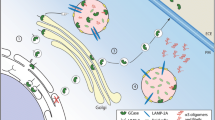
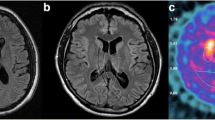
Abstract
An exciting development in the field of neurodegeneration is the association between the rare monogenic disorder Gaucher disease and the common complex disorder Parkinson disease (PD). Gaucher disease is a lysosomal storage disorder resulting from an inherited deficiency of the enzyme glucocerebrosidase, encoded by GBA1, which hydrolyses the glycosphingolipids glucosylceramide and glucosylsphingosine. The observation of parkinsonism in a rare subgroup of individuals with Gaucher disease first directed attention to the role of glucocerebrosidase deficiency in the pathogenesis of PD. PD occurs more frequently in people heterozygous for Gaucher GBA1 mutations, and 3–25% of people with Parkinson disease carry a GBA1 variant. However, only a small percentage of individuals with GBA1 variants develop parkinsonism, suggesting that the penetrance is low. Despite over a decade of intense research in this field, including clinical and radiological evaluations, genetic studies and investigations using model systems, the mechanism underlying GBA1-PD is still being pursued. Insights from this association have emphasized the role of lysosomal pathways in parkinsonism. Furthermore, different therapeutic strategies considered or developed for Gaucher disease can now inform drug development for PD.
Key points
-
The rare, autosomal recessively inherited disorder Gaucher disease is providing new insights into the pathogenesis of Parkinson disease.
-
Variants in GBA1, the gene encoding the lysosomal enzyme glucocerebrosidase, are the most common known genetic risk factor for Parkinson disease and dementia with Lewy bodies.
-
Most individuals with homozygous or heterozygous GBA1 variants do not develop parkinsonism. Identifying the factors that impact penetrance will be crucial to our understanding of disease mechanisms.
-
Therapeutic strategies under development for Gaucher disease, such as brain-penetrant enzyme replacement strategies, gene therapy approaches, and small molecule chaperones and activators, might inform new treatment approaches for Parkinson disease.
-
Improved clinical biomarkers are needed to identify GBA1 variant carriers early in their disease course to enable preventative therapies.



Similar content being viewed by others
References
Nalls, M. A. et al. A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol. 70, 727–735 (2013).
Sidransky, E. et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 361, 1651–1661 (2009).
Poewe, W. et al. Parkinson disease. Nat. Rev. Dis. Prim. 3, 17013 (2017).
Nalls, M. A. et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 18, 1091–1102 (2019).
Tayebi, N. et al. Gaucher disease with parkinsonian manifestations: does glucocerebrosidase deficiency contribute to a vulnerability to parkinsonism? Mol. Genet. Metab. 79, 104–109 (2003).
Neudorfer, O. et al. Occurrence of Parkinson’s syndrome in type I Gaucher disease. QJM 89, 691–694 (1996).
Goker-Alpan, O. et al. Parkinsonism among Gaucher disease carriers. J. Med. Genet. 41, 937–940 (2004).
Ran, C. et al. Strong association between glucocerebrosidase mutations and Parkinson’s disease in Sweden. Neurobiol. Aging 45, 212.e5–e11 (2016).
Emelyanov, A. K. et al. Mutation analysis of Parkinson’s disease genes in a Russian data set. Neurobiol. Aging 71, 267.e7– e10 (2018).
De Marco, E. V. et al. Glucocerebrosidase gene mutations are associated with Parkinson’s disease in southern Italy. Mov. Disord. 23, 460–463 (2008).
Yu, Z. et al. Mutations in the glucocerebrosidase gene are responsible for Chinese patients with Parkinson’s disease. J. Hum. Genet. 60, 85–90 (2015).
Lesage, S. et al. Large-scale screening of the Gaucher’s disease-related glucocerebrosidase gene in Europeans with Parkinson’s disease. Hum. Mol. Genet. 20, 202–210 (2011).
Mata, I. F. et al. Glucocerebrosidase gene mutations: a risk factor for Lewy body disorders. Arch. Neurol. 65, 379–382 (2008).
Robak, L. A. et al. Excessive burden of lysosomal storage disorder gene variants in Parkinson’s disease. Brain 140, 3191–3203 (2017).
Gaucher, P. C. E. De l’Epithelioma Primitif de la Rate: Hypertrophie Idiopathique de la Rate sans Leucémie [French]. Thesis, Faculté de Medécine de Paris (1882).
Aghion, H. La Maladie de Gaucher dans l’Enfance (Forme Cardio-Rénale) [French]. Thesis, Faculté de Médecine de Paris (1934).
Brady, R. O., Kanfer, J. N. & Shapiro, D. Metabolism of glucocerebrosides II. Evidence of an enzymatic deficiency in Gaucher’s disease. Biochem. Biophys. Res. Commun. 18, 221–225 (1965).
