Abstract
Williams syndrome (WS) is a relatively rare microdeletion disorder that occurs in as many as 1:7,500 individuals. WS arises due to the mispairing of low-copy DNA repetitive elements at meiosis. The deletion size is similar across most individuals with WS and leads to the loss of one copy of 25–27 genes on chromosome 7q11.23. The resulting unique disorder affects multiple systems, with cardinal features including but not limited to cardiovascular disease (characteristically stenosis of the great arteries and most notably supravalvar aortic stenosis), a distinctive craniofacial appearance, and a specific cognitive and behavioural profile that includes intellectual disability and hypersociability. Genotype–phenotype evidence is strongest for ELN, the gene encoding elastin, which is responsible for the vascular and connective tissue features of WS, and for the transcription factor genes GTF2I and GTF2IRD1, which are known to affect intellectual ability, social functioning and anxiety. Mounting evidence also ascribes phenotypic consequences to the deletion of BAZ1B, LIMK1, STX1A and MLXIPL, but more work is needed to understand the mechanism by which these deletions contribute to clinical outcomes. The age of diagnosis has fallen in regions of the world where technological advances, such as chromosomal microarray, enable clinicians to make the diagnosis of WS without formally suspecting it, allowing earlier intervention by medical and developmental specialists. Phenotypic variability is considerable for all cardinal features of WS but the specific sources of this variability remain unknown. Further investigation to identify the factors responsible for these differences may lead to mechanism-based rather than symptom-based therapies and should therefore be a high research priority.






Similar content being viewed by others
References
Beuren, A. J., Apitz, J. & Harmjanz, D. Supravalvular aortic stenosis in association with mental retardation and a certain facial appearance. Circulation 26, 1235–1240 (1962).
Williams, J. C., Barratt-Boyes, B. G. & Lowe, J. B. Supravalvular aortic stenosis. Circulation 24, 1311–1318 (1961).
Fanconi, G., Girardet, P., Schlesinger, B., Butler, N. & Black, J. Chronische hypercalcaemie kombiniert mit osteosklerose, hyperazotaemie, minderwuchs, und kongenitalen Missbildungen [Chronic hypercalcemia, combined with osteosclerosis, hyperazotemia, nanism, and congenital malformations]. Helv. Paediatr. Acta 7, 314–349 (1952).
Cha, S. G. et al. Long-term cardiovascular outcome of Williams syndrome. Congenit. Heart Dis. 14, 684–690 (2019).
Del Pasqua, A. et al. New findings concerning cardiovascular manifestations emerging from long-term follow-up of 150 patients with the Williams-Beuren-Beuren syndrome. Cardiol. Young. 19, 563–567 (2009). This paper reports the cardiovascular outcomes from a large number of individuals with WS over a range of 0.5–25 years (average 6 years) of age.
Collins, R. T. II Cardiovascular disease in Williams syndrome. Curr. Opin. Pediatr. 30, 609–615 (2018).
Li, D. Y. et al. Novel arterial pathology in mice and humans hemizygous for elastin. J. Clin. Invest. 102, 1783–1787 (1998). This paper describes the impact of elastin insufficiency in humans and mice, cementing its role in the vasculopathy of WS.
Bouchireb, K. et al. Clinical features and management of arterial hypertension in children with Williams-Beuren syndrome. Nephrol. Dial. Transpl. 25, 434–438 (2010).
Rose, C., Wessel, A., Pankau, R., Partsch, C. J. & Bursch, J. Anomalies of the abdominal aorta in Williams-Beuren syndrome-another cause of arterial hypertension. Eur. J. Pediatr. 160, 655–658 (2001).
Latham, G. J. et al. Perioperative morbidity in children with elastin arteriopathy. Paediatr. Anaesth. 26, 926–935 (2016). This is the largest study (n = 48) that discusses risks associated with anaesthesia in individuals with WS.
Furusawa, E. A. et al. Diagnosis and management of systemic hypertension due to renovascular and aortic stenosis in patients with Williams-Beuren syndrome. Rev. Assoc. Med. Bras. 64, 723–728 (2018).
Wessel, A. et al. Risk of sudden death in the Williams-Beuren syndrome. Am. J. Med. Genet. A 127A, 234–237 (2004). This is the first study to demonstrate an elevated relative risk of sudden cardiovascular death in individuals with WS.
Mervis, C. B. & Greiner de Magalhães, C. In Pediatric Neuropsychology: Research, theory, and practice (eds Beauchamp, M., et al.) Ch. Williams syndrome (Guilford Press, 2021). This article provides a thorough characterization of the WS behavioural phenotype with a special focus on intellectual disability, language and literacy development, memory, and executive function development along with suggested interventional approaches.
Mervis, C. B. & John, A. E. Cognitive and behavioral characteristics of children with Williams syndrome: implications for intervention approaches. Am. J. Med. Genet. C. Semin. Med. Genet. 154C, 229–248 (2010).
Jarvinen, A., Korenberg, J. R. & Bellugi, U. The social phenotype of Williams syndrome. Curr. Opin. Neurobiol. 23, 414–422 (2013).
Klein-Tasman, B. P. & Mervis, C. B. Distinctive personality characteristics of 8-, 9-, and 10-year-olds with Williams syndrome. Dev. Neuropsychol. 23, 269–290 (2003).
Ewart, A. K. et al. Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nat. Genet. 5, 11–16 (1993).
Perez Jurado, L. A., Peoples, R., Kaplan, P., Hamel, B. C. & Francke, U. Molecular definition of the chromosome 7 deletion in Williams syndrome and parent-of-origin effects on growth. Am. J. Hum. Genet. 59, 781–792 (1996).
Stromme, P., Bjornstad, P. G. & Ramstad, K. Prevalence estimation of Williams syndrome. J. Child. Neurol. 17, 269–271 (2002).
Greenberg, F. Williams syndrome professional symposium. Am. J. Med. Genet. 37, 85–88 (1990).
National Organization for Rare Disorders. Williams Syndrome https://rarediseases.org/rare-diseases/williams-syndrome/ (2006).
Honjo, R. S. et al. Williams-Beuren syndrome: a clinical study of 55 Brazilian patients and the diagnostic use of MLPA. Biomed. Res. Int. 2015, 903175 (2015).
Sharma, P. et al. Williams-Beuren syndrome: experience of 43 patients and a report of an atypical case from a tertiary care center in India. Cytogenet. Genome Res. 146, 187–194 (2015).
Gold, N. B. et al. Delayed diagnosis of Williams-Beuren syndrome in an adolescent of Jamaican descent: examining racial disparities in genetics education. Clin. Dysmorphol. 30, 69–70 (2021).
Lumaka, A. et al. Williams-Beuren syndrome: pitfalls for diagnosis in limited resources setting. Clin. Case Rep. 4, 294–297 (2016).
Tekendo-Ngongang, C. et al. Challenges in clinical diagnosis of Williams-Beuren syndrome in sub-Saharan Africans: case reports from Cameroon. Mol. Syndromol. 5, 287–292 (2014).
Kruszka, P. et al. Williams-Beuren syndrome in diverse populations. Am. J. Med. Genet. A 176, 1128–1136 (2018). This paper presents the largest series of facial photographs of individuals with WS from multiple regions throughout the world; this is crucial for increasing international awareness of WS.
Scheiber, D. et al. Echocardiographic findings in patients with Williams-Beuren syndrome. Wien. Klin. Wochenschr. 118, 538–542 (2006).
Parrish, P. C. R. et al. Whole exome sequencing in patients with Williams-Beuren syndrome followed by disease modeling in mice points to four novel pathways that may modify stenosis risk. Hum. Mol. Genet. 29, 2035–2050 (2020).
Sadler, L. S. et al. Differences by sex in cardiovascular disease in Williams syndrome. J. Pediatr. 139, 849–853 (2001).
Morris, C. A. et al. Alpha 1 antitrypsin deficiency alleles are associated with joint dislocation and scoliosis in Williams syndrome. Am. J. Med. Genet. C. Semin. Med. Genet. 154C, 299–306 (2010).
Wang, M. S. et al. Molecular and clinical correlation study of Williams-Beuren syndrome: no evidence of molecular factors in the deletion region or imprinting affecting clinical outcome. Am. J. Med. Genet. 86, 34–43 (1999).
Elison, S., Stinton, C. & Howlin, P. Health and social outcomes in adults with Williams syndrome: findings from cross-sectional and longitudinal cohorts. Res. Dev. Disabil. 31, 587–599 (2010).
