells, but big immune regulatory functions. Annu. Rev. Immunol. 37, 125–144 (2019).
Sreeramkumar, V. et al. Neutrophils scan for activated platelets to initiate inflammation. Science 346, 1234–1238 (2014).
Zuchtriegel, G. et al. Platelets guide leukocytes to their sites of extravasation. PLoS Biol. 14, e1002459 (2016).
Alard, J.-E. et al. Recruitment of classical monocytes can be inhibited by disturbing heteromers of neutrophil HNP1 and platelet CCL5. Sci. Transl Med. 7, 317ra196 (2015).
Badrnya, S. et al. Platelets mediate oxidized low-density lipoprotein-induced monocyte extravasation and foam cell formation. Arterioscler. Thromb. Vasc. Biol. 34, 571–580 (2014).
Elgueta, R. et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 229, 152–172 (2009).
Henn, V. et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391, 591–594 (1998).
Szczuko, M., Kozioł, I., Kotlęga, D., Brodowski, J. & Drozd, A. The role of thromboxane in the course and treatment of ischemic stroke: review. Int. J. Mol. Sci. 22, 11644 (2021).
Maouia, A., Rebetz, J., Kapur, R. & Semple, J. W. The immune nature of platelets revisited. Transf. Med. Rev. 34, 209–220 (2020).
Clark, S. R. et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 13, 463–469 (2007).
Assinger, A. et al. Efficient phagocytosis of periodontopathogens by neutrophils requires plasma factors, platelets and TLR2. J. Thromb. Haemost. 9, 799–809 (2011).
Andonegui, G. et al. Inhibition of human neutrophil apoptosis by platelets. J. Immunol. 158, 3372–3377 (1997).
Elzey, B. D. et al. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity 19, 9–19 (2003).
Carestia, A. et al. Platelets promote macrophage polarization toward pro-inflammatory phenotype and increase survival of septic mice. Cell Rep. 28, 896–908.e895 (2019).
Rolfes, V. et al. Platelets fuel the inflammasome activation of innate immune cells. Cell Rep. 31, 107615 (2020).
Hawwari, I. et al. Platelet-derived transcription factors license human monocyte inflammation. Preprint at bioRxiv https://doi.org/10.1101/2022.08.10.503291 (2023).
Totani, L. & Evangelista, V. Platelet–leukocyte interactions in cardiovascular disease and beyond. Arterioscler. Thromb. Vasc. Biol. 30, 2357–2361 (2010).
Kaiser, R., Escaig, R., Erber, J. & Nicolai, L. Neutrophil–platelet interactions as novel treatment targets in cardiovascular disease. Front. Cardiovasc. Med. 8, 824112 (2021).
Rolling, C. C., Barrett, T. J. & Berger, J. S. Platelet–monocyte aggregates: molecular mediators of thromboinflammation. Front. Cardiovasc. Med. 10, 960398 (2023).
Passacquale, G. et al. Monocyte–platelet interaction induces a pro-inflammatory phenotype in circulating monocytes. PLoS ONE 6, e25595 (2011).
Shi, P. et al. Platelet-specific p38α deficiency improved cardiac function after myocardial infarction in mice. Arterioscler. Thromb. Vasc. Biol. 37, e185–e196 (2017).
Abhilasha, K. V. et al. p38 MAP-kinase inhibitor protects against platelet-activating factor-induced death in mice. Free Radic. Biol. Med. 143, 275–287 (2019).
Margraf, A. & Zarbock, A. Platelets in inflammation and resolution. J. Immunol. 203, 2357–2367 (2019).
Scopelliti, F., Cattani, C., Dimartino, V., Mirisola, C. & Cavani, A. Platelet derivatives and the immunomodulation of wound healing. Int. J. Mol. Sci. 23, 8370 (2022).
Xiang, B. et al. Platelets protect from septic shock by inhibiting macrophage-dependent inflammation via the cyclooxygenase 1 signalling pathway. Nat. Commun. 4, 264 (2013).
Schroer, A. B. et al. Platelet factors attenuate inflammation and rescue cognition in ageing. Nature 620, 1071–1079 (2023).
Arora, K., Guleria, S., Jindal, A. K., Rawat, A. & Singh, S. Platelets in Kawasaki disease: is this only a numbers game or something beyond? Genes Dis. 7, 62–66 (2020).
Corrigan, J. J. Jr Kawasaki disease and the plight of the platelet. Am. J. Dis. Child. 140, 1223–1224, (1986).
Park, J. H. & Choi, H. J. Clinical implications of thrombocytosis in acute phase Kawasaki disease. Eur. J. Pediatr. 180, 1841–1846 (2021).