Patrick, A. A deficiency of glucocerebrosidase in Gaucher’s disease. Biochem. J. 97, 17C–24C (1965).
Barton, N. W. et al. Replacement therapy for inherited enzyme deficiency – macrophage-targeted glucocerebrosidase for Gaucher’s disease. N. Engl. J. Med. 324, 1464–1470 (1991).
Sidransky, E. Gaucher disease: complexity in a “simple” disorder. Mol. Genet. Metab. 83, 6–15 (2004).
Schiffmann, R. et al. The definition of neuronopathic Gaucher disease. J. Inherit. Metab. Dis. 43, 1056–1059 (2020).
Tylki-Szymanska, A., Keddache, M. & Grabowski, G. A. Characterization of neuronopathic Gaucher disease among ethnic Poles. Genet. Med. 8, 8–15 (2006).
Davidson, B. A., Hassan, S., Garcia, E. J., Tayebi, N. & Sidransky, E. Exploring genetic modifiers of Gaucher disease: the next horizon. Hum. Mutat. 39, 1739–1751 (2018).
Lachmann, R. H., Grant, I. R., Halsall, D. & Cox, T. M. Twin pairs showing discordance of phenotype in adult Gaucher’s disease. QJM 97, 199–204 (2004).
Lopez, G. et al. Clinical evaluation of sibling pairs with Gaucher disease discordant for parkinsonism. Mov. Disord. 35, 359–365 (2020).
Miao, S. et al. Identification of Glu340 as the active-site nucleophile in human glucocerebrosidase by use of electrospray tandem mass spectrometry. J. Biol. Chem. 269, 10975–10978 (1994).
Fabrega, S. et al. Human glucocerebrosidase: heterologous expression of active site mutants in murine null cells. Glycobiology 10, 1217–1224 (2000).
Dvir, H. et al. X-ray structure of human acid-β-glucosidase, the defective enzyme in Gaucher disease. EMBO Rep. 4, 704–709 (2003).
Takasaki, S. et al. Structure of the N-asparagine-linked oligosaccharide units of human placental β-glucocerebrosidase. J. Biol. Chem. 259, 10112–10117 (1984).
Hruska, K. S., LaMarca, M. E., Scott, C. R. & Sidransky, E. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA). Hum. Mutat. 29, 567–583 (2008).
Reczek, D. et al. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of β-glucocerebrosidase. Cell 131, 770–783 (2007).
Grabowski, G. A., Gaft, S., Horowitz, M. & Kolodny, E. H. Acid β-glucosidase: enzymology and molecular biology of Gaucher diseas. Crit. Rev. Biochem. Mol. Biol. 25, 385–414 (1990).
Beutler, E., Beutler, L. & West, C. Mutations in the gene encoding cytosolic β-glucosidase in Gaucher disease. J. Lab. Clin. Med. 144, 65–68 (2004).
Matern, H., Boermans, H., Lottspeich, F. & Matern, S. Molecular cloning and expression of human bile acid β-glucosidase. J. Biol. Chem. 276, 37929–37933 (2001).
Barneveld, R. A. et al. Assignment of the gene coding for human β-glucocerebrosidase to the region q21-q31 of chromosome 1 using monoclonal antibodies. Hum. Genet. 64, 227–231 (1983).
Reiner, O., Wigderson, M. & Horowitz, M. Structural analysis of the human glucocerebrosidase genes. DNA 7, 107–116 (1988).
Horowitz, M. et al. The human glucocerebrosidase gene and pseudogene: structure and evolution. Genomics 4, 87–96 (1989).
Tayebi, N. et al. Reciprocal and nonreciprocal recombination at the glucocerebrosidase gene region: implications for complexity in Gaucher disease. Am. J. Hum. Genet. 72, 519–534 (2003).
Gustavsson, E. K. et al. The annotation of GBA1 has been concealed by its protein-coding pseudogene GBAP1. Sci. Adv. 10, eadk1296 (2024).
Tsuji, S. et al. A mutation in the human glucocerebrosidase gene in neuronopathic Gaucher’s disease. N. Engl. J. Med. 316, 570–575 (1987).
Tayebi, N. et al. Genotypic heterogeneity and phenotypic variation among patients with type 2 Gaucher’s disease. Pediatr. Res. 43, 571–578 (1998).
Shemesh, E. et al. Enzyme replacement and substrate reduction therapy for Gaucher disease. Cochrane Database Syst. Rev. 2015, CD010324 (2015).
Woo, E. G., Tayebi, N. & Sidransky, E. Next-generation sequencing analysis of GBA1: the challenge of detecting complex recombinant alleles. Front. Genet. 12, 684067 (2021).
Toffoli, M. et al. Comprehensive short and long read sequencing analysis for the Gaucher and Parkinson’s disease-associated GBA gene. Commun. Biol. 5, 670 (2022).
Zampieri, S., Cattarossi, S., Bembi, B. & Dardis, A. GBA analysis in next-generation era: pitfalls, challenges, and possible solutions. J. Mol. Diagn. 19, 733–741 (2017).