Sauna-Aho, O., Bjelogrlic-Laakso, N., Siren, A., Kangasmaki, V. & Arvio, M. Cognition in adults with Williams syndrome-A 20-year follow-up study. Mol. Genet. Genom. Med. 7, e695 (2019). These authors provide long-term follow-up information on some of the oldest adults with WS reported to date and document difficulties across numerous medical and functional domains.
Sadler, L. S., Robinson, L. K., Verdaasdonk, K. R. & Gingell, R. The Williams syndrome: evidence for possible autosomal dominant inheritance. Am. J. Med. Genet. 47, 468–470 (1993).
Morris, C. A., Thomas, I. T. & Greenberg, F. Williams syndrome: autosomal dominant inheritance. Am. J. Med. Genet. 47, 478–481 (1993).
Metcalfe, K., Simeonov, E., Beckett, W., Donnai, D. & Tassabehji, M. Autosomal dominant inheritance of Williams-Beuren syndrome in a father and son with haploinsufficiency for FKBP6. Clin. Dysmorphol. 14, 61–65 (2005).
Pankau, R. et al. Familial Williams-Beuren syndrome showing varying clinical expression. Am. J. Med. Genet. 98, 324–329 (2001).
Bayes, M., Magano, L. F., Rivera, N., Flores, R. & Perez Jurado, L. A. Mutational mechanisms of Williams-Beuren syndrome deletions. Am. J. Hum. Genet. 73, 131–151 (2003). This paper dissects the complex make-up of the low-copy repeats that mediate the WS deletion.
Antonell, A. et al. Partial 7q11.23 deletions further implicate GTF2I and GTF2IRD1 as the main genes responsible for the Williams-Beuren syndrome neurocognitive profile. J. Med. Genet. 47, 312–320 (2010).
Cusco, I. et al. Copy number variation at the 7q11.23 segmental duplications is a susceptibility factor for the Williams-Beuren syndrome deletion. Genome Res. 18, 683–694 (2008).
Osborne, L. R. et al. A 1.5 million-base pair inversion polymorphism in families with Williams-Beuren syndrome. Nat. Genet. 29, 321–325 (2001). This paper identifies 7q11.23 inversion as a polymorphism that is a risk factor for the WS deletion.
Somerville, M. J. et al. Severe expressive-language delay related to duplication of the Williams-Beuren locus. N. Engl. J. Med. 353, 1694–1701 (2005).
Hobart, H. H. et al. Inversion of the Williams syndrome region is a common polymorphism found more frequently in parents of children with Williams syndrome. Am. J. Med. Genet. C. Semin. Med. Genet. 154C, 220–228 (2010).
Tam, E. et al. The common inversion of the Williams-Beuren syndrome region at 7q11.23 does not cause clinical symptoms. Am. J. Med. Genet. A 146A, 1797–1806 (2008).
Ramocki, M. B. et al. Recurrent distal 7q11.23 deletion including HIP1 and YWHAG identified in patients with intellectual disabilities, epilepsy, and neurobehavioral problems. Am. J. Hum. Genet. 87, 857–865 (2010).
Marshall, C. R. et al. Infantile spasms is associated with deletion of the MAGI2 gene on chromosome 7q11.23-q21.11. Am. J. Hum. Genet. 83, 106–111 (2008).
Nicita, F. et al. Epilepsy is a possible feature in Williams-Beuren syndrome patients harboring typical deletions of the 7q11.23 critical region. Am. J. Med. Genet. A 170A, 148–155 (2016).
Lugo, M. et al. Social, neurodevelopmental, endocrine, and head size differences associated with atypical deletions in Williams-Beuren syndrome. Am. J. Med. Genet. A 182, 1008–1020 (2020).
Fusco, C. et al. Smaller and larger deletions of the Williams Beuren syndrome region implicate genes involved in mild facial phenotype, epilepsy and autistic traits. Eur. J. Hum. Genet. 22, 64–70 (2014).
Stock, A. D. et al. Heat shock protein 27 gene: chromosomal and molecular location and relationship to Williams syndrome. Am. J. Med. Genet. A 120A, 320–325 (2003).
Vandeweyer, G., Van der Aa, N., Reyniers, E. & Kooy, R. F. The contribution of CLIP2 haploinsufficiency to the clinical manifestations of the Williams-Beuren syndrome. Am. J. Hum. Genet. 90, 1071–1078 (2012).
Tassabehji, M. et al. Williams syndrome: use of chromosomal microdeletions as a tool to dissect cognitive and physical phenotypes. Am. J. Hum. Genet. 64, 118–125 (1999).
Tassabehji, M. et al. GTF2IRD1 in craniofacial development of humans and mice. Science 310, 1184–1187 (2005).
Plaja, A. et al. A novel recurrent breakpoint responsible for rearrangements in the Williams-Beuren region. Cytogenet. Genome Res. 146, 181–186 (2015).
Morris, C. A. et al. GTF2I hemizygosity implicated in mental retardation in Williams syndrome: genotype-phenotype analysis of five families with deletions in the Williams syndrome region. Am. J. Med. Genet. A 123A, 45–59 (2003). This paper demonstrates the roles of the deletion of centromeric and telomeric portions of the WSCR in the WS neurocognitive profile.
Hoeft, F. et al. Mapping genetically controlled neural circuits of social behavior and visuo-motor integration by a preliminary examination of atypical deletions with Williams syndrome. PLoS One 9, e104088 (2014).
Hirota, H. et al. Williams syndrome deficits in visual spatial processing linked to GTF2IRD1 and GTF2I on chromosome 7q11.23. Genet. Med. 5, 311–321 (2003).
Gagliardi, C., Bonaglia, M. C., Selicorni, A., Borgatti, R. & Giorda, R. Unusual cognitive and behavioural profile in a Williams syndrome patient with atypical 7q11.23 deletion. J. Med. Genet. 40, 526–530 (2003).
Ferrero, G. B. et al. An atypical 7q11.23 deletion in a normal IQ Williams-Beuren syndrome patient. Eur. J. Hum. Genet. 18, 33–38 (2010).
Delgado, L. M. et al. A 1.3-mb 7q11.23 atypical deletion identified in a cohort of patients with Williams-Beuren syndrome. Mol. Syndromol. 4, 143–147 (2013).
Botta, A. et al. Detection of an atypical 7q11.23 deletion in Williams syndrome patients which does not include the STX1A and FZD3 genes. J. Med. Genet. 36, 478–480 (1999).
Alesi, V. et al. Atypical 7q11.23 deletions excluding ELN gene result in Williams-Beuren syndrome craniofacial features and neurocognitive profile. Am. J. Med. Genet. A 185, 242–249 (2021).
Frangiskakis, J. M. et al. LIM-kinase1 hemizygosity implicated in impaired visuospatial constructive cognition. Cell 86, 59–69 (1996).
Antonell, A., Vilardell, M. & Perez Jurado, L. A. Transcriptome profile in Williams-Beuren syndrome lymphoblast cells reveals gene pathways implicated in glucose intolerance and visuospatial construction deficits. Hum. Genet. 128, 27–37 (2010).
Kimura, R. et al. Integrated DNA methylation analysis reveals a potential role for ANKRD30B in Williams syndrome. Neuropsychopharmacology 45, 1627–1636 (2020).
Karagiannis, P. et al. Induced pluripotent stem cells and their use in human models of disease and development. Physiol. Rev. 99, 79–114 (2019).
Khattak, S. et al. Human induced pluripotent stem cell derived neurons as a model for Williams-Beuren syndrome. Mol. Brain 8, 77 (2015).
Adamo, A. et al. 7q11.23 dosage-dependent dysregulation in human pluripotent stem cells affects transcriptional programs in disease-relevant lineages. Nat. Genet. 47, 132–141 (2015). This paper uses iPSC models of 7q11.23 CNV to identify transcription changes associated with these genetic alterations.
Strong, E. et al. Symmetrical dose-dependent DNA-methylation profiles in children with deletion or duplication of 7q11.23. Am. J. Hum. Genet. 97, 216–227 (2015). This paper identifies extensive genome-wide changes in DNA methylation in WS that could have significant impacts on gene regulation.
Segura-Puimedon, M. et al. Heterozygous deletion of the Williams-Beuren syndrome critical interval in mice recapitulates most features of the human disorder. Hum. Mol. Genet. 23, 6481–6494 (2014).
