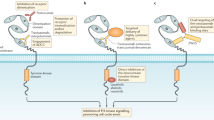
Abstract
The receptor tyrosine-kinase HER2 (also known as ErbB2) is a well-established therapeutic target in patients with breast or gastric cancer selected on the basis of HER2 overexpression on immunohistochemistry and/or ERBB2 amplification on in situ hybridization. With advances in cancer molecular profiling and increased implementation of precision medicine approaches into oncology practice, actionable HER2 alterations in solid tumours have expanded to include ERBB2 mutations in addition to traditional HER2 overexpression and ERBB2 amplification. These various HER2 alterations can be found in solid tumour types beyond breast and gastric cancer, although few HER2-targeted therapeutic options have been established for the other tumour types. Nevertheless, during the 5 years since our previous Review on this topic was published in this journal, obvious and fruitful progress in the development of HER2-targeted therapies has been made, including new disease indications, innovative drugs with diverse mechanisms of action and novel frameworks for approval by regulatory authorities. These advances have culminated in the recent histology-agnostic approval of the anti-HER2 antibody–drug conjugate trastuzumab deruxtecan for patients with HER2-overexpressing solid tumours. In this new Review, we provide an update on the current development landscape of HER2-targeted therapies beyond breast cancer, as well as anticipated future HER2-directed treatment strategies to overcome resistance and thereby improve efficacy and patient outcomes.
Key points
-
Over the past few years, the indications for HER2-targeted therapy have expanded beyond breast cancer and gastric or gastroesophageal junction cancer (G/GEJC) to include various other solid tumour types.
-
The anti-HER2 antibody–drug conjugate trastuzumab deruxtecan has become a new standard of care for patients with treatment-refractory HER2-positive G/GEJC, HER2-mutant non-small-cell lung cancer or any HER2-overexpressing (immunohistochemistry 3+) solid tumour, owing to its potent antitumour activity and impressive clinical efficacy.
-
Ten years after the ToGA study established the anti-HER2 antibody trastuzumab in combination with chemotherapy as the standard of care for patients with previously untreated advanced-stage HER2-positive G/GEJC, the addition of an immune-checkpoint inhibitor (the anti-PD-1 antibody pembrolizumab) to this regimen has presented a new first-line treatment option for this disease.
-
Several potential mechanisms of resistance to HER2-targeted therapies have been identified, such as alterations that impaired drug binding to HER2 or that constitutively activate signalling pathways downstream of or parallel to HER2.
-
Novel agents targeting HER2 and new combinations HER2-target therapies with various agents, including inhibitors of other receptor tyrosine kinases, immunotherapies and DNA damage repair inhibitors, are under investigation in clinical trials.




Similar content being viewed by others
References
Popescu, N. C., King, C. R. & Kraus, M. H. Localization of the human erbB-2 gene on normal and rearranged chromosomes 17 to bands q12-21.32. Genomics 4, 362–366 (1989).
Citri, A. & Yarden, Y. EGF-ERBB signalling: towards the systems level. Nat. Rev. Mol. Cell Biol. 7, 505–516 (2006).
Gutierrez, C. & Schiff, R. HER2: biology, detection, and clinical implications. Arch. Pathol. Lab. Med. 135, 55–62 (2011).
Oh, D. Y. & Bang, Y. J. HER2-targeted therapies — a role beyond breast cancer. Nat. Rev. Clin. Oncol. 17, 33–48 (2020).
Strickler, J. H. et al. Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): a multicentre, open-label, phase 2 study. Lancet Oncol. 24, 496–508 (2023).
FDA. FDA grants accelerated approval to tucatinib with trastuzumab for colorectal cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-tucatinib-trastuzumab-colorectal-cancer#:~:Text=FDA%20grants%20accelerated%20approval%20to%20tucatinib%20with%20trastuzumab%20for%20colorectal%20cancer,-Share&Text=On%20January%2019%2C%202023%2C%20the,(Tukysa%2C%20Seagen%20Inc (2023).
FDA. FDA grants accelerated approval to fam-trastuzumab deruxtecan-nxki for unresectable or metastatic HER2-positive solid tumors. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2 (2024).
Bang, Y. J. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 (2010).
Jorgensen, J. T. & Hersom, M. HER2 as a prognostic marker in gastric cancer — a systematic analysis of data from the literature. J. Cancer 3, 137–144 (2012).
Uchôa, B. C. D. M. et al. HER2-low and gastric cancer: a prognostic biomarker? J. Clin. Oncol. https://doi.org/10.1200/JCO.2021.39.15_suppl.e16086 (2021).
Riudavets, M., Sullivan, I., Abdayem, P. & Planchard, D. Targeting HER2 in non-small-cell lung cancer (NSCLC): a glimpse of hope? An updated review on therapeutic strategies in NSCLC harbouring HER2 alterations. ESMO Open 6, 100260 (2021).
Yoshizawa, A. et al. HER2 status in lung adenocarcinoma: a comparison of immunohistochemistry, fluorescence in situ hybridization (FISH), dual-ISH, and gene mutations. Lung Cancer 85, 373–378 (2014).
Connell, C. M. & Doherty, G. J. Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO Open 2, e000279 (2017).
Cocco, E., Lopez, S., Santin, A. D. & Scaltriti, M. Prevalence and role of HER2 mutations in cancer. Pharmacol. Ther. 199, 188–196 (2019).
Chmielecki, J. et al. Oncogenic alterations in ERBB2/HER2 represent potential therapeutic targets across tumors from diverse anatomic sites of origin. Oncologist 20, 7–12 (2015).
Li, B. T. et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N. Engl. J. Med. 386, 241–251 (2022).
Goto, K. et al. Trastuzumab deruxtecan in patients with HER2-mutant metastatic non-small-cell lung cancer: primary results from the randomized, phase II DESTINY-lung02 trial. J. Clin. Oncol. 41, 4852–4863 (2023).
FDA. FDA grants accelerated approval to fam-trastuzumab deruxtecan-nxki for HER2-mutant non-small cell lung cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-her2-mutant-non-small-cell-lung (2022).
Zabransky, D. J. et al. HER2 missense mutations have distinct effects on oncogenic signaling and migration. Proc. Natl Acad. Sci. USA 112, E6205–E6214 (2015).
Shigematsu, H. et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res. 65, 1642–1646 (2005).
Greulich, H. et al. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc. Natl Acad. Sci. USA 109, 14476–14481 (2012).