Biegstraaten, M. et al. A monozygotic twin pair with highly discordant Gaucher phenotypes. Blood Cell Mol. Dis. 46, 39–41 (2011).
Kurolap, A. et al. Gaucher disease type 3c: new patients with unique presentations and review of the literature. Mol. Genet. Metab. 127, 138–146 (2019).
Mallett, V. et al. GBA p.T369M substitution in Parkinson disease: polymorphism or association? A meta-analysis. Neurol. Genet. 2, e104 (2016).
Park, J. K. et al. The E326K mutation and Gaucher disease: mutation or polymorphism? Clin. Genet. 61, 32–34 (2002).
Duran, R. et al. The glucocerobrosidase E326K variant predisposes to Parkinson’s disease, but does not cause Gaucher’s disease. Mov. Disord. 28, 232–236 (2013).
Charrow, J. & Scott, C. R. Long-term treatment outcomes in Gaucher disease. Am. J. Hematol. 90, S19–S24 (2015).
El-Beshlawy, A. et al. Long-term hematological, visceral, and growth outcomes in children with Gaucher disease type 3 treated with imiglucerase in the International Collaborative Gaucher Group Gaucher Registry. Mol. Genet. Metab. 120, 47–56 (2017).
Cox, T. M. et al. Eliglustat compared with imiglucerase in patients with Gaucher’s disease type 1 stabilised on enzyme replacement therapy: a phase 3, randomised, open-label, non-inferiority trial. Lancet 385, 2355–2362 (2015).
Cox, T. et al. Novel oral treatment of Gaucher’s disease with N-butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. Lancet 355, 1481–1485 (2000).
Mistry, P. K. et al. Long-term effectiveness of eliglustat treatment: a real-world analysis from the International Collaborative Gaucher Group Gaucher Registry. Am. J. Hematol. 99, 1500–1510 (2024).
Schiffmann, R. et al. Venglustat combined with imiglucerase for neurological disease in adults with Gaucher disease type 3: the LEAP trial. Brain 146, 461–474 (2023).
Luan, Z. et al. Chaperone activity of bicyclic nojirimycin analogues for Gaucher mutations in comparison with N-(n-nonyl)deoxynojirimycin. Chembiochem 10, 2780–2792 (2009).
Han, T. U., Sam, R. & Sidransky, E. Small molecule chaperones for the treatment of Gaucher disease and GBA1-associated Parkinson disease. Front. Cell Dev. Biol. 8, 271 (2020).
Trapero, A., González-Bulnes, P., Butters, T. D. & Llebaria, A. Potent aminocyclitol glucocerebrosidase inhibitors are subnanomolar pharmacological chaperones for treating Gaucher disease. J. Med. Chem. 55, 4479–4488 (2012).
Maegawa, G. H. et al. Identification and characterization of ambroxol as an enzyme enhancement agent for Gaucher disease. J. Biol. Chem. 284, 23502–23516 (2009).
Narita, A. et al. Ambroxol chaperone therapy for neuronopathic Gaucher disease: a pilot study. Ann. Clin. Transl. Neurol. 3, 200–215 (2016).
Istaiti, M. et al. High-dose ambroxol therapy in type 1 Gaucher disease focusing on patients with poor response to enzyme replacement therapy or substrate reduction therapy. Int. J. Mol. Sci. 24, 6732 (2023).
Blauwendraat, C., Nalls, M. A. & Singleton, A. B. The genetic architecture of Parkinson’s disease. Lancet Neurol. 19, 170–178 (2020).
Cilia, R. et al. Survival and dementia in GBA-associated Parkinson’s disease: The mutation matters. Ann. Neurol. 80, 662–673 (2016).
Gan-Or, Z. et al. Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology 70, 2277–2283 (2008).
Pankratz, N. et al. Meta-analysis of Parkinson’s disease: identification of a novel locus, RIT2. Ann. Neurol. 71, 370–384 (2012).
Rizig, M. et al. Identification of genetic risk loci and causal insights associated with Parkinson’s disease in African and African admixed populations: a genome-wide association study. Lancet Neurol. 22, 1015–1025 (2023).
Mitsui, J. et al. Variants associated with Gaucher disease in multiple system atrophy. Ann. Clin. Transl. Neurol. 2, 417–426 (2015).
Sklerov, M. et al. Frequency of GBA variants in autopsy-proven multiple system atrophy. Mov. Disord. Clin. Pract. 4, 574–581 (2017).
Wernick, A. I. et al. GBA variation and susceptibility to multiple system atrophy. Parkinsonism Relat. Disord. 77, 64–69 (2020).
Alcalay, R. N. et al. Comparison of Parkinson risk in Ashkenazi Jewish patients with Gaucher disease and GBA heterozygotes. JAMA Neurol. 71, 752–757 (2014).
Bultron, G. et al. The risk of Parkinson’s disease in type 1 Gaucher disease. J. Inherit. Metab. Dis. 33, 167–173 (2010).