Borralleras, C. et al. Synaptic plasticity and spatial working memory are impaired in the CD mouse model of Williams-Beuren syndrome. Mol. Brain 9, 76 (2016).
Jimenez-Altayo, F. et al. Stenosis coexists with compromised alpha1-adrenergic contractions in the ascending aorta of a mouse model of Williams-Beuren syndrome. Sci. Rep. 10, 889 (2020).
Campuzano, V. et al. Reduction of NADPH-oxidase activity ameliorates the cardiovascular phenotype in a mouse model of Williams-Beuren Syndrome. PLoS Genet. 8, e1002458 (2012).
Lek, M. et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291 (2016).
Harkness, M. L., Harkness, R. D. & McDonald, D. A. The collagen and elastin content of the arterial wall in the dog. Proc. R. Soc. Lond. B Biol. Sci. 146, 541–551 (1957).
Leung, D. Y., Glagov, S. & Mathews, M. B. Elastin and collagen accumulation in rabbit ascending aorta and pulmonary trunk during postnatal growth. Correlation of cellular synthetic response with medial tension. Circ. Res. 41, 316–323 (1977).
Li, B. & Daggett, V. Molecular basis for the extensibility of elastin. J. Muscle Res. Cell Motil. 23, 561–573 (2002).
Kozel, B. A. & Mecham, R. P. Elastic fiber ultrastructure and assembly. Matrix Biol. 84, 31–40 (2019).
Shapiro, S. D., Endicott, S. K., Province, M. A., Pierce, J. A. & Campbell, E. J. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J. Clin. Invest. 87, 1828–1834 (1991).
Parks, W. C. Posttranscriptional regulation of lung elastin production. Am. J. Respir. Cell Mol. Biol. 17, 1–2 (1997).
Ott, C. E. et al. MicroRNAs differentially expressed in postnatal aortic development downregulate elastin via 3’ UTR and coding-sequence binding sites. PLoS One 6, e16250 (2011).
Zhang, M. & Parks, W. C. Posttranscriptional regulation of lung elastin expression involves binding of a developmentally regulated cytosolic protein to an open-reading frame cis-element in the messenger RNA. Chest 116, 74S (1999).
Li, D. Y. et al. Elastin point mutations cause an obstructive vascular disease, supravalvular aortic stenosis. Hum. Mol. Genet. 6, 1021–1028 (1997).
Tassabehji, M. et al. Elastin: genomic structure and point mutations in patients with supravalvular aortic stenosis. Hum. Mol. Genet. 6, 1029–1036 (1997).
Urban, Z. et al. Isolated supravalvular aortic stenosis: functional haploinsufficiency of the elastin gene as a result of nonsense-mediated decay. Hum. Genet. 106, 577–588 (2000).
Olson, T. M. et al. A 30 kb deletion within the elastin gene results in familial supravalvular aortic stenosis. Hum. Mol. Genet. 4, 1677–1679 (1995).
Pober, B. R., Johnson, M. & Urban, Z. Mechanisms and treatment of cardiovascular disease in Williams-Beuren syndrome. J. Clin. Invest. 118, 1606–1615 (2008).
Collins, R. T. II, Kaplan, P., Somes, G. W. & Rome, J. J. Long-term outcomes of patients with cardiovascular abnormalities and Williams syndrome. Am. J. Cardiol. 105, 874–878 (2010).
Collins, R. T. II Cardiovascular disease in Williams syndrome. Circulation 127, 2125–2134 (2013).
Kozel, B. A. et al. Williams syndrome predisposes to vascular stiffness modified by antihypertensive use and copy number changes in NCF1. Hypertension 63, 74–79 (2014).
Abu-Sultaneh, S., Gondim, M. J., Alexy, R. D. & Mastropietro, C. W. Sudden cardiac death associated with cardiac catheterization in Williams syndrome: a case report and review of literature. Cardiol. Young. https://doi.org/10.1017/S1047951119000295 (2019).
Matisoff, A. J., Olivieri, L., Schwartz, J. M. & Deutsch, N. Risk assessment and anesthetic management of patients with Williams syndrome: a comprehensive review. Paediatr. Anaesth. 25, 1207–1215 (2015).
Staudt, G. E. & Eagle, S. S. Anesthetic considerations for patients with Williams syndrome. J. Cardiothorac. Vasc. Anesth. 35, 176–186 (2021). This is a well-organized paper that reviews anaesthesia risks and provides management guidelines for both children and adults with WS.
Burch, T. M., McGowan, F. X. Jr., Kussman, B. D., Powell, A. J. & DiNardo, J. A. Congenital supravalvular aortic stenosis and sudden death associated with anesthesia: what’s the mystery? Anesth. Analg. 107, 1848–1854 (2008).
Hirano, E., Knutsen, R. H., Sugitani, H., Ciliberto, C. H. & Mecham, R. P. Functional rescue of elastin insufficiency in mice by the human elastin gene: implications for mouse models of human disease. Circ. Res. 101, 523–531 (2007).
Lin, C. J. et al. Heterogeneous cellular contributions to elastic laminae formation in arterial wall development. Circ. Res. 125, 1006–1018 (2019). This paper dissects the role of endothelial and smooth muscle cells in the elastin-insufficiency phenotype and is the first mouse model to show neointima formation in an elastin mutant.
Karnik, S. K. et al. A critical role for elastin signaling in vascular morphogenesis and disease. Development 130, 411–423 (2003).
Li, D. Y. et al. Elastin is an essential determinant of arterial morphogenesis. Nature 393, 276–280 (1998).
Urban, Z. et al. Connection between elastin haploinsufficiency and increased cell proliferation in patients with supravalvular aortic stenosis and Williams-Beuren syndrome. Am. J. Hum. Genet. 71, 30–44 (2002). This paper links elastin insufficiency to changes in smooth muscle cell proliferation.
Misra, A. et al. Integrin beta3 inhibition is a therapeutic strategy for supravalvular aortic stenosis. J. Exp. Med. 213, 451–463 (2016).
Jiao, Y. et al. Deficient circumferential growth is the primary determinant of aortic obstruction attributable to partial elastin deficiency. Arterioscler. Thromb. Vasc. Biol. 37, 930–941 (2017). This paper posits that elastin insufficiency impacts the circumferential growth of developing arteries.
Faury, G. et al. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J. Clin. Invest. 112, 1419–1428 (2003).
Jiao, Y. et al. mTOR (Mechanistic target of rapamycin) inhibition decreases mechanosignaling, collagen accumulation, and stiffening of the thoracic aorta in elastin-deficient mice. Arterioscler. Thromb. Vasc. Biol. 37, 1657–1666 (2017).
Kinnear, C. et al. Everolimus rescues the phenotype of elastin insufficiency in patient induced pluripotent stem cell-derived vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 40, 1325–1339 (2020).
Li, W. et al. Rapamycin inhibits smooth muscle cell proliferation and obstructive arteriopathy attributable to elastin deficiency. Arterioscler. Thromb. Vasc. Biol. 33, 1028–1035 (2013).
Wan, E. S., Pober, B. R., Washko, G. R., Raby, B. A. & Silverman, E. K. Pulmonary function and emphysema in Williams-Beuren syndrome. Am. J. Med. Genet. A 152A, 653–656 (2010).
Shifren, A., Durmowicz, A. G., Knutsen, R. H., Hirano, E. & Mecham, R. P. Elastin protein levels are a vital modifier affecting normal lung development and susceptibility to emphysema. Am. J. Physiol. Lung Cell Mol. Physiol. 292, L778–L787 (2007).
Pangallo, E. et al. Pulmonary function in Williams-Beuren syndrome: spirometric data of 22 Italian patients. Am. J. Med. Genet. A 185, 390–396 (2020).
Urban, Z. et al. Elastin gene deletions in Williams syndrome patients result in altered deposition of elastic fibers in skin and a subclinical dermal phenotype. Pediatr. Dermatol. 17, 12–20 (2000).
Kozel, B. A. et al. Skin findings in Williams syndrome. Am. J. Med. Genet. A 164A, 2217–2225 (2014).
Sammour, Z. M. et al. Congenital genitourinary abnormalities in children with Williams-Beuren syndrome. J. Pediatr. Urol. 10, 804–809 (2014).
Vaux, K. K., Wojtczak, H., Benirschke, K. & Jones, K. L. Vocal cord abnormalities in Williams syndrome: a further manifestation of elastin deficiency. Am. J. Med. Genet. A 119A, 302–304 (2003).