Meric-Bernstam, F. et al. Advances in HER2-targeted therapy: novel agents and opportunities beyond breast and gastric cancer. Clin. Cancer Res. 25, 2033–2041 (2019).
Xia, X., Gong, C., Zhang, Y. & Xiong, H. The history and development of HER2 inhibitors. Pharmaceuticals 16, 1450 (2023).
Banerji, U. et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 20, 1124–1135 (2019).
Xu, Y. et al. Phase I study of the recombinant humanized anti-HER2 monoclonal antibody–MMAE conjugate RC48-ADC in patients with HER2-positive advanced solid tumors. Gastric Cancer 24, 913–925 (2021).
Weisser, N. E. et al. An anti-HER2 biparatopic antibody that induces unique HER2 clustering and complement-dependent cytotoxicity. Nat. Commun. 14, 1394 (2023).
Xu, J. et al. KN026 (anti-HER2 bispecific antibody) in patients with previously treated, advanced HER2-expressing gastric or gastroesophageal junction cancer. Eur. J. Cancer 178, 1–12 (2023).
Back, J. et al. GBR1302: effect of CD3-HER2, a bispecific T cell engager antibody, in trastuzumab-resistant cancers. J. Clin. Oncol. https://doi.org/10.1200/JCO.2018.36.15_suppl.120 (2018).
Rudnik, M. et al. Combined investigation of anti-tumor efficacy and liver safety of bispecific T cell engagers in immune-competent high-throughput co-culturing platform. Cancer Res. 83, 2747 (2023).
Kim, K. M. et al. Human epidermal growth factor receptor 2 testing in gastric cancer: recommendations of an Asia-Pacific task force. Asia Pac. J. Clin. Oncol. 10, 297–307 (2014).
Valtorta, E. et al. Assessment of a HER2 scoring system for colorectal cancer: results from a validation study. Mod. Pathol. 28, 1481–1491 (2015).
Buza, N., English, D. P., Santin, A. D. & Hui, P. Toward standard HER2 testing of endometrial serous carcinoma: 4-year experience at a large academic center and recommendations for clinical practice. Mod. Pathol. 26, 1605–1612 (2013).
Lamarca, A. et al. The HER3 pathway as a potential target for inhibition in patients with biliary tract cancers. PLoS ONE 13, e0206007 (2018).
Meric-Bernstam, F. et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 phase II trial. J. Clin. Oncol. 42, 47–58 (2024).
DiPeri, T. P. et al. Discordance of HER2 expression and/or amplification on repeat testing. Mol. Cancer Ther. 22, 976–984 (2023).
Hashimoto, T. et al. A comprehensive appraisal of HER2 heterogeneity in HER2-amplified and HER2-low colorectal cancer. Br. J. Cancer 129, 1176–1183 (2023).
Zehir, A. et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 23, 703–713 (2017).
Hyman, D. M. et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554, 189–194 (2018).
Wu, L. et al. Landscape of somatic alterations in large-scale solid tumors from an Asian population. Nat. Commun. 13, 4264 (2022).
Bontoux, C. et al. Deciphering the impact of HER2 alterations on non-small-cell lung cancer: from biological mechanisms to therapeutic approaches. J. Pers. Med. 12, 1651 (2022).
Vaghi, C. et al. The predictive role of ERBB2 point mutations in metastatic colorectal cancer: a systematic review. Cancer Treat. Rev. 112, 102488 (2023).
Li, B. T. et al. Trastuzumab deruxtecan in patients with solid tumours harbouring specific activating HER2 mutations (DESTINY-PanTumor01): an international, phase 2 study. Lancet Oncol. 25, 707–719 (2024).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387, 9–20 (2022).
Uzunparmak, B. et al. HER2-low expression in patients with advanced or metastatic solid tumors. Ann. Oncol. 34, 1035–1046 (2023).
Yamaguchi, K. et al. Trastuzumab deruxtecan in anti-human epidermal growth factor receptor 2 treatment-naive patients with human epidermal growth factor receptor 2-low gastric or gastroesophageal junction adenocarcinoma: exploratory cohort results in a phase II trial. J. Clin. Oncol. 41, 816–825 (2023).
Ohba, A. et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): an investigator-initiated multicenter phase 2 study (HERB trial). J. Clin. Oncol. https://doi.org/10.1200/JCO.2022.40.16_suppl.4006 (2022).
Nishikawa, T. et al. Trastuzumab deruxtecan for human epidermal growth factor receptor 2-expressing advanced or recurrent uterine carcinosarcoma (NCCH1615): the STATICE trial. J. Clin. Oncol. 41, 2789–2799 (2023).
Yoshino, T. et al. Final results of DESTINY-CRC01 investigating trastuzumab deruxtecan in patients with HER2-expressing metastatic colorectal cancer. Nat. Commun. 14, 3332 (2023).
Meric-Bernstam, F. et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 20, 518–530 (2019).
Sartore-Bianchi, A. et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 17, 738–746 (2016).
Leyland-Jones, B. Trastuzumab: hopes and realities. Lancet Oncol. 3, 137–144 (2002).
Nahta, R. & Esteva, F. J. Trastuzumab: triumphs and tribulations. Oncogene 26, 3637–3643 (2007).
Fader, A. N. et al. Randomized phase II trial of carboplatin–paclitaxel versus carboplatin–paclitaxel–trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2/neu. J. Clin. Oncol. 36, 2044–2051 (2018).
Fader, A. N. et al. Randomized phase II trial of carboplatin-paclitaxel compared with carboplatin-paclitaxel-trastuzumab in advanced (stage III–IV) or recurrent uterine serous carcinomas that overexpress Her2/Neu (NCT01367002): updated overall survival analysis. Clin. Cancer Res. 26, 3928–3935 (2020).
Takahashi, H. et al. Phase II trial of trastuzumab and docetaxel in patients with human epidermal growth factor receptor 2-positive salivary duct carcinoma. J. Clin. Oncol. 37, 125–134 (2019).
Nam, A. R. et al. Therapeutic implication of HER2 in advanced biliary tract cancer. Oncotarget 7, 58007–58021 (2016).
Lee, C. K. et al. Trastuzumab plus FOLFOX for HER2-positive biliary tract cancer refractory to gemcitabine and cisplatin: a multi-institutional phase 2 trial of the Korean Cancer Study Group (KCSG-HB19-14). Lancet Gastroenterol. Hepatol. 8, 56–65 (2023).
