Abstract
Neoadjuvant pembrolizumab plus chemotherapy (P + CT) has emerged as a standard of care for stage II-III triple-negative breast cancer (TNBC). However, the best anthracycline-cyclophosphamide (AC) schedule remains to be determined. While the KEYNOTE-522 regimen employs AC every 3 weeks (q3w AC), previous studies have shown overall survival benefits of dose-dense regimens for early-stage breast cancer. The Neo-Real study (GBECAM-0123) is a real-world data effort evaluating patients with TNBC treated with neoadjuvant P + CT in ten cancer centers since July 2020. The objective of this analysis was to evaluate the effectiveness and safety of dose-dense AC (ddAC) versus q3w AC. Among 333 patients included until November 2023, 311 completed neoadjuvant therapy and 279 underwent surgery with pathology reports available; ddAC was used in 58.2% and q3w AC in 41.8% of the cases. Most patients (69.1%) had stage II TNBC. A pCR was observed in 65.4% with ddAC and 58.7% with q3w AC (P = 0.260), while RCB 0-1 occurred in 82.4% and 73.5%, respectively (P = 0.115). Patients with stage III disease had a numerically higher pCR with ddAC (59% vs 40%, P = 0.155), while pCR rates were similar regardless of AC regimen in stage II disease (66.6% vs 64.5%; P = 0.760). While no significant disparities in drug discontinuation was noted, ddAC showed a trend towards higher rates of grade ≥3 AE (40.5% vs. 30.7%, P = 0.092). The Neo-Real study could not rule out a difference between ddAC and q3w AC during neoadjuvant P + CT. The observation of a potentially higher pCR with ddAC in stage III disease warrants further investigation.
Similar content being viewed by others
Introduction
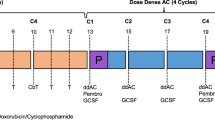
In recent years, significant improvements have been made in the treatment landscape of triple-negative breast cancer (TNBC), a subtype known for its worse prognosis. Notably, the KEYNOTE-522 study established the use of pembrolizumab as part of the treatment of patients with stage II-III TNBC1,2. This trial featured a regimen comprising four cycles of paclitaxel (administered weekly) alongside carboplatin (given weekly or every 3 weeks) followed by four cycles of anthracycline plus cyclophosphamide (AC) every 3 weeks. Concurrently, patients were randomized to receive 8 cycles of pembrolizumab at 200 mg or placebo every three weeks throughout the neoadjuvant phase. After surgery, pembrolizumab or placebo were continued regardless of pathologic response until a total of 17 cycles, and no other cytotoxic agent was allowed for patients not achieving pCR. The study demonstrated a notable improvement in pathologic complete response (64.8% vs 51.2%, P < 0.001) and event-free survival (EFS) (5-year EFS: 81.3% vs 72.3%, HR 0.63, 95% CI 0.49–0.81), although overall survival data remains immature1,2,3.
Conversely, investigations into other immune checkpoint inhibitors within the neoadjuvant setting yielded mixed outcomes. While trials such as IMpassion 031 and NeoTRIP assessing atezolizumab reported negative results for survival outcomes4,5,6,7, the phase II GeparNuevo trial suggested improved event-free survival and overall survival (OS) with neoadjuvant durvalumab8. Notably, a recent meta-analysis incorporating individual patient data and trial-level evidence revealed an OS benefit with neoadjuvant immunotherapy, translating to a remarkable 5-year survival gain of 7% (5-year OS: 90% in the immunotherapy arm vs 83% in the control arm, HR 0.62, 95% CI 0.46–0.82, P < 0.001)9.
As treatment standards evolve, the coalescence of unexplored therapeutic strategies prompts inquiries into optimal management approaches for TNBC. A pertinent query arises regarding the adoption of dose-dense anthracycline plus cyclophosphamide (AC) in lieu of the three-weekly regimen employed in the KEYNOTE-522 trial. This consideration stems from the established benefits of dose-dense chemotherapy in early-stage breast cancer, as corroborated by the comprehensive findings of a patient-level meta-analysis with over 35,000 patients and the updated results of the GIM2 trial that showed that patients with TNBC treated with dose-dense AC presented improvement in both disease-free and overall survival after a median follow-up of 15 years10,11. Despite critiques surrounding the use of every 3-week paclitaxel in the control arm, the GIM2 trial findings underscore the merits of dose-dense AC in clinical practice. In the KEYNOTE-522 trial, two additional drugs, carboplatin and pembrolizumab, were utilized compared to the regimen in GIM2. It remains uncertain whether a dose-dense regimen would yield similar benefits in this particular context. Notably, findings from the phase III BrighTNess trial demonstrated the efficacy of adding carboplatin to neoadjuvant chemotherapy for TNBC in both the dose-dense and every 3-week AC subgroups12.