Rosenbloom, B. et al. The incidence of Parkinsonism in patients with type 1 Gaucher disease: data from the ICGG Gaucher Registry. Blood Cell Mol. Dis. 46, 95–102 (2011).
Ali, A., Holman, A. P., Rodriguez, A., Osborne, L. & Kurouski, D. Elucidating the mechanisms of α-synuclein-lipid interactions using site-directed mutagenesis. Neurobiol. Dis. 198, 106553 (2024).
Zunke, F. et al. Reversible conformational conversion of α-synuclein into toxic assemblies by glucosylceramide. Neuron 97, 92–107.e10 (2018).
Ron, I., Rapaport, D. & Horowitz, M. Interaction between parkin and mutant glucocerebrosidase variants: a possible link between Parkinson disease and Gaucher disease. Hum. Mol. Genet. 19, 3771–3781 (2010).
Goker-Alpan, O. et al. Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology 67, 908–910 (2006).
Nichols, W. C. et al. Mutations in GBA are associated with familial Parkinson disease susceptibility and age at onset. Neurology 72, 310–316 (2009).
Ren, J. et al. Comparing the effects of GBA variants and onset age on clinical features and progression in Parkinson’s disease. CNS Neurosci. Ther. 30, e14387 (2024).
Malek, N. et al. Features of GBA-associated Parkinson’s disease at presentation in the UK Tracking Parkinson’s study. J. Neurol. Neurosurg. Psychiatry 89, 702–709 (2018).
Jesus, S. et al. GBA variants influence motor and non-motor features of Parkinson’s disease. PLoS ONE 11, e0167749 (2016).
Toffoli, M. et al. Phenotypic effect of GBA1 variants in individuals with and without Parkinson’s disease: The RAPSODI study. Neurobiol. Dis. 188, 106343 (2023).
Ren, J. et al. Association of GBA genotype with motor and cognitive decline in Chinese Parkinson’s disease patients. Front. Aging Neurosci. 15, 1091919 (2023).
Brockmann, K. et al. GBA-associated PD presents with nonmotor characteristics. Neurology 77, 276–280 (2011).
Straniero, L. et al. The SPID-GBA study: sex distribution, penetrance, incidence, and dementia in GBA-PD. Neurol. Genet. 6, e523 (2020).
Ortega, R. A. et al. Differences in sex-specific frequency of glucocerebrosidase variant carriers and familial Parkinsonism. Mov. Disord. 37, 2217–2225 (2022).
Thaler, A., Mirelman, A. & Alcalay, R. N. Differences in sex-specific frequency of glucocerebrosidase variant carriers and familial Parkinsonism. Mov. Disord. 38, 713–714 (2023).
Li, Q., Jing, Y., Lun, P., Liu, X. & Sun, P. Association of gender and age at onset with glucocerebrosidase associated Parkinson’s disease: a systematic review and meta-analysis. Neurol. Sci. 42, 2261–2271 (2021).
Panteghini, C. et al. Sex distribution and classification of GBA1 variants in an Italian cohort of Parkinson’s disease patients analyzed over the last seventeen years. Parkinsonism Relat. Disord. 117, 105919 (2023).
Zimmermann, M. et al. Patient’s perception: shorter and more severe prodromal phase in GBA-associated PD. Eur. J. Neurol. 26, 694–698 (2019).
Lopez, G. et al. Clinical course and prognosis in patients with Gaucher disease and parkinsonism. Neurol. Genet. 2, e57 (2016).
Simuni, T. et al. Clinical and dopamine transporter imaging characteristics of leucine rich repeat kinase 2 (LRRK2) and glucosylceramidase beta (GBA) Parkinson’s disease participants in the Parkinson’s Progression Markers Initiative: a cross-sectional study. Mov. Disord. 35, 833–844 (2020).
Chia, R. et al. Genome sequencing analysis identifies new loci associated with Lewy body dementia and provides insights into its genetic architecture. Nat. Genet. 53, 294–303 (2021).
Gaubert, S. et al. Exploring the link between GBA1 mutations and Dementia with Lewy bodies, a mini-review. Neurosci. Biobehav. Rev. 141, 104856 (2022).
Wong, K. et al. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol. Genet. Metab. 82, 192–207 (2004).
Balestrino, R. et al. Penetrance of glucocerebrosidase (GBA) mutations in Parkinson’s disease: a kin cohort study. Mov. Disord. 35, 2111–2114 (2020).
Beavan, M. et al. Evolution of prodromal clinical markers of Parkinson disease in a GBA mutation-positive cohort. JAMA Neurol. 72, 201–208 (2015).
Mullin, S. et al. Evolution and clustering of prodromal parkinsonian features in GBA1 carriers. Mov. Disord. 34, 1365–1373 (2019).