Drummond, G. R., Selemidis, S., Griendling, K. K. & Sobey, C. G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov. 10, 453–471 (2011).
Lassegue, B. & Griendling, K. K. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler. Thromb. Vasc. Biol. 30, 653–661 (2010).
Del Campo, M. et al. Hemizygosity at the NCF1 gene in patients with Williams-Beuren syndrome decreases their risk of hypertension. Am. J. Hum. Genet. 78, 533–542 (2006). This is the first paper to show a role for NCF1 gene dosage in modifying the risk of hypertension in WS.
Kozel, B. A. et al. Genetic modifiers of cardiovascular phenotype caused by elastin haploinsufficiency act by extrinsic noncomplementation. J. Biol. Chem. 286, 44926–44936 (2011).
Troia, A. et al. Inhibition of NOX1 mitigates blood pressure increases in elastin insufficiency. Function 2, zqab015 (2021).
Caraveo, G., van Rossum, D. B., Patterson, R. L., Snyder, S. H. & Desiderio, S. Action of TFII-I outside the nucleus as an inhibitor of agonist-induced calcium entry. Science 314, 122–125 (2006).
Hakre, S. et al. Opposing functions of TFII-I spliced isoforms in growth factor-induced gene expression. Mol. Cell 24, 301–308 (2006).
Roy, A. L. Signal-induced functions of the transcription factor TFII-I. Biochim. Biophys. Acta 1769, 613–621 (2007).
Enkhmandakh, B. et al. Essential functions of the Williams-Beuren syndrome-associated TFII-I genes in embryonic development. Proc. Natl Acad. Sci. USA 106, 181–186 (2009).
Hinsley, T. A., Cunliffe, P., Tipney, H. J., Brass, A. & Tassabehji, M. Comparison of TFII-I gene family members deleted in Williams-Beuren syndrome. Protein Sci. 13, 2588–2599 (2004).
Lucena, J. et al. Essential role of the N-terminal region of TFII-I in viability and behavior. BMC Med. Genet. 11, 61 (2010).
Roy, A. L. Biochemistry and biology of the inducible multifunctional transcription factor TFII-I: 10 years later. Gene 492, 32–41 (2012).
Cheriyath, V., Desgranges, Z. P. & Roy, A. L. c-Src-dependent transcriptional activation of TFII-I. J. Biol. Chem. 277, 22798–22805 (2002).
Desgranges, Z. P. et al. Inhibition of TFII-I-dependent cell cycle regulation by p53. Mol. Cell Biol. 25, 10940–10952 (2005).
Roy, A. L. Biochemistry and biology of the inducible multifunctional transcription factor TFII-I. Gene 274, 1–13 (2001).
Enkhmandakh, B., Bitchevaia, N., Ruddle, F. & Bayarsaihan, D. The early embryonic expression of TFII-I during mouse preimplantation development. Gene Expr. Patterns 4, 25–28 (2004).
Makeyev, A. V. & Bayarsaihan, D. New TFII-I family target genes involved in embryonic development. Biochem. Biophys. Res. Commun. 386, 554–558 (2009).
Ashworth, T. & Roy, A. L. Phase specific functions of the transcription factor TFII-I during cell cycle. Cell Cycle 8, 596–605 (2009).
Chimge, N. O., Makeyev, A. V., Ruddle, F. H. & Bayarsaihan, D. Identification of the TFII-I family target genes in the vertebrate genome. Proc. Natl Acad. Sci. USA 105, 9006–9010 (2008).
Tussie-Luna, M. I., Bayarsaihan, D., Seto, E., Ruddle, F. H. & Roy, A. L. Physical and functional interactions of histone deacetylase 3 with TFII-I family proteins and PIASxbeta. Proc. Natl Acad. Sci. USA 99, 12807–12812 (2002).
Bayarsaihan, D. What role does TFII-I have to play in epigenetic modulation during embryogenesis? Epigenomics 5, 9–11 (2013).
Howald, C. et al. Two high throughput technologies to detect segmental aneuploidies identify new Williams-Beuren syndrome patients with atypical deletions. J. Med. Genet. 43, 266–273 (2006).
Karmiloff-Smith, A. et al. Social cognition in Williams syndrome: genotype/phenotype insights from partial deletion patients. Front. Psychol. 3, 168 (2012).
van Hagen, J. M. et al. Contribution of CYLN2 and GTF2IRD1 to neurological and cognitive symptoms in Williams syndrome. Neurobiol. Dis. 26, 112–124 (2007).
Karmiloff-Smith, A. et al. Using case study comparisons to explore genotype-phenotype correlations in Williams-Beuren syndrome. J. Med. Genet. 40, 136–140 (2003).
Dai, L. et al. Is it Williams syndrome? GTF2IRD1 implicated in visual-spatial construction and GTF2I in sociability revealed by high resolution arrays. Am. J. Med. Genet. A 149A, 302–314 (2009).
Pinelli, M. et al. A small 7q11.23 microduplication involving GTF2I in a family with intellectual disability. Clin. Genet. 97, 940–942 (2020).
Sakurai, T. et al. Haploinsufficiency of Gtf2i, a gene deleted in Williams Syndrome, leads to increases in social interactions. Autism Res. 4, 28–39 (2011).
Martin, L. A., Iceberg, E. & Allaf, G. Consistent hypersocial behavior in mice carrying a deletion of Gtf2i but no evidence of hyposocial behavior with Gtf2i duplication: implications for Williams-Beuren syndrome and autism spectrum disorder. Brain Behav. 8, e00895 (2018).
Barak, B. et al. Neuronal deletion of Gtf2i, associated with Williams syndrome, causes behavioral and myelin alterations rescuable by a remyelinating drug. Nat. Neurosci. 22, 700–708 (2019). This paper demonstrates the neuronal functions of Gtf2i in mediating myelination properties in the mouse brain and that correcting myelination deficits rescues social and motor behaviour deficits; light is also shed on molecular and cellular defects related to myelination deficits in the brain of individuals with WS.
Osso, L. A. & Chan, J. R. A surprising role for myelin in Williams syndrome. Nat. Neurosci. 22, 681–683 (2019).
Borralleras, C., Sahun, I., Perez-Jurado, L. A. & Campuzano, V. Intracisternal Gtf2i gene therapy ameliorates deficits in cognition and synaptic plasticity of a mouse model of Williams-Beuren syndrome. Mol. Ther. 23, 1691–1699 (2015).
Mervis, C. B. et al. Duplication of GTF2I results in separation anxiety in mice and humans. Am. J. Hum. Genet. 90, 1064–1070 (2012).
Osborne, L. R. Animal models of Williams syndrome. Am. J. Med. Genet. C. Semin. Med. Genet. 154C, 209–219 (2010).
Mervis, C. B. et al. Children with 7q11.23 duplication syndrome: psychological characteristics. Am. J. Med. Genet. A 167, 1436–1450 (2015).
Morris, C. A. et al. 7q11.23 Duplication syndrome: physical characteristics and natural history. Am. J. Med. Genet. A 167A, 2916–2935 (2015).
Deurloo, M. H. S. et al. Transcription factor 2I regulates neuronal development via TRPC3 in 7q11.23 disorder models. Mol. Neurobiol. 56, 3313–3325 (2019).
Wang, Y. et al. Dlx5 and Dlx6 regulate the development of parvalbumin-expressing cortical interneurons. J. Neurosci. 30, 5334–5345 (2010).
Poitras, L. et al. An SNP in an ultraconserved regulatory element affects Dlx5/Dlx6 regulation in the forebrain. Development 137, 3089–3097 (2010).
Barak, B. & Feng, G. Neurobiology of social behavior abnormalities in autism and Williams syndrome. Nat. Neurosci. 19, 647–655 (2016).
Becerra, A. M. & Mervis, C. B. Age at onset of declarative gestures and 24-month expressive vocabulary predict later language and intellectual abilities in young children with Williams syndrome. Front. Psychol. 10, 2648 (2019).
Richards, C., Jones, C., Groves, L., Moss, J. & Oliver, C. Prevalence of autism spectrum disorder phenomenology in genetic disorders: a systematic review and meta-analysis. Lancet Psychiat. 2, 909–916 (2015).
Klein-Tasman, B. P. & Mervis, C. B. Autism spectrum symptomatology among children with duplication 7q11.23 syndrome. J. Autism Dev. Disord. 48, 1982–1994 (2018).