Nevertheless, extrapolating the benefits of dose-dense regimens to the realm of neoadjuvant chemoimmunotherapy introduces uncertainties. Concerns are compounded by logistical challenges associated with disparate schedules of dose-dense AC and pembrolizumab necessitating increased infusion service visits (at least two additional visits). Moreover, besides the fact that the use of granulocyte colony-stimulating factor (G-CSF) during dose-dense regimens increase the costs of treatment, the effectiveness of immune checkpoint inhibitor administered concomitantly with G-CSF remains ambiguous. Hypotheses posit that G-CSF stimulation may influence tumor-infiltrating immune cell profiles, potentially modulating immunotherapy efficacy13,14. Conversely, preclinical models suggest a potential synergy between G-CSF overexpression and immunotherapy efficacy, while neutrophil depletion may compromise checkpoint blockade response15.
Finally, safety concerns need to be considered in the neoadjuvant treatment of TNBC. The intensive five-drug regimen employed in the KEYNOTE-522 trial resulted in 32.5% of patients experiencing serious treatment-related adverse events, with 23.3% necessitating discontinuation of one or more drugs due to adverse events1. The use of dose-dense AC presents an additional challenge due to its heightened risk, particularly concerning hematological toxicities16. Therefore, assessing the safety profile of dose-dense AC within the context of neoadjuvant chemoimmunotherapy is imperative.
In response to these uncertainties, the Neo-Real study (GBECAM-0123) emerges as a collaborative, multicentric endeavor poised to generate real-world evidence elucidating the use of neoadjuvant immunotherapy in TNBC. This study aims to address existing gaps in patient management by evaluating the safety and effectiveness of dose-dense AC versus the three-weekly regimen during neoadjuvant chemoimmunotherapy.
Results
Patients’ characteristics
A total of 333 patients were included in the Neo-Real study (GBECAM-0123) until November 2023, with 311 who finished neoadjuvant therapy forming the safety cohort (22 patients had neoadjuvant therapy ongoing by the time of the analysis). The effectiveness cohort comprised 279 patients who underwent surgery and had available pathology reports (2 patients were not submitted to surgery due to disease progression during neoadjuvant therapy and 30 awaited surgery or pathology report).
Considering the 311 patients in the safety cohort, 58.2% received dose-dense AC while 41.8% received every 3-week AC. The median age was 43 years (range 24–83 years). Most patients (69.1%) had stage II TNBC. The baseline characteristics according to AC regimen are presented in Table 1. Baseline characteristics were well balanced between the dose-dense and every 3-week AC groups.
Effectiveness according to AC schedule
Among the 279 patients in the effectiveness cohort, 165 (59.1%) received dose-dense AC and 114 (40.9%) received every 3-week AC. During the neoadjuvant therapy, 2.2% (n = 4) of the patients in the dose-dense AC group and 4.6% (n = 6) of those in the every 3-week AC group had a clinical or radiological disease progression (P = 0.330).
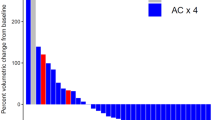
In terms of pathologic response, a pCR was observed in 65.4% with dose-dense AC and 58.7% with every 3-week AC (P = 0.260). The rates of RCB 0-1 were 82.4% and 73.5%, respectively (P = 0.115).
The pCR and RCB rates were highly influenced by the tumor stage, leading to the selection of this parameter for a subgroup analysis. Patients with stage III disease had a numerically higher pCR with dose-dense AC (59%) than every 3-week AC (40%), with an absolute difference of 19%. However, the difference did not reach statistical significance (P = 0.155). The same trend was observed for RCB 0-1 rates (70.7% versus 50%, P = 0.128).
For stage II tumors, the pCR and RCB rates were similar regardless of the AC regimen, with a pCR of 66.6% with dose-dense AC and 64.5% with every 3-week AC (P = 0.760) and a RCB 0–1 of 86% and 81.4%, respectively (P = 0.523).
Figures 1 and 2 illustrate the pCR and RCB rates according to AC regimen in the overall cohort and in the subgroups defined by tumor stage.