Burns, J. C., Glode, M. P., Clarke, S. H., Wiggins, J. Jr. & Hathaway, W. E. Coagulopathy and platelet activation in Kawasaki syndrome: identification of patients at high risk for development of coronary artery aneurysms. J. Pediatr. 105, 206–211 (1984).
Levin, M. et al. Platelet immune-complex interaction in pathogenesis of Kawasaki disease and childhood polyarteritis. Brit Med. J. 290, 1456–1460 (1985).
Tremoulet, A. H. et al. Evolution of laboratory values in patients with Kawasaki disease. Pediatr. Infect. Dis. J. 30, 1022–1026 (2011).
Hara, T. et al. Thrombocytopenia: a complication of Kawasaki disease. Eur. J. Pediatr. 147, 51–53 (1988).
Niwa, K. et al. Thrombocytopenia: a risk factor for acute myocardial infarction during the acute phase of Kawasaki disease. Coron. Artery Dis. 6, 857–864 (1995).
Ae, R. et al. Platelet count variation and risk for coronary artery abnormalities in Kawasaki disease. Pediatr. Infect. Dis. J. 39, 197–203 (2020).
Taki, M. et al. Spontaneous platelet aggregation in Kawasaki disease using the particle counting method. Pediatr. Int. 45, 649–652 (2003).
Yahata, T., Suzuki, C., Yoshioka, A., Hamaoka, A. & Ikeda, K. Platelet activation dynamics evaluated using platelet-derived microparticles in Kawasaki disease. Circ. J. 78, 188–193 (2014).
Yokoyama, T., Kato, H. & Ichinose, E. Aspirin treatment and platelet function in Kawasaki disease. Kurume Med. J. 27, 57–61 (1980).
Straface, E. et al. Oxidative stress and defective platelet apoptosis in naive patients with Kawasaki disease. Biochem. Biophys. Res. Commun. 392, 426–430 (2010).
Zheng, X., Wu, W., Zhang, Y. & Wu, G. Changes in and significance of platelet function and parameters in Kawasaki disease. Sci. Rep. 9, 17641 (2019).
Liu, R., Gao, F., Huo, J. & Yi, Q. Study on the relationship between mean platelet volume and platelet distribution width with coronary artery lesion in children with Kawasaki disease. Platelets 23, 11–16 (2012).
Nofech-Mozes, Y. & Garty, B. Z. Thrombocytopenia in Kawasaki disease: a risk factor for the development of coronary artery aneurysms. Pediatr. Hematol. Oncol. 20, 597–601 (2003).
Kobayashi, T. et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation 113, 2606–2612 (2006).
Egami, K. et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J. Pediatr. 149, 237–240 (2006).
Sano, T. et al. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur. J. Pediatr. 166, 131–137 (2007).
Rigante, D. et al. Critical overview of the risk scoring systems to predict non-responsiveness to intravenous immunoglobulin in Kawasaki syndrome. Int. J. Mol. Sci. 17, 278 (2016).
Kanegaye, J. T. et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics 123, e783–789, (2009).
Takahashi, M. in Kawasaki Disease: Current Understanding of the Mechanism and Evidence-Based Treatment (eds Saji, B. T. et al.) 355–372 (Springer, 2017).
Hidaka, T., Nakano, M., Ueta, T., Komatsu, Y. & Yamamoto, M. Increased synthesis of thromboxane A2 by platelets from patients with Kawasaki disease. J. Pediatr. 102, 94–96 (1983).
Yamada, K., Fukumoto, T., Shinkai, A., Shirahata, A. & Meguro, T. The platelet functions in acute febrile mucocutaneous lymph node syndrome and a trial of prevention for thrombosis by antiplatelet agent. Nihon Ketsueki Gakkai Zasshi 41, 791–802 (1978).
Stark, K. & Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 18, 666–682 (2021).
Laurito, M. et al. Endothelial and platelet function in children with previous Kawasaki disease. Angiology 65, 716–722 (2014).
Shah, V. et al. Cardiovascular status after Kawasaki disease in the UK. Heart 101, 1646–1655 (2015).
Qiu, Y., Zhang, Y., Li, Y., Hua, Y. & Zhang, Y. Molecular mechanisms of endothelial dysfunction in Kawasaki-disease-associated vasculitis. Front. Cardiovasc. Med. 9, 981010 (2022).
Dhillon, R. et al. Endothelial dysfunction late after Kawasaki disease. Circulation 94, 2103–2106 (1996).