Becker-Cohen, M. et al. A comprehensive assessment of qualitative and quantitative prodromal parkinsonian features in carriers of Gaucher disease-identifying those at the greatest risk. Int. J. Mol. Sci. 23, 12211 (2022).
Avenali, M. et al. Evolution of prodromal parkinsonian features in a cohort of GBA mutation-positive individuals: a 6-year longitudinal study. J. Neurol. Neurosurg. Psychiatry 90, 1091–1097 (2019).
Lopez, G. J. et al. Longitudinal evaluation of olfactory function in individuals with Gaucher disease and GBA1 mutation carriers with and without Parkinson’s disease. Front. Neurol. 13, 1039214 (2022).
Filippi, M., Balestrino, R., Basaia, S. & Agosta, F. Neuroimaging in glucocerebrosidase-associated parkinsonism: a systematic review. Mov. Disord. 37, 1375–1393 (2022).
Goker-Alpan, O. et al. The neurobiology of glucocerebrosidase-associated parkinsonism: a positron emission tomography study of dopamine synthesis and regional cerebral blood flow. Brain 135, 2440–2448 (2012).
Slingerland, S. et al. Cholinergic innervation topography in GBA-associated de novo Parkinson’s disease patients. Brain 147, 900–910 (2024).
Greuel, A. et al. GBA variants in Parkinson’s disease: clinical, metabolomic, and multimodal neuroimaging phenotypes. Mov. Disord. 35, 2201–2210 (2020).
Lopez, G. et al. Longitudinal positron emission tomography of dopamine synthesis in subjects with GBA1 mutations. Ann. Neurol. 87, 652–657 (2020).
Marek, K. et al. The Parkinson’s Progression Markers Initiative (PPMI) – establishing a PD biomarker cohort. Ann. Clin. Transl. Neurol. 5, 1460–1477 (2018).
Simuni, T. et al. Clinical and dopamine transporter imaging characteristics of non-manifest LRRK2 and GBA mutation carriers in the Parkinson’s Progression Markers Initiative (PPMI): a cross-sectional study. Lancet Neurol. 19, 71–80 (2020).
Mullin, S. et al. Brain microglial activation increased in glucocerebrosidase (GBA) mutation carriers without Parkinson’s disease. Mov. Disord. 36, 774–779 (2021).
Berg, D., Godau, J. & Walter, U. Transcranial sonography in movement disorders. Lancet Neurol. 7, 1044–1055 (2008).
Li, D. H., He, Y. C., Liu, J. & Chen, S. D. Diagnostic accuracy of transcranial sonography of the substantia nigra in Parkinson’s disease: a systematic review and meta-analysis. Sci. Rep. 6, 20863 (2016).
Kresojevic, N. et al. Transcranial sonography in patients with Parkinson’s disease with glucocerebrosidase mutations. Parkinsonism Relat. Disord. 19, 431–435 (2013).
Saunders-Pullman, R. et al. Gaucher disease ascertained through a Parkinson’s center: imaging and clinical characterization. Mov. Disord. 25, 1364–1372 (2010).
Eisenberg, D. P., Lopez, G., Gregory, M. D., Berman, K. F. & Sidransky, E. Comparison of transcranial sonography and [18F]-fluorodopa PET imaging in GBA1 mutation carriers. Mov. Disord. 37, 629–634 (2022).
Murugesan, V. et al. Glucosylsphingosine is a key biomarker of Gaucher disease. Am. J. Hematol. 91, 1082–1089 (2016).
Elstein, D. et al. Reductions in glucosylsphingosine (lyso-Gb1) in treatment-naive and previously treated patients receiving velaglucerase alfa for type 1 Gaucher disease: data from phase 3 clinical trials. Mol. Genet. Metab. 122, 113–120 (2017).
Lerche, S. et al. The mutation matters: CSF profiles of GCase, sphingolipids, α-synuclein in PDGBA. Mov. Disord. 36, 1216–1228 (2021).
Parnetti, L. et al. Cerebrospinal fluid β-glucocerebrosidase activity is reduced in Parkinson’s disease patients. Mov. Disord. 32, 1423–1431 (2017).
Surface, M. et al. Plasma glucosylsphingosine in GBA1 mutation carriers with and without Parkinson’s disease. Mov. Disord. 37, 416–421 (2022).
Omer, N. et al. Glucocerebrosidase activity is not associated with Parkinson’s disease risk or severity. Mov. Disord. 37, 651–652 (2022).
den Heijer, J. M. et al. A biomarker study in patients with GBA1-Parkinson’s disease and healthy controls. Mov. Disord. 38, 783–795 (2023).
Siderowf, A. et al. Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol. 22, 407–417 (2023).
Neumann, J. et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain 132, 1783–1794 (2009).
Clark, L. N. et al. Association of glucocerebrosidase mutations with dementia with Lewy bodies. Arch. Neurol. 66, 578–583 (2009).
Huebecker, M. et al. Reduced sphingolipid hydrolase activities, substrate accumulation and ganglioside decline in Parkinson’s disease. Mol. Neurodegener. 14, 40 (2019).