Kim, M. et al. Phase II study of a trastuzumab biosimilar in combination with paclitaxel for HER2-positive recurrent or metastatic urothelial carcinoma: KCSG GU18-18. ESMO Open 8, 101588 (2023).
Barthelemy, P., Leblanc, J., Goldbarg, V., Wendling, F. & Kurtz, J. E. Pertuzumab: development beyond breast cancer. Anticancer Res. 34, 1483–1491 (2014).
Oh, D. Y. & Bang, Y. J. Pertuzumab in gastrointestinal cancer. Expert Opin. Biol. Ther. 16, 243–253 (2016).
Tabernero, J. et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 19, 1372–1384 (2018).
Tabernero, J. et al. Pertuzumab, trastuzumab, and chemotherapy in HER2-positive gastric/gastroesophageal junction cancer: end-of-study analysis of the JACOB phase III randomized clinical trial. Gastric Cancer 26, 123–131 (2023).
Javle, M. et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 22, 1290–1300 (2021).
Sweeney, C. J. et al. MyPathway human epidermal growth factor receptor 2 basket study: pertuzumab + trastuzumab treatment of a tissue-agnostic cohort of patients with human epidermal growth factor receptor 2-altered advanced solid tumors. J. Clin. Oncol. 42, 258–265 (2024).
Markham, A. Margetuximab: first approval. Drugs 81, 599–604 (2021).
Bang, Y. J. et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann. Oncol. 28, 855–861 (2017).
Catenacci, D. V. T. et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b-2 trial. Lancet Oncol. 21, 1066–1076 (2020).
Catenacci, D. V. T. et al. Margetuximab with retifanlimab as first-line therapy in HER2+/PD-L1+ unresectable or metastatic gastroesophageal adenocarcinoma: MAHOGANY cohort A. ESMO Open 7, 100563 (2022).
Voigtlaender, M., Schneider-Merck, T. & Trepel, M. Lapatinib. Recent Results Cancer Res. 211, 19–44 (2018).
Satoh, T. et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN — a randomized, phase III study. J. Clin. Oncol. 32, 2039–2049 (2014).
Hecht, J. R. et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC — a randomized phase III trial. J. Clin. Oncol. 34, 443–451 (2016).
Bose, R. et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 3, 224–237 (2013).
Friedman, C. F. et al. Targeting HER2-mutant metastatic cervical cancer with neratinib: final results from the phase 2 SUMMIT basket trial. Gynecol. Oncol. 181, 162–169 (2024).
Harding, J. J. et al. Antitumour activity of neratinib in patients with HER2-mutant advanced biliary tract cancers. Nat. Commun. 14, 630 (2023).
Liu, D. et al. Pyrotinib alone or in combination with docetaxel in refractory HER2-positive gastric cancer: a dose-escalation phase I study. Cancer Med. 12, 10704–10714 (2023).
Chang, J. et al. Dual HER2 targeted therapy with pyrotinib and trastuzumab in refractory HER2 positive metastatic colorectal cancer: a result from HER2-FUSCC-G study. Clin. Colorectal Cancer 21, 347–353 (2022).
Zhou, C. et al. Pyrotinib in HER2-mutant advanced lung adenocarcinoma after platinum-based chemotherapy: a multicenter, open-label, single-arm, phase II study. J. Clin. Oncol. 38, 2753–2761 (2020).
Liu, S. M. et al. First-line pyrotinib in advanced HER2-mutant non-small-cell lung cancer: a patient-centric phase 2 trial. Nat. Med. 29, 2079–2086 (2023).
Kim, T. M. et al. Phase 1 studies of poziotinib, an irreversible pan-HER tyrosine kinase inhibitor in patients with advanced solid tumors. Cancer Res. Treat. 50, 835–842 (2018).
Kim, T. Y. et al. A phase I/II study of poziotinib combined with paclitaxel and trastuzumab in patients with HER2-positive advanced gastric cancer. Gastric Cancer 22, 1206–1214 (2019).
Le, X. et al. Poziotinib in non-small-cell lung cancer harboring HER2 exon 20 insertion mutations after prior therapies: ZENITH20-2 trial. J. Clin. Oncol. 40, 710–718 (2022).
Cornelissen, R. et al. Poziotinib in treatment-naive NSCLC harboring HER2 exon 20 mutations: ZENITH20-4, a multicenter, multicohort, open-label, phase 2 trial (cohort 4). J. Thorac. Oncol. 18, 1031–1041 (2023).
Olson, D. J. et al. Preclinical characterization of tucatinib in HER2-amplified xenograft and CNS implanted tumors. Cancer Res. 80, 1962 (2020).
Moulder, S. L. et al. Phase I study of ONT-380, a HER2 inhibitor, in patients with HER2(+)-advanced solid tumors, with an expansion cohort in HER2(+) metastatic breast cancer (MBC). Clin. Cancer Res. 23, 3529–3536 (2017).
Bekaii-Saab, T. S. et al. MOUNTAINEER-03: phase 3 study of tucatinib, trastuzumab, and mFOLFOX6 as first-line treatment in HER2+ metastatic colorectal cancer — trial in progress. J. Clin. Oncol. 41, https://doi.org/10.1200/JCO.2023.41.4_suppl.TPS261 (2023).
Tehfe, M. et al. Phase II dose optimization results from MOUNTAINEER-02: a study of tucatinib, trastuzumab, ramucirumab, and paclitaxel for HER2+ gastroesophageal cancer (GEC). Ann. Oncol. 34, S857–S858 (2023).
Reck, M. et al. SGNTUC-019: phase II basket study of tucatinib and trastuzumab in previously treated solid tumors with HER2 alterations. Ann. Oncol. 32, S614–S615 (2021).
Nakamura, Y. et al. Tucatinib and trastuzumab for previously treated human epidermal growth factor receptor 2-positive metastatic biliary tract cancer (SGNTUC-019): a phase II basket study. J. Clin. Oncol. 41, 5569–5578 (2023).
Yu, E. Y., Jin, F., Ramos, J., Tan, Q. & Galsky, M. D. A phase 2 basket study of tucatinib and trastuzumab in previously treated solid tumors with HER2 alterations: urothelial cancer cohort J. Clin. Oncol. https://doi.org/10.1200/JCO.2023.41.6_suppl.TPS587 (2023).