Factors associated with pathologic complete response
The univariate logistic regression identified Ki67 ≥ 50%, tumor grade 3, and TILs ≥ 30%) as predictors of higher pCR rates, while tumor stage III and receiving less than 6 cycles of neoadjuvant pembrolizumab were associated with a decreased pCR rate. The use of G-CSF or antibiotics were not associated with pCR.
The factors identified in the univariate model were selected for a multivariable model together with the AC schedule. Due to the high proportion of missing data for TILs, this variable was not included in the model. Lymph node status was also not included due to its correlation with disease stage. The multivariate analysis confirmed the impact of Ki67 ≥ 50% and the number of neoadjuvant pembrolizumab cycles in the pCR rate (Table 2).
Similar results were observed in the univariate and multivariable analysis when RCB 0-1 was evaluated as the outcome, although in this case a trend was observed for the association between AC schedule and RCB 0-1 in the multivariable model (Supplementary Table 1).
Supplementary Table 2 shows pCR and RCB 0-1 rates according to the predictive factors identified.
Safety and adherence according to AC schedule
Patients receiving dose-dense AC or every 3-week AC had similar rates of discontinuation of any neoadjuvant drug due to toxicities (25.1% versus 20.1%, P = 0.339), dose reduction of any drug (12.8% versus 14.8%, P = 0.618) or treatment delay greater than 7 days due to toxicity (28% versus 21.8%, P = 0.234).
Patients receiving dose-dense AC had a tendency towards a higher frequency of adverse events of grade 3 or higher (40.5%) compared with those treated with every 3-week AC (30.7%, P = 0.092). Adverse events of grade 3 or more that tended to be higher in the dose-dense AC group were febrile neutropenia (16% versus 9.2%), elevated liver enzymes (4.4% versus 0.7%), nausea (2.7% versus 0%), and vomiting (2.7% versus 0%).
Notably, 40.1% of patients in the every 3-week AC group received G-CSF. The need for antibiotics during neoadjuvant therapy was high in both groups (31.8% with dose-dense AC and 24.4% with every 3-week AC, P = 0.162). Frequent reasons for antibiotic use other than febrile neutropenia were upper respiratory tract infection (n = 13), pneumonia (n = 8), and urinary tract infection (n = 5).
Immune-related adverse events of grade 3 or higher occurred in 8.2% and 6.9% of the patients receiving dose-dense AC and every 3-week AC, respectively (P = 0.830). The most common grade ≥3 immune-related adverse events were hepatitis (n = 10), pneumonitis (n = 5), and cutaneous reaction (n = 4).
The two groups differed in terms of the number of neoadjuvant pembrolizumab cycles received. Forty—three (23.9%) patients in the dose-dense AC group and 17 (13.1%) in the every 3-week AC group received less than 6 cycles of neoadjuvant pembrolizumab (P = 0.020. Among those who received less than 6 cycles, the main reasons were delay in drug coverage by the medical insurance (44.2% in the dose-dense AC group and 5.9% in the every 3-week AC group) and toxicity (37.2% and 58.8%, respectively). Among patients who had pembrolizumab initiation delayed due to drug coverage issues (n = 19), half of them (n = 11) started neoadjuvant treatment with dose dense-AC, which was followed by carboplatin and paclitaxel with pembrolizumab added after approval. This observation explains the difference in the proportion of patients who received less than 6 cycles of neoadjuvant pembrolizumab and who started treatment with AC (followed by carboplatin and paclitaxel) between the AC groups.
Since the number of neoadjuvant pembrolizumab cycles were not balanced between AC groups (higher proportion of patients with less than 6 cycles of neoadjuvant pembrolizumab in the dose-dense AC group) and receiving less than 6 cycles was identified as a negative predictive factor, we repeated the effectiveness analyses excluding patients who received less than 6 cycles of neoadjuvant pembrolizumab. The results observed in this analysis were similar to the main effectiveness analysis observed in the overall cohort.
Table 3 details the adherence and safety outcomes according to AC regimen.
Discussion
The Neo-Real study contributes valuable insights into the safety and effectiveness of neoadjuvant chemoimmunotherapy for early-stage TNBC, aligning with the pivotal findings of the KEYNOTE-522 trial. Our study demonstrated comparable pCR rates (62.7% in Neo-Real and 64.8% in the KEYNOTE-522) and safety outcomes (drug discontinuation rates of 23% in Neo-Real and 23.3% in the KEYNOTE-522) to those reported in the phase III trial, reinforcing the real-world applicability of pembrolizumab in this setting1.