Murphy, K. E. et al. Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson’s disease. Brain 137, 834–848 (2014).
Rocha, E. M. et al. Progressive decline of glucocerebrosidase in aging and Parkinson’s disease. Ann. Clin. Transl. Neurol. 2, 433–438 (2015).
Milenkovic, I., Blumenreich, S. & Futerman, A. H. GBA mutations, glucosylceramide and Parkinson’s disease. Curr. Opin. Neurobiol. 72, 148–154 (2022).
Orvisky, E. et al. Glucosylsphingosine accumulation in tissues from patients with Gaucher disease: correlation with phenotype and genotype. Mol. Genet. Metab. 76, 262–270 (2002).
Gegg, M. E. et al. No evidence for substrate accumulation in Parkinson brains with GBA mutations. Mov. Disord. 30, 1085–1089 (2015).
Leyns, C. E. G. et al. Glucocerebrosidase activity and lipid levels are related to protein pathologies in Parkinson’s disease. NPJ Parkinsons Dis. 9, 74 (2023).
Blumenreich, S. et al. Elevation of gangliosides in four brain regions from Parkinson’s disease patients with a GBA mutation. NPJ Parkinsons Dis. 8, 99 (2022).
Walton, R. L. et al. Role of GBA variants in Lewy body disease neuropathology. Acta Neuropathol. 147, 54 (2024).
Velayati, A. et al. A mutation in SCARB2 is a modifier in Gaucher disease. Hum. Mutat. 32, 1232–1238 (2011).
Mistry, P. K. et al. Glucocerebrosidase 2 gene deletion rescues type 1 Gaucher disease. Proc. Natl Acad. Sci. USA 111, 4934–4939 (2014).
Yildiz, Y. et al. Functional and genetic characterization of the non-lysosomal glucosylceramidase 2 as a modifier for Gaucher disease. Orphanet J. Rare Dis. 8, 151 (2013).
Zhang, C. K. et al. Genome-wide association study of N370S homozygous Gaucher disease reveals the candidacy of CLN8 gene as a genetic modifier contributing to extreme phenotypic variation. Am. J. Hematol. 87, 377–383 (2012).
di Ronza, A. et al. CLN8 is an endoplasmic reticulum cargo receptor that regulates lysosome biogenesis. Nat. Cell Biol. 20, 1370–1377 (2018).
Klein, A. D. et al. Identification model of Gaucher disease. Cell Rep. 16, 2546–2553 (2016).
Blauwendraat, C. et al. Genetic modifiers of risk and age at onset in GBA associated Parkinson’s disease and Lewy body dementia. Brain 143, 234–248 (2020).
Fredriksen, K. et al. Pathological α-syn aggregation is mediated by glycosphingolipid chain length and the physiological state of α-syn in vivo. Proc. Natl Acad. Sci. USA 118, e2108489118 (2021).
Henderson, M. X. et al. Glucocerebrosidase activity modulates neuronal susceptibility to pathological α-synuclein insult. Neuron 105, 822–836.e7 (2020).
Jo, J. et al. Lewy body-like inclusions in human midbrain organoids carrying glucocerebrosidase and α-synuclein mutations. Ann. Neurol. 90, 490–505 (2021).
Mazzulli, J. R. et al. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 146, 37–52 (2011).
Paul, A. et al. Glucosylceramide associated with Gaucher disease forms amyloid-like twisted ribbon fibrils that induce α-synuclein aggregation. ACS Nano 15, 11854–11868 (2021).
Taguchi, Y. V. et al. Glucosylsphingosine promotes α-synuclein pathology in mutant GBA-associated Parkinson’s disease. J. Neurosci. 37, 9617–9631 (2017).
Galvagnion, C. et al. Sphingolipid changes in Parkinson L444P GBA mutation fibroblasts promote α-synuclein aggregation. Brain 145, 1038–1051 (2022).
Schondorf, D. C. et al. iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat. Commun. 5, 4028 (2014).
Fernandes, H. J. et al. ER stress and autophagic perturbations lead to elevated extracellular α-synuclein in GBA-N370S Parkinson’s iPSC-derived dopamine neurons. Stem Cell Rep. 6, 342–356 (2016).
Zurbruegg, M., Chan, M. Y. & Svenningsson, P. GBA RNAi but not catalytic inhibition of glucocerebrosidase with conduritol-β-epoxide increases levels of total α-synuclein in SH-SY5Y cells. Neurosci. Lett. 706, 217–222 (2019).
Maor, G., Rapaport, D. & Horowitz, M. The effect of mutant GBA1 on accumulation and aggregation of α-synuclein. Hum. Mol. Genet. 28, 1768–1781 (2019).
Maor, G. et al. The contribution of mutant GBA to the development of Parkinson disease in Drosophila. Hum. Mol. Genet. 25, 2712–2727 (2016).