Monk, B., Jin, F., Ramos, J., Tan, Q. & O’Malley, D. A phase 2 basket study of tucatinib and trastuzumab in solid tumors with human epidermal growth factor receptor 2 alterations: uterine and cervical cancer cohorts (SGNTUC-019, trial in progress). Gynecol. Oncol. 176, S165–S166 (2023).
Heymach, J. et al. A phase I, open-label, dose confirmation, escalation, and expansion trial of BI 1810631 as monotherapy in patients with advanced or metastatic solid tumors with HER2 aberrations. Clin. Lung Cancer 24, e65–e68 (2023).
Hong, M. H. et al. 1333P A global phase 1b study of ORIC-114, a highly selective, brain penetrant EGFR and HER2 inhibitor, in patients with advanced solid tumors harboring EGFR exon 20 or HER2 alterations. Ann. Oncol. 34, S769 (2023).
Liu, J. et al. Mechanism and treatment of diarrhea associated with tyrosine kinase inhibitors. Heliyon 10, e27531 (2024).
Thuss-Patience, P. C. et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 18, 640–653 (2017).
Li, B. T. et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J. Clin. Oncol. 36, 2532–2537 (2018).
Peters, S. et al. Trastuzumab emtansine (T-DM1) in patients with previously treated HER2-overexpressing metastatic non-small cell lung cancer: efficacy, safety, and biomarkers. Clin. Cancer Res. 25, 64–72 (2019).
Sartore-Bianchi, A. et al. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: the phase II HERACLES-B trial. ESMO Open 5, e000911 (2020).
Nakada, T. et al. Novel antibody–drug conjugates containing exatecan derivative-based cytotoxic payloads. Bioorg. Med. Chem. Lett. 26, 1542–1545 (2016).
Ogitani, Y., Hagihara, K., Oitate, M., Naito, H. & Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 107, 1039–1046 (2016).
Doi, T. et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody–drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 18, 1512–1522 (2017).
Shitara, K. et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 382, 2419–2430 (2020).
Swain, S. M. et al. Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)-related interstitial lung disease/pneumonitis — focus on proactive monitoring, diagnosis, and management. Cancer Treat. Rev. 106, 102378 (2022).
Errisuriz, K., Bazan, D. Z., Verduzco, R. Jr. & Guedez, R. Trastuzumab-induced interstitial pneumonitis. Cureus 15, e42116 (2023).
Van Cutsem, E. et al. Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol. 24, 744–756 (2023).
Shitara, K. et al. Trastuzumab deruxtecan (T-DXd) in patients with HER2-positive gastric cancer (GC) or gastroesophageal junction (GEJ) adenocarcinoma who have progressed on or after a trastuzumab-containing regimen (DESTINY-Gastric04): a randomized phase 3 study. Ann. Oncol. 33, S306–S307 (2022).
Smit, E. F. et al. Trastuzumab deruxtecan in patients with metastatic non-small-cell lung cancer (DESTINY-Lung01): primary results of the HER2-overexpressing cohorts from a single-arm, phase 2 trial. Lancet Oncol. 25, 439–454 (2024).
Raghav, K. P. S. et al. Trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-overexpressing/amplified (HER2+) metastatic colorectal cancer (mCRC): primary results from the multicenter, randomized, phase 2 DESTINY-CRC02 study. J. Clin. Oncol. https://doi.org/10.1200/JCO.2023.41.16_suppl.3501 (2023).
Elgersma, R. C. et al. Design, synthesis, and evaluation of linker-duocarmycin payloads: toward selection of HER2-targeting antibody–drug conjugate SYD985. Mol. Pharm. 12, 1813–1835 (2015).
Li, H. W. et al. An anti-HER2 antibody conjugated with monomethyl auristatin E is highly effective in HER2-positive human gastric cancer. Cancer Biol. Ther. 17, 346–354 (2016).
Peng, Z. et al. Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: a single-arm phase II study. Cancer Commun (Lond.) 41, 1173–1182 (2021).
Sheng, X. et al. Open-label, multicenter, phase II study of RC48-ADC, a HER2-targeting antibody–drug conjugate, in patients with locally advanced or metastatic urothelial carcinoma. Clin. Cancer Res. 27, 43–51 (2021).
Barnscher, S. D., Rojas, A. H., Hamblett, K. J. & Escalante, N. Zanidatamab zovodotin (ZW49) induces hallmarks of immunogenic cell death and is active in patient-derived xenograft models of gastric cancer. Cancer Res. 83, 2633 (2023).
Jhaveri, K. et al. Preliminary results from a phase I study using the bispecific, human epidermal growth factor 2 (HER2)-targeting antibody–drug conjugate (ADC) zanidatamab zovodotin (ZW49) in solid cancers. Ann. Oncol. https://doi.org/10.1016/j.annonc.2022.07.589 (2022).
Zhang, T. et al. SHR-A1811, a novel anti-HER2 ADC with superior bystander effect, optimal DAR and favorable safety profiles. Cancer Res. 83, LB031 (2023).
Yao, H. et al. The HER2-targeting ADC SHR-A1811 in HER2-expressing/mutated advanced non-breast solid tumors (STs): results from the global phase I study. Ann. Oncol. 34, S461–S462 (2023).
Yao, H. et al. Safety, efficacy, and pharmacokinetics of shr-a1811, a human epidermal growth factor receptor 2-directed antibody–drug conjugate, in human epidermal growth factor receptor 2-expressing or mutated advanced solid tumors: a global phase I trial. J. Clin. Oncol. https://doi.org/10.1200/JCO.23.02044 (2024).
Lin, F. et al. Phase I study of ZV0203, a first in class pertuzumab ADC, in patients with HER2+ advanced solid tumors. Ann. Oncol. 34, S365 (2023).
Miao, Z. et al. Phase 1 study of ZV0203, a first in class pertuzumab ADC, in patients with HER2 positive advanced solid tumors. J. Clin. Oncol. https://doi.org/10.1200/JCO.2024.42.16_suppl.e15004 (2024).
Meric-Bernstam, F. et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: a phase 1, dose-escalation and expansion study. Lancet Oncol. 23, 1558–1570 (2022).
Harding, J. J. et al. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study. Lancet Oncol. 24, 772–782 (2023).
Lamarca, A. et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 22, 690–701 (2021).
Meric-Bernstam, F. et al. Zanidatamab (ZW25) in HER2-expressing gastroesophageal adenocarcinoma (GEA): results from a phase I study. J. Clin. Oncol. https://doi.org/10.1200/JCO.2021.39.3_suppl.164 (2021).