Burns, J. C. et al. Conjunctival biopsy in patients with Kawasaki disease. Pediatr. Pathol. Lab. Med. 15, 547–553 (1995).
Irazuzta, J. E., Elbl, F. & Rees, A. R. Factor VIII related antigen (von Willebrand’s factor) in Kawasaki disease. Clin. Pediatr. 29, 347–348 (1990).
Luo, L. et al. Serum levels of syndecan-1 in patients with Kawasaki disease. Pediatr. Infect. Dis. J. 38, 89–94 (2019).
Takeshita, S. et al. Elevated serum levels of matrix metalloproteinase-9 (MMP-9) in Kawasaki disease. Clin. Exp. Immunol. 125, 340–344 (2001).
Tian, F. et al. Correlation between matrix metalloproteinases with coronary artery lesion caused by kawasaki disease. Front. Pediatr. 10, 802217 (2022).
Verheul, H. M. et al. Platelet: transporter of vascular endothelial growth factor. Clin. Cancer Res. 3, 2187–2190 (1997).
Geindreau, M., Ghiringhelli, F. & Bruchard, M. Vascular endothelial growth factor, a key modulator of the anti-tumor immune response. Int. J. Mol. Sci. 22, 4871 (2021).
Ueno, K. et al. Platelet vascular endothelial growth factor is a useful predictor for prognosis in Kawasaki syndrome. Br. J. Haematol. 148, 285–292 (2010).
Maeno, N. et al. Increased serum levels of vascular endothelial growth factor in Kawasaki disease. Pediatr. Res. 44, 596–599 (1998).
Ohno, T. et al. Serum vascular endothelial growth factor: a new predictive indicator for the occurrence of coronary artery lesions in Kawasaki disease. Eur. J. Pediatr. 159, 424–429 (2000).
Terai, M. et al. Vascular endothelial growth factor in acute Kawasaki disease. Am. J. Cardiol. 83, 337–339 (1999).
Eicher, J. D. et al. Characterization of the platelet transcriptome by RNA sequencing in patients with acute myocardial infarction. Platelets 27, 230–239 (2016).
Rowley, J. W. et al. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood 118, e101–e111 (2011).
Kocatürk, B. et al. Platelets exacerbate cardiovascular inflammation in a murine model of Kawasaki disease vasculitis. JCI Insight 8, e169855 (2023).
Zhang, Y. et al. Reduced platelet miR-223 induction in kawasaki disease leads to severe coronary artery pathology through a miR-223/PDGFRβ vascular smooth muscle cell axis. Circ. Res. 127, 855–873 (2020).
Wang, C. L. et al. Expression of CD40 ligand on CD4+ T-cells and platelets correlated to the coronary artery lesion and disease progress in Kawasaki disease. Pediatrics 111, E140–E147 (2003).
Pi, L. et al. Immature platelets and antiplatelet therapy response to aspirin in Kawasaki disease. Drug Des. Devel. Ther. 12, 1353–1362 (2018).
Fu, L. et al. Thymic stromal lymphopoietin induces platelet mitophagy and promotes thrombosis in Kawasaki disease. Br. J. Haematol. 200, 776–791 (2023).
Inamo, Y. Studies on plasma thromboxane B2 levels in patients with Kawasaki disease; as an indicator of coronary aneurysm formation. Pediatr. Int. 25, 230–236 (1983).
Furui, J. Soluble forms of P-, E- and L-selectin in children with Kawasaki disease. Kurume Med. J. 48, 135–143 (2001).
Furui, J. et al. Soluble forms of the selectin family in children with Kawasaki disease: prediction for coronary artery lesions. Acta Paediatr. 91, 1183–1188 (2002).
Yi, L., Zhang, J., Zhong, J. & Zheng, Y. Elevated levels of platelet activating factor and its acetylhydrolase indicate high risk of Kawasaki disease. J. Interferon Cytokine Res. 40, 159–167 (2020).
Wilson, H. M. SOCS proteins in macrophage polarization and function. Front. Immunol. 5, 357 (2014).
Barrett, T. J. et al. Platelet regulation of myeloid suppressor of cytokine signaling 3 accelerates atherosclerosis. Sci. Transl Med. 11, eaax0481 (2019).
Porritt, R. A. et al. Interleukin-1β-mediated sex differences in Kawasaki disease vasculitis development and response to treatment. Arterioscler. Thromb. Vasc. Biol. 40, 802–818 (2020).
Shimizu, C. et al. Differential expression of miR-145 in children with Kawasaki disease. PLoS ONE 8, e58159 (2013).