Zanella, M. et al. Dosage analysis of the 7q11.23 Williams region identifies BAZ1B as a major human gene patterning the modern human face and underlying self-domestication. Sci. Adv. 5, eaaw7908 (2019).
Barnett, C. et al. Williams syndrome transcription factor is critical for neural crest cell function in Xenopus laevis. Mech. Dev. 129, 324–338 (2012).
Nagy, N. & Goldstein, A. M. Enteric nervous system development: a crest cell’s journey from neural tube to colon. Semin. Cell Dev. Biol. 66, 94–106 (2017).
Gregory, M. D. et al. Williams syndrome hemideletion and LIMK1 variation both affect dorsal stream functional connectivity. Brain 142, 3963–3974 (2019).
Ravindran, S., Nalavadi, V. C. & Muddashetty, R. S. BDNF induced translation of Limk1 in developing neurons regulates dendrite growth by fine-tuning cofilin1 activity. Front. Mol. Neurosci. 12, 64 (2019).
Todorovski, Z. et al. LIMK1 regulates long-term memory and synaptic plasticity via the transcriptional factor CREB. Mol. Cell. Biol. 35, 1316–1328 (2015).
Durdiakova, J., Warrier, V., Banerjee-Basu, S., Baron-Cohen, S. & Chakrabarti, B. STX1A and Asperger syndrome: a replication study. Mol. Autism 5, 14 (2014).
Sanchez-Mora, C. et al. Evaluation of common variants in 16 genes involved in the regulation of neurotransmitter release in ADHD. Eur. Neuropsychopharmacol. 23, 426–435 (2013).
Aslamy, A. & Thurmond, D. C. Exocytosis proteins as novel targets for diabetes prevention and/or remediation? Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R739–R752 (2017).
Andersson, S. A. et al. Reduced insulin secretion correlates with decreased expression of exocytotic genes in pancreatic islets from patients with type 2 diabetes. Mol. Cell Endocrinol. 364, 36–45 (2012).
Pober, B. R. et al. High prevalence of diabetes and pre-diabetes in adults with Williams syndrome. Am. J. Med. Genet. C. Semin. Med. Genet. 154C, 291–298 (2010).
Morigny, P. et al. Interaction between hormone-sensitive lipase and ChREBP in fat cells controls insulin sensitivity. Nat. Metab. 1, 133–146 (2019).
Vijayakumar, A. et al. Absence of carbohydrate response element binding protein in adipocytes causes systemic insulin resistance and impairs glucose transport. Cell Rep. 21, 1021–1035 (2017).
Mejhert, N. et al. Partitioning of MLX-family transcription factors to lipid droplets regulates metabolic gene expression. Mol. Cell 77, 1251–1264.e9 (2020).
Stenton, S. L. et al. Impaired complex I repair causes recessive Leber’s hereditary optic neuropathy. J. Clin. Invest. 131, e138267 (2021).
Tebbenkamp, A. T. N. et al. The 7q11.23 protein DNAJC30 interacts with ATP synthase and links mitochondria to brain development. Cell 175, 1088–1104.e23 (2018).
Pober, B. R. Williams-Beuren syndrome. N. Engl. J. Med. 362, 239–252 (2010).
Pober, B. R. & Morris, C. A. Diagnosis and management of medical problems in adults with Williams-Beuren syndrome. Am. J. Med. Genet. C. Semin. Med. Genet. 145C, 280–290 (2007).
Morris, C. A. in GeneReviews® (University of Washington, 1999).
Sindhar, S. et al. Hypercalcemia in patients with Williams-Beuren syndrome. J. Pediatr. 178, 254–260.e4 (2016). This is a large series showing a relatively low frequency of ‘actionable’ hypercalcaemia in children with WS, along with recommendations for medical management.
de Sousa Lima Strafacci, A., Fernandes Camargo, J., Bertapelli, F. & Guerra Junior, G. Growth assessment in children with Williams-Beuren syndrome: a systematic review. J. Appl. Genet. 61, 205–212 (2020).
Morris, C. A., Braddock, S. R. & Council On Genetics. Health care supervision for children with Williams syndrome. Pediatrics 145, e20193761 (2020). These are the most recent US general health-care recommendations for children with WS.
Martin, N. D., Smith, W. R., Cole, T. J. & Preece, M. A. New height, weight and head circumference charts for British children with Williams syndrome. Arch. Dis. Child. 92, 598–601 (2007).
Jehee, F. S. et al. Using a combination of MLPA kits to detect chromosomal imbalances in patients with multiple congenital anomalies and mental retardation is a valuable choice for developing countries. Eur. J. Med. Genet. 54, e425–e432 (2011).
Dutra, R. L. et al. Copy number variation in Williams-Beuren syndrome: suitable diagnostic strategy for developing countries. BMC Res. Notes 5, 13 (2012).
Lumaka, A. et al. Facial dysmorphism is influenced by ethnic background of the patient and of the evaluator. Clin. Genet. 92, 166–171 (2017).
Mishima, H. et al. Evaluation of Face2Gene using facial images of patients with congenital dysmorphic syndromes recruited in Japan. J. Hum. Genet. 64, 789–794 (2019).
Elmas, M. & Gogus, B. Success of face analysis technology in rare genetic diseases diagnosed by whole-exome sequencing: a single-center experience. Mol. Syndromol. 11, 4–14 (2020).
Scherer, S. W. et al. Observation of a parental inversion variant in a rare Williams-Beuren syndrome family with two affected children. Hum. Genet. 117, 383–388 (2005).
Kara-Mostefa, A. et al. Recurrent Williams-Beuren syndrome in a sibship suggestive of maternal germ-line mosaicism. Am. J. Hum. Genet. 64, 1475–1478 (1999).
Mulik, V. V., Temple, K. I. & Howe, D. T. Two pregnancies in a woman with Williams syndrome. BJOG 111, 511–512 (2004).
van der Tuuk, K., Drenthen, W., Moons, P. & Budts, W. Three live-birth pregnancies in a woman with Williams syndrome. Congenit. Heart Dis. 2, 139–142 (2007).
Yuan, M., Deng, L., Yang, Y. & Sun, L. Intrauterine phenotype features of fetuses with Williams-Beuren syndrome and literature review. Ann. Hum. Genet. 84, 169–176 (2020).
Stanley, T. L., Leong, A. & Pober, B. R. Growth, body composition, and endocrine issues in Williams syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 28, 64–74 (2021).
Riby, D. M. & Porter, M. A. Williams syndrome. Adv. Child. Dev. Behav. 39, 163–209 (2010).
Porter, M. A. In Magill’s Medical Guide (eds Auday, B., Buratovich, M., Marrocco, G., & Moglia, P.) 2385–2387 (Salem Press Inc., 2013).
Castro, T., de Paula Martins Santos, C., de Oliveira Lira Ortega, A. & Gallottini, M. Oral characteristics and medical considerations in the dental treatment of individuals with Williams syndrome. Spec. Care Dent. 39, 108–113 (2019).
Hertzberg, J., Nakisbendi, L., Needleman, H. L. & Pober, B. Williams syndrome–oral presentation of 45 cases. Pediatr. Dent. 16, 262–267 (1994).
Williams Syndrome Guideline Development Group. Management of Williams Syndrome. A Clinical Guideline. https://williams-syndrome.org.uk/wp-content/uploads/2018/07/williams_syndrome_guidelines_pdf.pdf (The Williams Syndrome Foundation UK, London, 2017).
Wu, F. Y. et al. Long-term surgical prognosis of primary supravalvular aortic stenosis repair. Ann. Thorac. Surg. 108, 1202–1209 (2019).
Rastelli, G. C., McGoon, D. C., Ongley, P. A., Mankin, H. T. & Kirklin, J. W. Surgical treatment of supravalvular aortic stenosis. Report of 16 cases and review of literature. J. Thorac. Cardiovasc. Surg. 51, 873–882 (1966).
McGoon, D. C., Mankin, H. T., Vlad, P. & Kirklin, J. W. The surgical treatment of supravalvular aortic stenosis. J. Thorac. Cardiovasc. Surg. 41, 123–133 (1961).
Doty, D. B., Polansky, D. B. & Jenson, C. B. Supravalvular aortic stenosis. Repair by extended aortoplasty. J. Thorac. Cardiovasc. Surg. 74, 362–371 (1977).
Brom, A. G. in Cardiac surgery: safeguards and pitfalls in operative technique (ed. Khonsari, S.) 276–280 (Aspen Publishers, 1988).