Ku, G. et al. Phase (Ph) II study of zanidatamab + chemotherapy (chemo) in first-line (1L) HER2 expressing gastroesophageal adenocarcinoma (GEA). Ann. Oncol. 32, S1044–S1045 (2021).
Wei, H. et al. Structural basis of a novel heterodimeric Fc for bispecific antibody production. Oncotarget 8, 51037–51049 (2017).
Labrijn, A. F., Janmaat, M. L., Reichert, J. M. & Parren, P. Bispecific antibodies: a mechanistic review of the pipeline. Nat. Rev. Drug Discov. 18, 585–608 (2019).
Back, J. GBR1302-BEAT® bispecific antibody targeting CD3 and HER2 demonstrates a higher anti-tumor potential than current HER2-targeting therapies. Ann. Oncol. https://doi.org/10.1093/annonc/mdw525.04 (2016).
Wermke, M. et al. Preliminary results from a phase I study of GBR 1302, a bispecific antibody T-cell engager, in HER2 positive cancers. Ann. Oncol. 29, VIII408–VIII409 (2018).
Vinay, D. S. & Kwon, B. S. 4-1BB (CD137), an inducible costimulatory receptor, as a specific target for cancer therapy. BMB Rep. 47, 122–129 (2014).
Hinner, M. J. et al. Tumor-localized costimulatory T-cell engagement by the 4-1BB/HER2 bispecific antibody–anticalin fusion PRS-343. Clin. Cancer Res. 25, 5878–5889 (2019).
Ku, G. et al. A phase I dose escalation study of PRS-343, a HER2/4-1BB bispecific molecule, in patients with HER2-positive malignancies. Ann. Oncol. https://doi.org/10.1016/j.annonc.2020.08.639 (2020).
Ku, G. et al. A phase 2, multi-center, open-label study of cinrebafusp alfa (PRS-343) in patients with HER2-high and HER2-low gastric or gastroesophageal junction (GEJ) adenocarcinoma. Ann. Oncol. 33, S265 (2022).
Ku, G. et al. Combination of cinrebafusp alfa with ramucirumab and paclitaxel is well tolerated and elicits encouraging clinical activity in patients with HER2-positive gastric/gastroesophageal junction (GEJ) adenocarcinoma. Cancer Res. https://doi.org/10.1158/1538-7445.AM2023-CT154 (2023).
Lee, E. et al. A novel HER2/4-1BB bispecific antibody, YH32367 (ABL105) exerts significant anti-tumor effects through tumor-directed T cell activation. Ann. Oncol. 32, S607–S608 (2021).
Jiang, Z., Sun, H., Yu, J., Tian, W. & Song, Y. Targeting CD47 for cancer immunotherapy. J. Hematol. Oncol. 14, 180 (2021).
Zhang, B. et al. Preclinical development of a novel bispecific mAb-Trap fusion protein, IMM2902, targeting both HER2 and CD47 as cancer immunotherapy. Cancer Res. 83, 2938 (2023).
Meng, Y. et al. Preliminary results of a phase I, first-in-human, dose escalation study of IMM2902 in patients with HER2-expressing advanced solid tumors. J. Clin. Oncol. https://doi.org/10.1200/JCO.2023.41.16_suppl.e151 (2023).
Safran, H. et al. Phase 1/2 study of DF1001, a novel tri-specific, NK cell engager therapy targeting HER2, in patients with advanced solid tumors: phase 1 DF1001 monotherapy dose-escalation results. J. Clin. Oncol. https://doi.org/10.1200/JCO.2023.41.16_suppl.2508 (2023).
Conilh, L., Sadilkova, L., Viricel, W. & Dumontet, C. Payload diversification: a key step in the development of antibody–drug conjugates. J. Hematol. Oncol. 16, 3 (2023).
Li, B. T. et al. A phase 1/2 study of a first-in-human immune-stimulating antibody conjugate (ISAC) BDC-1001 in patients with advanced HER2-expressing solid tumors. J. Clin. Oncol. https://doi.org/10.1200/JCO.2023.41.16_suppl.2538 (2023).
Metz, H. et al. SBT6050, a HER2-directed TLR8 therapeutic, as a systemically administered, tumor-targeted human myeloid cell agonist. J. Clin. Oncol. https://doi.org/10.1200/JCO.2020.38.15_suppl.311 (2020).
Klempner, S. J. et al. Interim results of a phase I/Ib study of SBT6050 monotherapy and pembrolizumab combination in patients with advanced HER2-expressing or amplified solid tumors. Ann. Oncol. https://doi.org/10.1016/j.annonc.2021.08.491 (2021).
Albelda, S. M. CAR T cell therapy for patients with solid tumours: key lessons to learn and unlearn. Nat. Rev. Clin. Oncol. 21, 47–66 (2024).
Morgan, R. A. et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 18, 843–851 (2010).
Sen, M. et al. Use of anti-CD3 x anti-HER2/neu bispecific antibody for redirecting cytotoxicity of activated T cells toward HER2/neu+ tumors. J. Hematother. Stem Cell Res. 10, 247–260 (2001).
Vaishampayan, U. N. et al. Phase II trial of pembrolizumab and anti-CD3 × anti-HER2 bispecific antibody-armed activated T cells in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 29, 122–133 (2023).
Gabrusiewicz, K. et al. CT-0508, a novel CAR macrophage product directed against HER2, promotes a proinflammatory tumor microenvironment. Cancer Immunol. Res. 8, B65 (2020).
Reiss, K. et al. A phase 1, first-in-human (FIH) clinical trial of the anti-HER2 CAR macrophage CT-0508 in participants with HER2 overexpressing solid tumors. J. Immunother. Cancer https://doi.org/10.1136/jitc-2022-SITC2022.0634 (2022).
Wang, L. et al. Patient-derived TAC01-HER2 TAC T cells produced in Cocoon® Platform is highly functional in models of solid tumors. Cancer Res. https://doi.org/10.1158/1538-7445.AM2023-3188 (2023).
Schlechter, B. L. et al. A phase I/II trial investigating safety and efficacy of autologous TAC01-HER2 in relapsed or refractory solid tumors. J. Clin. Oncol. https://doi.org/10.1200/JCO.2024.42.3_suppl.747 (2024).
Lin, M. J. et al. Cancer vaccines: the next immunotherapy frontier. Nat. Cancer 3, 911–926 (2022).