Rowley, A. H. et al. A study of cardiovascular miRNA biomarkers for Kawasaki disease. Pediatr. Infect. Dis. J. 33, 1296–1299 (2014).
Kuo, H.-C. et al. Next-generation sequencing identifies micro-RNA-based biomarker panel for Kawasaki disease. J. Allergy Clin. Immunol. 138, 1227–1230 (2016).
Ebina-Shibuya, R. & Leonard, W. J. Role of thymic stromal lymphopoietin in allergy and beyond. Nat. Rev. Immunol. 23, 24–37 (2023).
Truchetet, M. E. et al. Platelets induce thymic stromal lymphopoietin production by endothelial cells: contribution to fibrosis in human systemic sclerosis. Arthritis Rheumatol. 68, 2784–2794 (2016).
Pietraforte, D. et al. Platelets in Kawasaki patients: two different populations with different mitochondrial functions. Int. J. Cardiol. 172, 526–528 (2014).
Ueno, K. et al. Circulating platelet–neutrophil aggregates play a significant role in Kawasaki disease. Circ. J. 79, 1349–1356 (2015).
Vignesh, P. et al. Monocyte platelet aggregates in children with Kawasaki disease- a preliminary study from a tertiary care centre in North-West India. Pediatr. Rheumatol. Online J. 19, 25 (2021).
Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 18, 134–147 (2018).
Finsterbusch, M., Schrottmaier, W. C., Kral-Pointner, J. B., Salzmann, M. & Assinger, A. Measuring and interpreting platelet–leukocyte aggregates. Platelets 29, 677–685 (2018).
Kral, J. B., Schrottmaier, W. C., Salzmann, M. & Assinger, A. Platelet interaction with innate immune cells. Transfus. Med. Hemother. 43, 78–88 (2016).
Allen, N. et al. Circulating monocyte–platelet aggregates are a robust marker of platelet activity in cardiovascular disease. Atherosclerosis 282, 11–18 (2019).
Klinkhardt, U. et al. Clopidogrel but not aspirin reduces P-selectin expression and formation of platelet–leukocyte aggregates in patients with atherosclerotic vascular disease. Clin. Pharmacol. Ther. 73, 232–241 (2003).
Dorsam, R. T. & Kunapuli, S. P. Central role of the P2Y12 receptor in platelet activation. J. Clin. Invest. 113, 340–345 (2004).
Rolling, C. C. et al. P2Y12 inhibition suppresses proinflammatory platelet–monocyte interactions. Thromb. Haemost. 123, 231–244 (2023).
Lehman, T. J., Walker, S. M., Mahnovski, V. & McCurdy, D. Coronary arteritis in mice following the systemic injection of group B Lactobacillus casei cell walls in aqueous suspension. Arthritis Rheum. 28, 652–659 (1985).
Murata, H. Experimental candida-induced arteritis in mice. Relation to arteritis in the mucocutaneous lymph node syndrome. Microbiol. Immunol. 23, 825–831 (1979).
Nishio, H. et al. Nod1 ligands induce site-specific vascular inflammation. Arterioscler. Thromb. Vasc. Biol. 31, 1093–1099 (2011).
Lee, Y. et al. Interleukin-1β is crucial for the induction of coronary artery inflammation in a mouse model of Kawasaki disease. Circulation 125, 1542–1550 (2012).
Porritt, R. A. et al. NLRP3 inflammasome mediates immune–stromal interactions in vasculitis. Circ. Res. 129, e183–e200 (2021).
Lin, I. C. et al. Vascular endothelial growth factor-a in Lactobacillus casei cell wall extract-induced coronary arteritis of a murine model. Circ. J. 78, 752–762 (2014).
Anzai, F. et al. Crucial role of NLRP3 inflammasome in a murine model of Kawasaki disease. J. Mol. Cell Cardiol. 138, 185–196 (2019).
Lee, Y. et al. IL-1 signaling is critically required in stromal cells in Kawasaki disease vasculitis mouse model: role of both IL-1α and IL-1β. Arterioscler. Thromb. Vasc. Biol. 35, 2605–2616 (2015).
Wakita, D. et al. Role of interleukin-1 signaling in a mouse model of Kawasaki disease-associated abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 36, 886–897 (2016).
Marek-Iannucci, S. et al. Autophagy–mitophagy induction attenuates cardiovascular inflammation in a murine model of Kawasaki disease vasculitis. JCI Insight 6, e151981 (2021).
Lee, S. H. et al. Inducing mitophagy in diabetic platelets protects against severe oxidative stress. EMBO Mol. Med. 8, 779–795 (2016).