Sardi, S. P. et al. CNS expression of glucocerebrosidase corrects α-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc. Natl Acad. Sci. USA 108, 12101–12106 (2011).
Bogetofte, H. et al. Post-translational proteomics platform identifies neurite outgrowth impairments in Parkinson’s disease GBA-N370S dopamine neurons. Cell Rep. 42, 112180 (2023).
Smith, L. J., Bolsinger, M. M., Chau, K. Y., Gegg, M. E. & Schapira, A. H. V. The GBA variant E326K is associated with α-synuclein aggregation and lipid droplet accumulation in human cell lines. Hum. Mol. Genet. 32, 773–789 (2023).
Kuo, S. H. et al. Mutant glucocerebrosidase impairs α-synuclein degradation by blockade of chaperone-mediated autophagy. Sci. Adv. 8, eabm6393 (2022).
Kim, S., Wong, Y. C., Gao, F. & Krainc, D. Dysregulation of mitochondria-lysosome contacts by GBA1 dysfunction in dopaminergic neuronal models of Parkinson’s disease. Nat. Commun. 12, 1807 (2021).
Wong, Y. C., Ysselstein, D. & Krainc, D. Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature 554, 382–386 (2018).
Kim, S., Coukos, R., Gao, F. & Krainc, D. Dysregulation of organelle membrane contact sites in neurological diseases. Neuron 110, 2386–2408 (2022).
Baden, P. et al. Glucocerebrosidase is imported into mitochondria and preserves complex I integrity and energy metabolism. Nat. Commun. 14, 1930 (2023).
Rosety, I. et al. Impaired neuron differentiation in GBA-associated Parkinson’s disease is linked to cell cycle defects in organoids. NPJ Parkinsons Dis. 9, 166 (2023).
Udayar, V., Chen, Y., Sidransky, E. & Jagasia, R. Lysosomal dysfunction in neurodegeneration: emerging concepts and methods. Trends Neurosci. 45, 184–199 (2022).
Abu-Remaileh, M. et al. Lysosomal metabolomics reveals V-ATPase- and mTOR-dependent regulation of amino acid efflux from lysosomes. Science 358, 807–813 (2017).
Gehrlein, A. et al. Targeting neuronal lysosomal dysfunction caused by β-glucocerebrosidase deficiency with an enzyme-based brain shuttle construct. Nat. Commun. 14, 2057 (2023).
Kampmann, M. CRISPR-based functional genomics for neurological disease. Nat. Rev. Neurol. 16, 465–480 (2020).
Tian, R. et al. Genome-wide CRISPRi/a screens in human neurons link lysosomal failure to ferroptosis. Nat. Neurosci. 24, 1020–1034 (2021).
Meng, Y. et al. Putaminal recombinant glucocerebrosidase delivery with magnetic resonance-guided focused ultrasound in Parkinson’s disease: a phase I study. Mov. Disord. 37, 2134–2139 (2022).
Logan, T. et al. Rescue of a lysosomal storage disorder caused by Grn loss of function with a brain penetrant progranulin biologic. Cell 184, 4651–4668.e25 (2021).
Giladi, N. et al. Safety and efficacy of venglustat in GBA1-associated Parkinson’s disease: an international, multicentre, double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 22, 661–671 (2023).
Sidransky, E. et al. Substrate reduction therapy for GBA1-associated Parkinsonism: are we betting on the wrong mouse? Mov. Disord. 35, 228–230 (2020).
Sardi, S. P. et al. Glucosylceramide synthase inhibition alleviates aberrations in synucleinopathy models. Proc. Natl Acad. Sci. USA 114, 2699–2704 (2017).
Mullin, S. et al. Ambroxol for the treatment of patients with Parkinson disease with and without glucocerebrosidase gene mutations: a nonrandomized, noncontrolled trial. JAMA Neurol. 77, 427–434 (2020).
Colucci, F. et al. Ambroxol as a disease-modifying treatment to reduce the risk of cognitive impairment in GBA-associated Parkinson’s disease: a multicentre, randomised, double-blind, placebo-controlled, phase II trial. The AMBITIOUS study protocol. BMJ Neurol. Open. 5, e000535 (2023).
Chwiszczuk, L. J. et al. The ANeED study – ambroxol in new and early dementia with Lewy bodies (DLB): protocol for a phase IIa multicentre, randomised, double-blinded and placebo-controlled trial. Front. Aging Neurosci. 15, 1163184 (2023).
den Heijer, J. M. et al. A phase 1B trial in GBA1-associated Parkinson’s disease of BIA-28-6156, a glucocerebrosidase activator. Mov. Disord. 38, 1197–1208 (2023).
Oftedal, L. et al. Association of CSF glucocerebrosidase activity with the risk of incident dementia in patients with Parkinson disease. Neurology 100, e388–e395 (2023).
Chiasserini, D. et al. Selective loss of glucocerebrosidase activity in sporadic Parkinson’s disease and dementia with Lewy bodies. Mol. Neurodegener. 10, 15 (2015).