Ede, N. J. et al. Development of the B cell cancer vaccine HER-vaxx for the treatment of her-2 expressing cancers. Front Oncol. 12, 939356 (2022).
Wiedermann, U. et al. Clinical and immunologic responses to a B-cell epitope vaccine in patients with HER2/neu-overexpressing advanced gastric cancer — results from phase Ib trial IMU.ACS.001. Clin. Cancer Res. 27, 3649–3660 (2021).
Maglakelidze, M. et al. HERIZON: a phase 2 study of HER-Vaxx (IMU-131), a HER2-targeting peptide vaccine, plus standard of care chemotherapy in patients with HER2-overexpressing metastatic or advanced gastric/GEJ adenocarcinoma — overall survival analysis. J. Clin. Oncol. https://doi.org/10.1200/JCO.2023.41.4_suppl.289 (2023).
Bekes, M., Langley, D. R. & Crews, C. M. PROTAC targeted protein degraders: the past is prologue. Nat. Rev. Drug Discov. 21, 181–200 (2022).
Hu, M. et al. Discovery of potent and selective HER2 PROTAC degrader based tucatinib with improved efficacy against HER2 positive cancers. Eur. J. Med. Chem. 244, 114775 (2022).
Maneiro, M. A. et al. Antibody–PROTAC conjugates enable HER2-dependent targeted protein degradation of BRD4. ACS Chem. Biol. 15, 1306–1312 (2020).
Le Joncour, V. et al. A novel anti-HER2 antibody–drug conjugate XMT-1522 for HER2-positive breast and gastric cancers resistant to trastuzumab emtansine. Mol. Cancer Ther. 18, 1721–1730 (2019).
Skidmore, L. et al. ARX788, a site-specific anti-HER2 antibody–drug conjugate, demonstrates potent and selective activity in HER2-low and T-DM1-resistant breast and gastric cancers. Mol. Cancer Ther. 19, 1833–1843 (2020).
Pietrantonio, F. et al. HER2 loss in HER2-positive gastric or gastroesophageal cancer after trastuzumab therapy: implication for further clinical research. Int. J. Cancer 139, 2859–2864 (2016).
Saeki, H. et al. Re-evaluation of HER2 status in patients with HER2-positive advanced or recurrent gastric cancer refractory to trastuzumab (KSCC1604). Eur. J. Cancer 105, 41–49 (2018).
Seo, S. et al. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: results of the GASTric cancer HER2 reassessment study 3 (GASTHER3). Gastric Cancer 22, 527–535 (2019).
Yu, M., Liang, Y., Li, L., Zhao, L. & Kong, F. Research progress of antibody–drug conjugates therapy for HER2-low expressing gastric cancer. Transl. Oncol. 29, 101624 (2023).
Hong, J. et al. Regulation of ERBB2 receptor by t-DARPP mediates trastuzumab resistance in human esophageal adenocarcinoma. Cancer Res. 72, 4504–4514 (2012).
Shi, M. et al. Catecholamine-induced beta2-adrenergic receptor activation mediates desensitization of gastric cancer cells to trastuzumab by upregulating MUC4 expression. J. Immunol. 190, 5600–5608 (2013).
Duarte, H. O. et al. ST6Gal1 targets the ectodomain of ErbB2 in a site-specific manner and regulates gastric cancer cell sensitivity to trastuzumab. Oncogene 40, 3719–3733 (2021).
Wang, D. S. et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut 68, 1152–1161 (2019).
Ohba, A. et al. Circulating tumor DNA (ctDNA) analyses in patients with HER2-positive biliary tract cancer (BTC) treated with trastuzumab deruxtecan (T-DXd): exploratory results from the HERB trial. J. Clin. Oncol. https://doi.org/10.1200/JCO.2023.41.16_suppl.4097 (2023).
Siravegna, G. et al. Radiologic and genomic evolution of individual metastases during HER2 blockade in colorectal cancer. Cancer Cell 34, 148–162.e7 (2018).
Ding, X. et al. Systematic molecular profiling of inhibitor response to the clinical missense mutations of ErbB family kinases in human gastric cancer. J. Mol. Graph. Model. 96, 107526 (2020).
Ma, C., Wang, X., Guo, J., Yang, B. & Li, Y. Challenges and future of HER2-positive gastric cancer therapy. Front. Oncol. 13, 1080990 (2023).
Kaito, A. et al. HER2 heterogeneity is a poor prognosticator for HER2-positive gastric cancer. World J. Clin. Cases 7, 1964–1977 (2019).
Bang, K. et al. Association between HER2 heterogeneity and clinical outcomes of HER2-positive gastric cancer patients treated with trastuzumab. Gastric Cancer 25, 794–803 (2022).
Haffner, I. et al. HER2 expression, test deviations, and their impact on survival in metastatic gastric cancer: results from the prospective multicenter VARIANZ study. J. Clin. Oncol. 39, 1468–1478 (2021).
Suzuki, M. et al. Visualization of intratumor pharmacokinetics of [fam-] trastuzumab deruxtecan (DS-8201a) in HER2 heterogeneous model using phosphor-integrated dots imaging analysis. Clin. Cancer Res. 27, 3970–3979 (2021).
Eto, K. et al. The microRNA-21/PTEN pathway regulates the sensitivity of HER2-positive gastric cancer cells to trastuzumab. Ann. Surg. Oncol. 21, 343–350 (2014).
Tang, L. et al. NES1/KLK10 promotes trastuzumab resistance via activation of PI3K/AKT signaling pathway in gastric cancer. J. Cell. Biochem. 119, 6398–6407 (2018).
Deguchi, Y. et al. PTEN loss is associated with a poor response to trastuzumab in HER2-overexpressing gastroesophageal adenocarcinoma. Gastric Cancer 20, 416–427 (2017).
Mezynski, M. J. et al. Targeting the PI3K and MAPK pathways to improve response to HER2-targeted therapies in HER2-positive gastric cancer. J. Transl. Med. 19, 184 (2021).
Ning, G. et al. A novel treatment strategy for lapatinib resistance in a subset of HER2-amplified gastric cancer. BMC Cancer 21, 923 (2021).
Hong, Y. S. et al. Src mutation induces acquired lapatinib resistance in ERBB2-amplified human gastroesophageal adenocarcinoma models. PLoS ONE 9, e109440 (2014).