Kimura, M. Y., Koyama-Nasu, R., Yagi, R. & Nakayama, T. A new therapeutic target: the CD69–Myl9 system in immune responses. Semin. Immunopathol. 41, 349–358 (2019).
Kobayashi, H. et al. Increased myosin light chain 9 expression during Kawasaki disease vasculitis. Front. Immunol. 13, 1036672 (2022).
Burns, J. C. et al. Review: found in translation: international initiatives pursuing interleukin-1 blockade for treatment of acute Kawasaki disease. Arthritis Rheumatol. 69, 268–276 (2017).
Dusser, P. & Kone-Paut, I. IL-1 inhibition may have an important role in treating refractory Kawasaki disease. Front. Pharmacol. 8, 163 (2017).
Kone-Paut, I. et al. The use of interleukin 1 receptor antagonist (anakinra) in Kawasaki disease: a retrospective cases series. Autoimmun. Rev. 17, 768–774 (2018).
Newburger, J. W. et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 110, 2747–2771 (2004).
Baumer, J. H. et al. Salicylate for the treatment of Kawasaki disease in children. Cochrane Database Syst. Rev. 2006, Cd004175 (2006).
Terai, M. & Shulman, S. T. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J. Pediatr. 131, 888–893 (1997).
Kuo, H. C., Lo, M. H., Hsieh, K. S., Guo, M. M. & Huang, Y. H. High-dose aspirin is associated with anemia and does not confer benefit to disease outcomes in Kawasaki disease. PLoS ONE 10, e0144603 (2015).
Amarilyo, G. et al. High-dose aspirin for Kawasaki disease: outdated myth or effective aid? Clin. Exp. Rheumatol. 35, 209–212 (2017).
Lee, G., Lee, S. E., Hong, Y. M. & Sohn, S. Is high-dose aspirin necessary in the acute phase of Kawasaki disease? Korean Circ. J. 43, 182–186, (2013).
Dallaire, F. et al. Aspirin dose and prevention of coronary abnormalities in Kawasaki disease. Pediatrics 139, 249 (2017).
Burns, J. C. & Franco, A. The immunomodulatory effects of intravenous immunoglobulin therapy in Kawasaki disease. Expert Rev. Clin. Immunol. 11, 819–825 (2015).
Nadig, P. L. et al. Intravenous immunoglobulin in Kawasaki disease — evolution and pathogenic mechanisms. Diagnostics 13, 2338 (2023).
Zhu, Y. P. et al. Immune response to intravenous immunoglobulin in patients with Kawasaki disease and MIS-C. J. Clin. Invest 131, e147046 (2021).
Inagaki, M. & Yamada, K. Inhibitory effects of high doses of intravenous gamma-globulin on platelet interaction with the vessel wall in Kawasaki disease. Acta Paediatr. Jpn 33, 791–798 (1991).
Thomas, M. R. et al. Platelet P2Y12 inhibitors reduce systemic inflammation and its prothrombotic effects in an experimental human model. Arterioscler. Thromb. Vasc. Biol. 35, 2562–2570 (2015).
Ataga, K. I. et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N. Engl. J. Med. 376, 429–439 (2016).
Low, T. et al. Bleeding risk associated with combination thromboprophylaxis therapy is low for patients with coronary artery aneurysms after Kawasaki disease. Int. J. Cardiol. 321, 6–11 (2020).
Gupta, M. et al. Serum interleukin-6 (IL-6) levels in children with Kawasaki’s disease (KD) [abstract 115]. Pediatr. Res. 43, 22–22 (1998).
Porritt, R. A. et al. Inhibition of IL-6 in the LCWE mouse model of Kawasaki disease inhibits acute phase reactant serum amyloid A but fails to attenuate vasculitis. Front. Immunol. 12, 630196 (2021).
Ishida-Okawara, A. et al. Neutrophil activation and arteritis induced by C. albicans water-soluble mannoprotein-β-glucan complex (CAWS). Exp. Mol. Pathol. 82, 220–226 (2007).
Ohashi, R. et al. Characterization of a murine model with arteritis induced by Nod1 ligand, FK565: a comparative study with a CAWS-induced model. Mod. Rheumatol. 27, 1024–1030 (2017).
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Jane Burns, Tessa Barrett and Ho-Chang Kuo for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Noval Rivas, M., Kocatürk, B., Franklin, B.S. et al. Platelets in Kawasaki disease: mediators of vascular inflammation. Nat Rev Rheumatol 20, 459–472 (2024). https://doi.org/10.1038/s41584-024-01119-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-024-01119-3
- Springer Nature Limited