Farfel-Becker, T., Do, J., Tayebi, N. & Sidransky, E. Can GBA1-associated Parkinson disease be modeled in the mouse? Trends Neurosci. 42, 631–643 (2019).
Cullen, V. et al. Acid β-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter α-synuclein processing. Ann. Neurol. 69, 940–953 (2011).
Zhao, X. et al. PGRN deficiency exacerbates, whereas a brain penetrant PGRN derivative protects, GBA1 mutation-associated pathologies and diseases. Proc. Natl Acad. Sci. USA 120, e2210442120 (2023).
Tayebi, N. et al. Glucocerebrosidase haploinsufficiency in A53T α-synuclein mice impacts disease onset and course. Mol. Genet. Metab. 122, 198–208 (2017).
Mahoney-Crane, C. L. et al. Neuronopathic GBA1L444P mutation accelerates glucosylsphingosine levels and formation of hippocampal α-synuclein inclusions. J. Neurosci. 43, 501–521 (2023).
Ramos, D. M., Skarnes, W. C., Singleton, A. B., Cookson, M. R. & Ward, M. E. Tackling neurodegenerative diseases with genomic engineering: a new stem cell initiative from the NIH. Neuron 109, 1080–1083 (2021).
Bressan, E. et al. The foundational data initiative for Parkinson disease: enabling efficient translation from genetic maps to mechanism. Cell Genom. 3, 100261 (2023).
Deen, M. C. et al. A versatile fluorescence-quenched substrate for quantitative measurement of glucocerebrosidase activity within live cells. Proc. Natl Acad. Sci. USA 119, e2200553119 (2022).
Zhu, S. et al. A fixable fluorescence-quenched substrate for quantitation of lysosomal glucocerebrosidase activity in both live and fixed cells. Angew. Chem. Int. Ed. Engl. 62, e202309306 (2023).
Jong, T., Gehrlein, A., Sidransky, E., Jagasia, R. & Chen, Y. Characterization of novel human β-glucocerebrosidase antibodies for Parkinson’s disease research. J. Parkinsons Dis. 14, 65–78 (2024).
Scharenberg, S. G. et al. An SPNS1-dependent lysosomal lipid transport pathway that enables cell survival under choline limitation. Sci. Adv. 9, eadf8966 (2023).
Medoh, U. N. et al. The Batten disease gene product CLN5 is the lysosomal bis(monoacylglycero)phosphate synthase. Science 381, 1182–1189 (2023).
Davis, O. B. et al. NPC1-mTORC1 signaling couples cholesterol sensing to organelle homeostasis and is a targetable pathway in Niemann–Pick type C. Dev. Cell. 56, 260–276.e7 (2021).
Tian, R. et al. CRISPR interference-based platform for multimodal genetic screens in human iPSC-derived neurons. Neuron 104, 239–255.e12 (2019).
Gilbert, L. A. et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159, 647–661 (2014).
Magalhaes, J. et al. Autophagic lysosome reformation dysfunction in glucocerebrosidase deficient cells: relevance to Parkinson disease. Hum. Mol. Genet. 25, 3432–3445 (2016).
Kim, S. et al. GBA1 deficiency negatively affects physiological α-synuclein tetramers and related multimers. Proc. Natl Acad. Sci. USA 115, 798–803 (2018).
Rocha, E. M. et al. Sustained systemic glucocerebrosidase inhibition induces brain α-synuclein aggregation, microglia and complement C1q activation in mice. Antioxid. Redox Signal. 23, 550–564 (2015).
Osellame, L. D. et al. Mitochondria and quality control defects in a mouse model of Gaucher disease – links to Parkinson’s disease. Cell Metab. 17, 941–953 (2013).
Holleran, W. M. et al. Consequences of β-glucocerebrosidase deficiency in epidermis. Ultrastructure and permeability barrier alterations in Gaucher disease. J. Clin. Invest. 93, 1756–1764 (1994).
Laqtom, N. N. et al. CLN3 is required for the clearance of glycerophosphodiesters from lysosomes. Nature 609, 1005–1011 (2022).
Wyant, G. A. et al. NUFIP1 is a ribosome receptor for starvation-induced ribophagy. Science 360, 751–758 (2018).
Park, H. et al. Spatial snapshots of amyloid precursor protein intramembrane processing via early endosome proteomics. Nat. Commun. 13, 6112 (2022).
Acknowledgements
This work was supported by the Intramural Research Programs of the National Human Genome Research Institute and the National Institutes of Health. The authors thank J. Fekecs for her assistance with drafting the figures.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks Hugo Fernandes, Kathrin Brockmann and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hertz, E., Chen, Y. & Sidransky, E. Gaucher disease provides a unique window into Parkinson disease pathogenesis. Nat Rev Neurol 20, 526–540 (2024). https://doi.org/10.1038/s41582-024-00999-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-024-00999-z
- Springer Nature Limited