While the dose-dense AC regimen was more commonly selected by physicians in the real-world cohort (58.2%), our analysis revealed no statistically significant differences in effectiveness or safety between dose-dense and every 3-week AC schedules. Nevertheless, grade 3 or higher adverse events, including febrile neutropenia, tended to be higher with the dose-dense regimen. Anyhow, the high rates of treatment discontinuation and antibiotic use underscore the need for vigilant monitoring of toxicity, particularly focusing on myelosuppression and immune-related side effects, irrespective of the AC schedule.
Although the pCR did not differ between groups in the overall cohort, one hypothesis-generating observation was a numerically higher pCR with dose-dense AC in the subgroup of stage III disease (59% vs 40%). However, caution is warranted due to the small sample size in this group. Nonetheless, the hypothesis warrants further investigation, as this subgroup consistently exhibits lower pathologic complete response rates with chemoimmunotherapy and worse long-term outcomes. For instance, in the KEYNOTE-522 trial, 65.4% of patients with stage II disease achieved a complete response compared to 56.1% of those with stage III3. Similarly, in the single-arm phase II NeoPACT trial, with neoadjuvant carboplatin, paclitaxel, and pembrolizumab (without AC), these figures were 59% and 43%, respectively17.
Insights were also gained through the identification of predictive factors for pCR, aiming to differentiate patients for whom treatment could be de-escalated from those requiring additional intervention. The KEYNOTE-522 trial’s deeper analysis revealed that 51.2% of patients in the control arm achieved a pCR with favorable long-term outcomes, suggesting that they may not necessitate a five-drug neoadjuvant regimen1,2. In contrast, among patients with residual disease and an RCB score of 3, the 3-year EFS rate was only 26.2%, even in the arm receiving chemotherapy plus pembrolizumab, indicating a subset requiring different or additional strategies18. In our study, a Ki67 index higher than 50%, tumor grade 3, and TILs of 30% or more were associated with higher pathologic complete response rates. TILs levels were also predictors of pCR with neoadjuvant chemoimmunotherapy in the NeoPACT trial17, implying the potential utility of these biomarkers in tailoring treatment selection for TNBC in future trials19.
Concerning the use of G-CSF during immune checkpoint inhibitor therapy, our study suggested that while patients using dose-dense AC requires prophylactic G-CSF, the need for this supportive medication was also high among patients receiving AC every 3 weeks. Moreover, the study provided reassuring results indicating that, in the regression model, G-CSF did not impact pathologic response, suggesting it does not impair immunotherapy activity. On the other hand, receiving less than 6 cycles of neoadjuvant pembrolizumab was associated with a lower pCR rate, reinforcing the importance of a proper adherence and completion of the proposed neoadjuvant therapy for improved outcomes.
The main limitations of our study include the retrospective nature of data collection for most patients and the heterogeneous availability of a few variables, particularly TILs. In addition, to more accurately assess the comparison between the AC schedules, a randomized controlled trial would be ideal, allowing for better control of potential biases. Moreover, a larger sample size would be necessary to achieve statistical significance for the observed differences in pCR rates. For instance, based on a power of 80% and a two-sided alpha error of 5%, the study would require a sample of 1646 participants to confirm a difference of 6.7% in pCR rates (65.4% versus 58.7%) in the overall cohort. Similarly, for the observed 19% difference in the stage III subgroup (59% versus 40%), a sample of 216 participants with stage III disease would be needed.
Nonetheless, the study provides valuable Real-World information from a large multicentric population that supports KEYNOTE-522 trial results. The study addresses a pertinent clinical practice question regarding the impact of AC schedule during neoadjuvant chemoimmunotherapy and offers hypothesis-generating observations. Despite the observational nature of the study, it measured an objective short-term outcome (pathologic response) and groups were comparable regarding baseline characteristics. Importantly, the pathologic response has been shown to be an excellent marker of long-term outcomes in TNBC20.
In conclusion, while this study did not find statistically significant differences in effectiveness or safety outcomes between dose-dense AC and every 3-week AC during neoadjuvant chemoimmunotherapy in the overall stage II-III TNBC cohort, it’s important to note that the study’s limitations mean that the possibility of a difference between the AC schedules cannot be entirely ruled out. The numerically higher rates of pCR with dose-dense AC in patients with stage III disease are intriguing and suggest a potential benefit of ddAC in this subgroup that warrants further investigation. The Neo-Real study will continue to collect real-world evidence to further evaluate this question and determine whether the AC schedule impacts survival outcomes.