Kaushal, S., Backer, C. L., Patel, S., Gossett, J. G. & Mavroudis, C. Midterm outcomes in supravalvular aortic stenosis demonstrate the superiority of multisinus aortoplasty. Ann. Thorac. Surg. 89, 1371–1377 (2010).
Pham, P. P., Moller, J. H., Hills, C., Larson, V. & Pyles, L. Cardiac catheterization and operative outcomes from a multicenter consortium for children with Williams syndrome. Pediatr. Cardiol. 30, 9–14 (2009).
Brown, M. L., Nasr, V. G., Toohey, R. & DiNardo, J. A. Williams syndrome and anesthesia for non-cardiac surgery: high risk can be mitigated with appropriate planning. Pediatr. Cardiol. 39, 1123–1128 (2018).
Collins Ii, R. T., Collins, M. G., Schmitz, M. L. & Hamrick, J. T. Peri-procedural risk stratification and management of patients with Williams syndrome. Congenit. Heart Dis. 12, 133–142 (2017).
Hayashi, A. et al. Minoxidil stimulates elastin expression in aortic smooth muscle cells. Arch. Biochem. Biophys. 315, 137–141 (1994).
Slove, S. et al. Potassium channel openers increase aortic elastic fiber formation and reverse the genetically determined elastin deficit in the BN rat. Hypertension 62, 794–801 (2013).
Knutsen, R. H. et al. Minoxidil improves vascular compliance, restores cerebral blood flow, and alters extracellular matrix gene expression in a model of chronic vascular stiffness. Am. J. Physiol. Heart Circ. Physiol. 315, H18–H32 (2018).
Coquand-Gandit, M. et al. Chronic treatment with minoxidil induces elastic fiber neosynthesis and functional improvement in the aorta of aged mice. Rejuvenation Res. 20, 218–230 (2017).
Fhayli, W. et al. Chronic administration of minoxidil protects elastic fibers and stimulates their neosynthesis with improvement of the aorta mechanics in mice. Cell Signal. 62, 109333 (2019).
Kassai, B. et al. Minoxidil versus placebo in the treatment of arterial wall hypertrophy in children with Williams Beuren syndrome: a randomized controlled trial. BMC Pediatr. 19, 170 (2019).
Zhang, P. et al. Inhibition of microRNA-29 enhances elastin levels in cells haploinsufficient for elastin and in bioengineered vessels–brief report. Arterioscler. Thromb. Vasc. Biol. 32, 756–759 (2012).
French, J. W. & Guntheroth, W. G. An explanation of asymmetric upper extremity blood pressures in supravalvular aortic stenosis: the Coanda effect. Circulation 42, 31–36 (1970).
Cherniske, E. M. et al. Multisystem study of 20 older adults with Williams syndrome. Am. J. Med. Genet. A 131, 255–264 (2004). This article presents multisystem assessments on adults with WS, emphasizing the major medical conditions in adults with WS, and provides recommendations for monitoring guidelines.
Walton, J. R., Martens, M. A. & Pober, B. R. The proceedings of the 15th professional conference on Williams syndrome. Am. J. Med. Genet. A 173, 1159–1171 (2017).
Halabi, C. M. et al. Chronic antihypertensive treatment improves pulse pressure but not large artery mechanics in a mouse model of congenital vascular stiffness. Am. J. Physiol. Heart Circ. Physiol. 309, H1008–H1016 (2015).
Ferrari, M. & Stagi, S. Oxidative stress in Down and Williams-Beuren syndromes: an overview. Molecules 26, 3139 (2021).
Martens, M. A., Wilson, S. J. & Reutens, D. C. Research review: Williams syndrome: a critical review of the cognitive, behavioral, and neuroanatomical phenotype. J. Child. Psychol. Psychiat. 49, 576–608 (2008).
Miezah, D. et al. Cognitive profile of young children with Williams syndrome. J. Intellect. Disabil. Res. https://doi.org/10.1111/jir.12860 (2021).
Meyer-Lindenberg, A., Mervis, C. B. & Berman, K. F. Neural mechanisms in Williams syndrome: a unique window to genetic influences on cognition and behaviour. Nat. Rev. Neurosci. 7, 380–393 (2006).
Mervis, C. B. & Pitts, C. H. Children with Williams syndrome: developmental trajectories for intellectual abilities, vocabulary abilities, and adaptive behavior. Am. J. Med. Genet. C. Semin. Med. Genet. 169, 158–171 (2015).
Porter, M. & Dodd, H. A longitudinal study of cognitive abilities in Williams syndrome. Dev. Neuropsychol. 36, 255–272 (2011).
Fisher, M. H., Lense, M. D. & Dykens, E. M. Longitudinal trajectories of intellectual and adaptive functioning in adolescents and adults with Williams syndrome. J. Intellect. Disabil. Res. 60, 920–932 (2016).
Mervis, C. B., Kistler, D. J., John, A. E. & Morris, C. A. Longitudinal assessment of intellectual abilities of children with Williams syndrome: multilevel modeling of performance on the Kaufman brief intelligence test-second edition. Am. J. Intellect. Dev. Disabil. 117, 134–155 (2012).
Zitzer-Comfort, C., Doyle, T., Masataka, N., Korenberg, J. & Bellugi, U. Nature and nurture: Williams syndrome across cultures. Dev. Sci. 10, 755–762 (2007).
Leyfer, O. T., Woodruff-Borden, J., Klein-Tasman, B. P., Fricke, J. S. & Mervis, C. B. Prevalence of psychiatric disorders in 4 to 16-year-olds with Williams syndrome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 141B, 615–622 (2006). Clinical diagnostic interview of children and teens with WS that demonstrated a high frequency of ADHD and an increasing frequency of generalized anxiety disorder with age.
Perez-Garcia, D., Brun-Gasca, C., Perez-Jurado, L. A. & Mervis, C. B. Behavioral profiles of children with Williams syndrome from Spain and the United States: cross-cultural similarities and differences. Am. J. Intellect. Dev. Disabil. 122, 156–172 (2017).
Tomc, S. A., Williamson, N. K. & Pauli, R. M. Temperament in Williams syndrome. Am. J. Med. Genet. 36, 345–352 (1990).
Vonarnim, G. & Engel, P. Mental retardation related to hypercalcaemia. Dev. Med. Child. Neurol. 6, 366–377 (1964).
Thurman, A. J. & Fisher, M. H. The Williams syndrome social phenotypes: disentangling the contributions of social interest and social difficulties. Int. Rev. Res. Dev. Disabil. 49, 191–227 (2015).
American Psychiatric Association. DSM-4: Diagnostic and Statistical Manual of Mental Disorders 4th ed. (American Psychiatric Association, 2000).
Copes, L. E., Pober, B. R. & Terilli, C. A. Description of common musculoskeletal findings in Williams syndrome and implications for therapies. Clin. Anat. 29, 578–589 (2016).
Van Herwegen, J., Ashworth, M. & Palikara, O. Parental views on special educational needs provision: cross-syndrome comparisons in Williams syndrome, Down syndrome, and autism spectrum disorders. Res. Dev. Disabil. 80, 102–111 (2018).
Klein-Tasman, B. P., van der Fluit, F. & Mervis, C. B. Autism spectrum symptomatology in children with Williams syndrome who have phrase speech or fluent language. J. Autism Dev. Disord. 48, 3037–3050 (2018).
Klein-Tasman, B. P. & Albano, A. M. Intensive, short-term cognitive-behavioral treatment of OCD-like behavior with a young adult with Williams syndrome. Clin. Case Stud. 6, 483–492 (2017).
Phillips, K. D. & Klein-Tasman, B. P. Mental health concerns in Williams syndrome: Intervention considerations and illustrations from case examples. J. Ment. Health Res. Intellect. Disabil. 2, 110–133 (2009).
Thom, R. P., Keary, C. J., Waxler, J. L., Pober, B. R. & McDougle, C. J. Buspirone for the treatment of generalized anxiety disorder in Williams syndrome: a case series. J. Autism Dev. Disord. 50, 676–682 (2020).
Green, T. et al. Phenotypic psychiatric characterization of children with Williams syndrome and response of those with ADHD to methylphenidate treatment. Am. J. Med. Genet. B Neuropsychiatr. Genet. 159B, 13–20 (2012).
Thom, R. P., Pober, B. R. & McDougle, C. J. Psychopharmacology of Williams syndrome: safety, tolerability, and effectiveness. Expert Opin. Drug Saf. 20, 293–306 (2020).