Jin, M. H. et al. Resistance mechanism against trastuzumab in HER2-positive cancer cells and its negation by src inhibition. Mol. Cancer Ther. 16, 1145–1154 (2017).
Su, B. et al. Apatinib exhibits synergistic effect with pyrotinib and reverses acquired pyrotinib resistance in HER2-positive gastric cancer via stem cell factor/c-kit signaling and its downstream pathways. Gastric Cancer 24, 352–367 (2021).
Liu, J. et al. A new mechanism of trastuzumab resistance in gastric cancer: MACC1 promotes the Warburg effect via activation of the PI3K/AKT signaling pathway. J. Hematol. Oncol. 9, 76 (2016).
Sanchez-Vega, F. et al. EGFR and MET amplifications determine response to HER2 inhibition in ERBB2-amplified esophagogastric cancer. Cancer Discov. 9, 199–209 (2019).
Chen, C. T. et al. MET activation mediates resistance to lapatinib inhibition of HER2-amplified gastric cancer cells. Mol. Cancer Ther. 11, 660–669 (2012).
Lee, Y. Y. et al. Phosphoproteomic analysis identifies activated MET-axis PI3K/AKT and MAPK/ERK in lapatinib-resistant cancer cell line. Exp. Mol. Med. 45, e64 (2013).
Park, J. et al. FOXO1 suppression is a determinant of acquired lapatinib-resistance in HER2-positive gastric cancer cells through MET upregulation. Cancer Res. Treat. 50, 239–254 (2018).
Yoshioka, T. et al. Acquired resistance mechanisms to afatinib in HER2-amplified gastric cancer cells. Cancer Sci. 110, 2549–2557 (2019).
Liao, H. et al. The significance of MET expression and strategies of targeting MET treatment in advanced gastric cancer. Front. Oncol. 11, 719217 (2021).
Ughetto, S. et al. Personalized therapeutic strategies in HER2-driven gastric cancer. Gastric Cancer 24, 897–912 (2021).
Piro, G. et al. An FGFR3 autocrine loop sustains acquired resistance to trastuzumab in gastric cancer patients. Clin. Cancer Res. 22, 6164–6175 (2016).
Yu, Y., Yu, X., Liu, H., Song, Q. & Yang, Y. miR-494 inhibits cancer-initiating cell phenotypes and reverses resistance to lapatinib by downregulating FGFR2 in HER2-positive gastric cancer. Int. J. Mol. Med. 42, 998–1007 (2018).
Kim, J. et al. Preexisting oncogenic events impact trastuzumab sensitivity in ERBB2-amplified gastroesophageal adenocarcinoma. J. Clin. Invest. 124, 5145–5158 (2014).
Jin, M. H. et al. WEE1 inhibition reverses trastuzumab resistance in HER2-positive cancers. Gastric Cancer 24, 1003–1020 (2021).
Oh, K. S. et al. A synthetic lethal strategy using PARP and ATM inhibition for overcoming trastuzumab resistance in HER2-positive cancers. Oncogene 41, 3939–3952 (2022).
DiPeri, T. P. et al. Adavosertib enhances antitumor activity of trastuzumab deruxtecan in HER2-expressing cancers. Clin. Cancer Res. 29, 4385–4398 (2023).
Shi, W. et al. Hyperactivation of HER2–SHCBP1–PLK1 axis promotes tumor cell mitosis and impairs trastuzumab sensitivity to gastric cancer. Nat. Commun. 12, 2812 (2021).
Kim, H. P. et al. Testican-1-mediated epithelial–mesenchymal transition signaling confers acquired resistance to lapatinib in HER2-positive gastric cancer. Oncogene 33, 3334–3341 (2014).
Zhou, X. et al. miR-200c inhibits TGF-beta-induced-EMT to restore trastuzumab sensitivity by targeting ZEB1 and ZEB2 in gastric cancer. Cancer Gene Ther. 25, 68–76 (2018).
Shi, J. et al. The HER4–YAP1 axis promotes trastuzumab resistance in HER2-positive gastric cancer by inducing epithelial and mesenchymal transition. Oncogene 37, 3022–3038 (2018).
Ebbing, E. A. et al. Esophageal adenocarcinoma cells and xenograft tumors exposed to Erb-b2 receptor tyrosine kinase 2 and 3 inhibitors activate transforming growth factor beta signaling, which induces epithelial to mesenchymal transition. Gastroenterology 153, 63–76 (2017).
Nam, A. R. et al. Targeting the hippo transducer YAP overcomes trastuzumab-resistance in HER2-positive cancers. Cancer Res. 2022, 5334 (2022).
Liu, W., Yuan, J., Liu, Z., Zhang, J. & Chang, J. Label-free quantitative proteomics combined with biological validation reveals activation of Wnt/beta-catenin pathway contributing to trastuzumab resistance in gastric cancer. Int. J. Mol. Sci. https://doi.org/10.3390/ijms19071981 (2018).
Danzi, F. et al. To metabolomics and beyond: a technological portfolio to investigate cancer metabolism. Signal. Transduct. Target Ther. 8, 137 (2023).
Hu, X. et al. Glutamine metabolic microenvironment drives M2 macrophage polarization to mediate trastuzumab resistance in HER2-positive gastric cancer. Cancer Commun. 43, 909–937 (2023).
Castagnoli, L. et al. Fatty acid synthase as a new therapeutic target for HER2-positive gastric cancer. Cell Oncol. 46, 661–676 (2023).
Suh, K. J. et al. EGFR or HER2 inhibition modulates the tumor microenvironment by suppression of PD-L1 and cytokines release. Oncotarget 8, 63901–63910 (2017).
Fukai, S. et al. Down-regulation of stimulator of interferon genes (STING) expression and CD8(+) T-cell infiltration depending on HER2 heterogeneity in HER2-positive gastric cancer. Gastric Cancer 26, 878–890 (2023).
Yuan, Q. et al. Integration of transcriptomics, proteomics, and metabolomics data to reveal HER2-associated metabolic heterogeneity in gastric cancer with response to immunotherapy and neoadjuvant chemotherapy. Front. Immunol. 13, 951137 (2022).
Janjigian, Y. Y. et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet 402, 2197–2208 (2023).
MERCK. Merck Announces Phase 3 KEYNOTE-811 Trial Met Dual Primary Endpoint of Overall Survival (OS) as First-Line Treatment in Patients With HER2-Positive Advanced Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma. https://www.merck.com/news/merck-announces-phase-3-keynote-811-trial-met-dual-primary-endpoint-of-overall-survival-os-as-first-line-treatment-in-patients-with-her2-positive-advanced-gastric-or-gastroesophageal-junction-gej/ (2024).