Reilly, C., Senior, J. & Murtagh, L. A comparative study of educational provision for children with neurogenetic syndromes: parent and teacher survey. J. Intellect. Disabil. Res. 59, 1094–1107 (2015).
UNESCO. Education and disability: analysis of data from 49 countries (Information Paper N. 49) (UNESCO, 2018).
Karr, V., Hayes, A. & Hayford, S. Inclusion of children with learning difficulties in literacy and numeracy in Ghana: a literature review. Int. J. Disabil. Dev. Educ. https://doi.org/10.1080/1034912X.2020.1792419 (2020).
Fisher, M. H., Josol, C. K. & Shivers, C. M. An examination of social skills, friendship quality, and loneliness for adults with Williams syndrome. J. Autism Dev. Disord. 50, 3649–3660 (2020).
Howlin, P. & Udwin, O. Outcome in adult life for people with Williams syndrome – results from a survey of 239 families. J. Intellect. Disabil. Res. 50, 151–160 (2006).
Pagon, R. A., Bennett, F. C., LaVeck, B., Stewart, K. B. & Johnson, J. Williams syndrome: features in late childhood and adolescence. Pediatrics 80, 85–91 (1987).
Brawn, G., Kohnen, S., Tassabehji, M. & Porter, M. Functional basic reading skills in Williams syndrome. Dev. Neuropsychol. 43, 454–477 (2018).
Levy, Y. & Antebi, V. Word reading and reading-related skills in Hebrew-speaking adolescents with Williams syndrome. Neurocase 10, 444–451 (2004).
Mervis, C. B. Language and literacy development of children with Williams syndrome. Top. Lang. Disord. 29, 149–169 (2009).
Mervis, C. B., Greiner de Magalhães, C. & Cardoso-Martins, C. Concurrent predictors of word reading and reading comprehension for 9-year-olds with Williams syndrome. Read. Writ. https://doi.org/10.1007/s11145-021-10163-4 (2021).
Van Herwegen, J. & Simms, V. Mathematical development in Williams syndrome: a systematic review. Res. Dev. Disabil. 100, 103609 (2020).
Brawn, G. & Porter, M. Adaptive functioning in Williams syndrome: a systematic review. Int. J. Disabil. Dev. Educ. 65, 123–147 (2018).
Pitts, C. H., Klein-Tasman, B. P., Osborne, J. W. & Mervis, C. B. Predictors of specific phobia in children with Williams syndrome. J. Intellect. Disabil. Res. 60, 1031–1042 (2016).
Edgin, J. O., Pennington, B. F. & Mervis, C. B. Neuropsychological components of intellectual disability: the contributions of immediate, working, and associative memory. J. Intellect. Disabil. Res. 54, 406–417 (2010).
Fu, T. J., Lincoln, A. J., Bellugi, U. & Searcy, Y. M. The association of intelligence, visual-motor functioning, and personality characteristics with adaptive behavior in individuals with Williams syndrome. Am. J. Intellect. Dev. Disabil. 120, 273–288 (2015).
Palikara, O., Ashworth, M. & Van Herwegen, J. Addressing the educational needs of children with Williams syndrome: a rather neglected area of research? J. Autism Dev. Disord. 48, 3256–3259 (2018).
Gillooly, A. E., Riby, D. M., Durkin, K. & Rhodes, S. M. Peer relationships in children with Williams syndrome: parent and teacher insights. J. Autism Dev. Disord. 51, 169–178 (2020).
Lough, E. & Fisher, M. H. Parent and self-report ratings on the perceived levels of social vulnerability of adults with Williams syndrome. J. Autism Dev. Disord. 46, 3424–3433 (2016).
Fisher, M. H., Moskowitz, A. L. & Hodapp, R. M. Differences in social vulnerability among individuals with autism spectrum disorder, Williams syndrome, and down syndrome. Res. Autism Spectr. Disord. 7, 931–937 (2013).
Ridley, E., Riby, D. M. & Leekam, S. R. A cross-syndrome approach to the social phenotype of neurodevelopmental disorders: Focusing on social vulnerability and social interaction style. Res. Dev. Disabil. 100, 103604 (2020).
Fisher, M. H. Evaluation of a stranger safety training programme for adults with Williams syndrome. J. Intellect. Disabil. Res. 58, 903–914 (2014).
Riby, D. M., Kirk, H., Hanley, M. & Riby, L. M. Stranger danger awareness in Williams syndrome. J. Intellect. Disabil. Res. 58, 572–582 (2014).
Fisher, M. H. & Morin, L. Addressing social skills deficits in adults with Williams syndrome. Res. Dev. Disabil. 71, 77–87 (2017).
Morris, C. A., Demsey, S. A., Leonard, C. O., Dilts, C. & Blackburn, B. L. Natural history of Williams syndrome: physical characteristics. J. Pediatr. 113, 318–326 (1988). A hallmark paper in delineating the natural history of WS, in both children and adults.
Udwin, O., Howlin, P., Davies, M. & Mannion, E. Community care for adults with Williams syndrome: how families cope and the availability of support networks. J. Intellect. Disabil. Res. 42, 238–245 (1998).
Pao, M. & Bosk, A. Anxiety in medically ill children/adolescents. Depress. Anxiety 28, 40–49 (2011).
American Heart Association. American Heart Association Recommendations for Physical Activity in Adults and Kids https://www.heart.org/en/healthy-living/fitness/fitness-basics/aha-recs-for-physical-activity-in-adults (2018).
CDC National Center on Birth Defects and Developmental Disabilities. Physical Activity for People with Disability https://www.cdc.gov/ncbddd/disabilityandhealth/features/physical-activity-for-all.html (2020).
Leyfer, O., Woodruff-Borden, J. & Mervis, C. B. Anxiety disorders in children with williams syndrome, their mothers, and their siblings: implications for the etiology of anxiety disorders. J. Neurodev. Disord. 1, 4–14 (2009).
Papaeliou, C. et al. Behavioural profile and maternal stress in Greek young children with Williams syndrome. Child Care Health Dev. 38, 844–853 (2012).
Sarimski, K. Behavioural phenotypes and family stress in three mental retardation syndromes. Eur. Child. Adolesc. Psychiat. 6, 26–31 (1997).
John, A. E. & Mervis, C. B. Sensory modulation impairments in children with Williams syndrome. Am. J. Med. Genet. C. Semin. Med. Genet. 154C, 266–276 (2010).
Fidler, D. J., Hodapp, R. M. & Dykens, E. M. Stress in families of young children with Down syndrome, Williams syndrome, and Smith-Magenis syndrome. Early Educ. Dev. 11, 395–406 (2000).
Reilly, C., Murtagh, L. & Senior, J. The impact on the family of four neurogenetic syndromes: a comparative study of parental views. J. Genet. Couns. 24, 851–861 (2015).
Ashworth, M., Palikara, O. & Van Herwegen, J. Comparing parental stress of children with neurodevelopmental disorders: the case of Williams syndrome, Down syndrome and autism spectrum disorders. J. Appl. Res. Intellect. Disabil. 32, 1047–1057 (2019).
Nir, A. & Barak, B. White matter alterations in Williams syndrome related to behavioral and motor impairments. Glia 69, 5–19 (2021).
Chailangkarn, T. et al. A human neurodevelopmental model for Williams syndrome. Nature 536, 338–343 (2016).
Lalli, M. A. et al. Haploinsufficiency of BAZ1B contributes to Williams syndrome through transcriptional dysregulation of neurodevelopmental pathways. Hum. Mol. Genet. 25, 1294–1306 (2016).
Kinnear, C. et al. Modeling and rescue of the vascular phenotype of Williams-Beuren syndrome in patient induced pluripotent stem cells. Stem Cell Transl. Med. 2, 2–15 (2013).
Amin, N. D. & Pasca, S. P. Building models of brain disorders with three-dimensional organoids. Neuron 100, 389–405 (2018).
Baldassari, S. et al. Brain organoids as model systems for genetic neurodevelopmental disorders. Front. Cell Dev. Biol. 8, 590119 (2020).
Wimmer, R. A. et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 565, 505–510 (2019).
Huang, A. H. et al. Biaxial stretch improves elastic fiber maturation, collagen arrangement, and mechanical properties in engineered arteries. Tissue Eng. C. Methods 22, 524–533 (2016).
Liu, C., Niu, K. & Xiao, Q. Updated perspectives on vascular cell specification and pluripotent stem cell-derived vascular organoids for studying vasculopathies. Cardiovasc. Res. https://doi.org/10.1093/cvr/cvaa313 (2020).