FDA. FDA Amends Pembrolizumab’s Gastric Cancer Indication. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-amends-pembrolizumabs-gastric-cancer-indication (2023).
Lee, K. W. et al. Zanidatamab (zani), a HER2-targeted bispecific antibody, in combination with chemotherapy (chemo) and tislelizumab (TIS) as first-line (1L) therapy for patients (pts) with advanced HER2-positive gastric/gastroesophageal junction adenocarcinoma (G/GEJC): preliminary results from a phase 1b/2 study. J. Clin. Oncol. https://doi.org/10.1200/JCO.2022.40.16_suppl.4032 (2023).
Tabernero, J. et al. HERIZON-GEA-01: zanidatamab + chemo ± tislelizumab for 1L treatment of HER2-positive gastroesophageal adenocarcinoma. Future Oncol. 18, 3255–3266 (2022).
Candas-Green, D. et al. Dual blockade of CD47 and HER2 eliminates radioresistant breast cancer cells. Nat. Commun. 11, 4591 (2020).
Kauder, S. E. et al. ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. PLoS ONE 13, e0201832 (2018).
ALX Oncology. ALX Oncology reports positive interim phase 2 ASPEN-06 clinical trial results of evorpacept for the treatment of advanced HER2-positive gastric cancer. ALX Oncology, https://ir.alxoncology.com/news-releases/news-release-details/alx-oncology-reports-positive-interim-phase-2-aspen-06-clinical/ (2023).
Xia, L. et al. HER2-targeted antibody–drug conjugate induces host immunity against cancer stem cells. Cell Chem. Biol. 28, 610–624.e5 (2021).
Wu, X. et al. A HER2-targeting antibody–MMAE conjugate RC48 sensitizes immunotherapy in HER2-positive colon cancer by triggering the cGAS–STING pathway. Cell. Death Dis. 14, 550 (2023).
Yadav, G. et al. Synergistic activity of neratinib in combination with olaparib in uterine serous carcinoma overexpressing HER2/neu. Gynecol. Oncol. 166, 351–357 (2022).
Wainberg, Z. A. et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 23, 1430–1440 (2022).
Yasui, H. et al. Prospective analysis of the expression status of FGFR2 and HER2 in colorectal and gastric cancer populations: DS-Screen Study. Int. J. Colorectal Dis. 37, 1393–1402 (2022).
Krzyscik, M. A., Porebska, N., Opalinski, L. & Otlewski, J. Targeting HER2 and FGFR-positive cancer cells with a bispecific cytotoxic conjugate combining anti-HER2 Affibody and FGF2. Int. J. Biol. Macromol. 254, 127657 (2024).
Fukuoka, S. et al. p70S6K/Akt dual inhibitor DIACC3010 is efficacious in preclinical models of gastric cancer alone and in combination with trastuzumab. Sci. Rep. 13, 16017 (2023).
Egebjerg, K. et al. HER2 positivity in histological subtypes of salivary gland carcinoma: a systematic review and meta-analysis. Front. Oncol. 11, 693394 (2011).
Hiraoka, N. et al. Details of human epidermal growth factor receptor 2 status in 454 cases of biliary tract cancer. Hum. Pathol. 105, 9–19 (2020).
Nasioudis, D. et al. Molecular landscape of ERBB2/HER2 gene amplification among patients with gynecologic malignancies; clinical implications and future directions. Gynecol. Oncol. 180, 1–5 (2024).
Hirsch, F. R. et al. Evaluation of HER-2/neu gene amplification and protein expression in non-small cell lung carcinomas. Br. J. Cancer 86, 1449–1456 (2002).
Van Cutsem, E. et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 18, 476–484 (2015).
Han, S. H., Ryu, K. H. & Kwon, A. Y. The prognostic impact of HER2 genetic and protein expression in pancreatic carcinoma-HER2 protein and gene in pancreatic cancer. Diagnostics 11, 653 (2021).
Seo, A. N. et al. HER2 status in colorectal cancer: its clinical significance and the relationship between HER2 gene amplification and expression. PLoS ONE 9, e98528 (2014).
Scherrer, E., Kang, A., Bloudek, L. M. & Koshkin, V. S. HER2 expression in urothelial carcinoma, a systematic literature review. Front. Oncol. 12, 1011885 (2022).
Estephan, F. et al. The prevalence and clinical significance of HER2 expression in prostate adenocarcinoma. Ann. Diagn. Pathol. 67, 152219 (2023).
Shayeb, A. M. et al. Comprehensive analysis of human epidermal growth factor receptor 2 through DNA, mRNA, and protein in diverse malignancies. JCO Precis. Oncol. 7, e2200604 (2023).
Mishra, R., Hanker, A. B. & Garrett, J. T. Genomic alterations of ERBB receptors in cancer: clinical implications. Oncotarget 8, 114371–114392 (2017).
Acknowledgements
The work of the authors is supported by a National Research Foundation of Korea grant funded by the Korea government (MSIT; grant no. 2021R1A2C2007430 to D.-Y.O.) and the Institute of Smart Healthcare Innovative Medical Sciences, a Brain Korea 21 four programme, Seoul National University (to D.-Y.O.).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
D.-Y.O. has acted as a consultant or adviser for Abbvie, Arcus Biosciences, ASLAN, Astellas, AstraZeneca, Basilea, Bayer, BeiGene, Bristol Myers Squibb/Celgene, Eutilex, Genentech/Roche, Halozyme, Idience, IQVIA, J-Pharma, LG Chem, Merck Serono, Mirati Therapeutics, Moderna, MSD, Novartis, Taiho, Turning Point, Yuhan and Zymeworks, and has received research grants from Array, AstraZeneca, BeiGene, Eli Lilly, Handok, MSD, Novartis and Servier. J.Y. declares no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks F. Lordick, M. Nagasaka, A. Sartore-Bianchi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
cBioPortal: cbioportal.org
ClinicalTrials.gov: https://clinicaltrials.gov/
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoon, J., Oh, DY. HER2-targeted therapies beyond breast cancer — an update. Nat Rev Clin Oncol 21, 675–700 (2024). https://doi.org/10.1038/s41571-024-00924-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-024-00924-9
- Springer Nature Limited