Ellis, M. W., Luo, J. & Qyang, Y. Modeling elastin-associated vasculopathy with patient induced pluripotent stem cells and tissue engineering. Cell Mol. Life Sci. 76, 893–901 (2019).
Kurki, M. I. et al. Contribution of rare and common variants to intellectual disability in a sub-isolate of northern Finland. Nat. Commun. 10, 410 (2019).
Magavern, E. F. et al. An academic clinician’s road map to hypertension genomics: recent advances and future directions MMXX. Hypertension 77, 284–295 (2021).
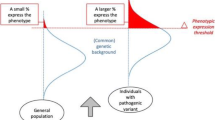
Girirajan, S. & Eichler, E. E. Phenotypic variability and genetic susceptibility to genomic disorders. Hum. Mol. Genet. 19, R176–R187 (2010).
Levitin, D. J., Cole, K., Lincoln, A. & Bellugi, U. Aversion, awareness, and attraction: investigating claims of hyperacusis in the Williams syndrome phenotype. J. Child. Psychol. Psychiat. 46, 514–523 (2005).
Levy, G. & Barak, B. Postnatal therapeutic approaches in genetic neurodevelopmental disorders. Neural Regen. Res. 16, 414–422 (2021).
Powell, S. K., Gregory, J., Akbarian, S. & Brennand, K. J. Application of CRISPR/Cas9 to the study of brain development and neuropsychiatric disease. Mol. Cell. Neurosci. 82, 157–166 (2017).
Ramdas, S. & Servais, L. New treatments in spinal muscular atrophy: an overview of currently available data. Expert Opin. Pharmacother. 21, 307–315 (2020).
Oetjens, M. T., Kelly, M. A., Sturm, A. C., Martin, C. L. & Ledbetter, D. H. Quantifying the polygenic contribution to variable expressivity in eleven rare genetic disorders. Nat. Commun. 10, 4897 (2019).
Osborne, L. R. & Mervis, C. B. 7q11.23 deletion and duplication. Curr. Opin. Genet. Dev. 68, 41–48 (2021).
Wing, L. & Gould, J. Severe impairments of social interaction and associated abnormalities in children: epidemiology and classification. J. Autism Dev. Disord. 9, 11–29 (1979).
Schubert, C. The genomic basis of the Williams-Beuren syndrome. Cell Mol. Life Sci. 66, 1178–1197 (2009).
Scherer, S. W. & Osborne, L. R. in Genomic disorders. The genomic basis of disease. (eds Lupinski, J. R. & Stankiewicz, P.) 221–236 (Humana Press, Totowa, NJ, 2006).
Masataka, N. Why early developmental milestones are delayed in children with Williams syndrome: late onset of hand banging as a possible rate-limiting constraint on the emergence of canonical babbling. Dev. Sci. 4, 158–164 (2001).
Plissart, L. & Fryns, J. P. Early development (5 to 48 months) in Williams syndrome. A study of 14 children. Genet. Couns. 10, 151–156 (1999).
Sarimski, K. Early development of children with Williams syndrome. Genet. Couns. 10, 141–150 (1999).
WHO Multicentre Growth Reference Study Group. WHO motor development study: windows of achievement for six gross motor development milestones. Acta Paediatr. Suppl. 450, 86–95 (2006).
Bayley, N. Manual for the Bayley Scales of Infant Development 2nd edn, (Psychological Corp., 1993).
Fenson, L. et al. Variability in early communicative development. Monogr. Soc. Res. Child. Dev. 59, 1–173 (1994).
Fenson, L. et al. MacArthur-Bates Communicative Development Inventories: User’s guide and technical manual 2nd edn, (Brookes, 2007).
Mervis, C. B., Becerra, A. M., Pitts, C. H. & Marchman, V. A. in Symposium on Research in Child Language Disorders (Madison, WI, 2019).
Weisberg, D. S. Pretend play. Wiley Interdiscip. Rev. Cogn. Sci. 6, 249–261 (2015).
Lincoln, A. J., Searcy, Y. M., Jones, W. & Lord, C. Social interaction behaviors discriminate young children with autism and Williams syndrome. J. Am. Acad. Child. Adolesc. Psychiat. 46, 323–331 (2007).
Dodd, H. F. & Porter, M. A. Psychopathology in Williams syndrome: the effect of individual differences across the life span. J. Ment. Health Res. Intellect. Disabil. 2, 89–109 (2009). Clinical diagnostic interview of psychopathology and behaviour, as opposed to just a symptom checklist. This study looks at children and adults separately.
Dykens, E. M. Anxiety, fears, and phobias in persons with Williams syndrome. Dev. Neuropsychol. 23, 291–316 (2003).
Kennedy, J. C., Kaye, D. L. & LS, S. Psychiatric diagnoses in patients with Williams syndrome and their families. Jefferson J. Psychiat. 20, 22–31 (2006).
Zarchi, O. et al. A comparative study of the neuropsychiatric and neurocognitive phenotype in two microdeletion syndromes: velocardiofacial (22q11.2 deletion) and Williams (7q11.23 deletion) syndromes. Eur. Psychiat. 29, 203–210 (2014).
Lord, C. et al. Autism spectrum disorder. Nat. Rev. Dis. Prim. 6, 5 (2020).
Acknowledgements
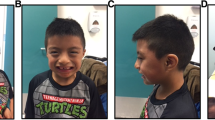
The authors thank the anonymous individual with WS and her parents who provided the family experience interview as well as those who provided facial photographs for Fig. 4 and Supplementary Table 1. The authors thank the persons with WS and their families belonging to the Associação Brasileira de Síndrome de Williams, Canadian Association for Williams Syndrome, WIlliams Syndrome Australia Limited, and Williams Syndrome Association (USA) for continued participation in studies and the support of research that allows the knowledge of WS to grow. The authors thank Z. Urban for discussion of elastin biology and management strategies for vascular disease in WS. B.A.K. was supported by the Department of Intramural Research at the National Heart, Lung and Blood Institute of the National Institutes of Health. C.B.M. was supported by the Williams Syndrome Association (grant number 0111). B.R.P. was supported by the Williams Syndrome Association and the Williams Syndrome Charitable Foundation, USA. M.P. was funded by Williams Syndrome Australia Limited. B.B. was funded by the Fritz Thyssen Stiftung and the Israel Science Foundation (Grant No.2305/20). L.R.O. was supported by a Canada Research Chair in the Genetics of Neurodevelopmental Disorders. C.A.K. was supported by FAPESP 2019/21644-0 and CNPq 304897/2020-5.
Author information
Authors and Affiliations
Contributions
B.A.K. and B.R.P. developed the outline for the manuscript, engaged the contributors and synthesized the final manuscript. All authors participated in the research, writing, and editing of the document and all approved the final version of the manuscript. C.A.K. provided clinical photos and M.P. conducted the family experience interview.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Additional information
Informed consent
The authors affirm that human research participants provided informed consent for: publication of the photographs in Fig. 4 and Supplementary Table 1 and taking part in the family experience interview.
Peer review information
Nature Reviews Disease Primers thanks A. Selicorni, D. Gothelf, J. Van Herwegen, P. Ortiz-Romero, who co-reviewed with V. Campuzano, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
NCBI Gene: https://www.ncbi.nlm.nih.gov/gene/
Supplementary information
Rights and permissions
About this article
Cite this article
Kozel, B.A., Barak, B., Kim, C.A. et al. Williams syndrome. Nat Rev Dis Primers 7, 42 (2021). https://doi.org/10.1038/s41572-021-00276-z
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-021-00276-z
- Springer Nature Limited
This article is cited by
-
The role of social motivation in sharing and fairness: insights from Williams syndrome
Journal of Neurodevelopmental Disorders (2024)
-
Concurrent predictors of mathematics achievement for 9-year-old children with Williams syndrome
Scientific Reports (2024)
-
Prenatal diagnosis and molecular cytogenetic characterization of fetuses with central nervous system anomalies using chromosomal microarray analysis: a seven-year single-center retrospective study
Scientific Reports (2024)
-
Contrasting neurofunctional correlates of face- and visuospatial-processing in children and adolescents with Williams syndrome: convergent results from four fMRI paradigms
Scientific Reports (2024)
-
ChIP-seq analysis found IL21R, a target gene of GTF2I–the susceptibility gene for primary biliary cholangitis in Chinese Han
Hepatology International (2024)