Methods
Study design and participants
This observational real-world cohort study evaluated patients diagnosed with early-stage TNBC who received neoadjuvant chemotherapy plus pembrolizumab across ten Brazilian cancer centers from July 2020 to November 2023. Eligible participants included those with histologically confirmed TNBC of any histological subtype who underwent at least one cycle of neoadjuvant immunotherapy. Patients had estrogen-receptor and progesterone-receptor lower than 10% and did not have HER2-overexpression (defined as immunohistochemistry +3 or +2 with positive in-situ hybridization).
Patient records were scrutinized to gather pertinent data, including the date of diagnosis, patient age, tumor stage, estrogen receptor status, progesterone receptor status, HER2 status, Ki67 index, tumor-infiltrating lymphocytes, date of neoadjuvant chemotherapy initiation, type of chemotherapy regimen and schedule, date of pembrolizumab initiation, use of granulocyte colony-stimulating factor (G-CSF), toxicities, drug discontinuation and dose reduction, use of antibiotics, date and type of surgery, pathologic response, use of adjuvant radiotherapy and systemic therapies, recurrence, and death. Data collection utilized a centralized RedCap case report form.
The study was conducted within the GBECAM (Grupo Brasileiro de Estudos em Câncer de Mama) and was approved by the Institutional Review Board at each of the participating centers: Instituto D’Or de Pesquisa e Ensino, A.C.Camargo Cancer Center, Hospital Moinhos de Vento, Hospital Sírio-Libanês, Hospital Beneficência Portuguesa, Hospital 9 de Julho, Hospital Santa Paula, Clínica AMO, Instituto do Câncer do Ceará, and Brasilia Hospital. The study complied with ethical regulations for studies with human participants, including the Declaration of Helsinki. A written informed consent was obtained from participants who could be located, while a waiver of consent was granted by the Institutional Review Boards for participants who were dead or could not be located since the study only collected de-identified information. Data were collected and analyzed by the Research Unit at IDOR (Instituto D’Or de Pesquisa e Ensino, São Paulo, Brazil). The manuscript was written by the first and last authors without industry medical-writing support. All authors reviewed the manuscript and affirm the accuracy and completeness of the data.
Objectives and endpoints
The study aimed to assess the effectiveness and safety of dose-dense AC compared to every 3-week AC in patients with early-stage TNBC undergoing neoadjuvant chemotherapy plus pembrolizumab. Dose-dense AC consisted in administering AC every 2 weeks with G-CSF support.
Effectiveness endpoints included rates of pathologic complete response and residual cancer burden (RCB) 0–1. Additionally, we explored the association between these outcomes and factors such as AC schedule, tumor stage, nodal status, tumor grade, Ki67 index, tumor-infiltrating lymphocytes, germline BRCA status, number of neoadjuvant pembrolizumab cycles, use of G-CSF, and body mass index. For the effectiveness analysis, we included patients who already underwent surgery and have the pathology report available.
Safety endpoints encompassed rates of grade 3 or higher adverse events, grade 3 or higher immune-related adverse events, febrile neutropenia, drug discontinuation, drug reduction, delay to conclude neoadjuvant therapy, and use of antibiotics. The delay to conclude neoadjuvant therapy was considered as a delay of at least seven days for treatment completion compared to the estimated date of completion based on the date of initiation and expected treatment duration. Toxicities were graded based on the Common Terminology Criteria for Adverse Events Version 5.0. The safety cohort was composed of patients who had received at least one cycle of neoadjuvant pembrolizumab and had finished the neoadjuvant therapy phase.
Statistical analysis
Baseline characteristics were summarized using descriptive statistics, with continuous variables presented as median and range, and categorical variables expressed as absolute and relative numbers. Normality of the distribution of continuous variables was assessed using the Shapiro–Wilk test. The Student’s T Test was chosen for comparing normally distributed continuous variables, while the Mann–Whitney test was employed for skewed data. Fisher’s exact test was used to compare categorical variables between groups. P-values below 0.05 were considered statistically significant.
Univariate and multivariable logistic regression were conducted to assess factors associated with pathologic complete response and RCB 0-1. Variables for the multivariable analysis were selected based on a p-value below 0.1 in the univariate analysis or their relevance to the research question. If variables were correlated, the most significant predictor was included. Statistical analyses were performed using Stata software, version 15.0 (StataCorp, Texas, USA).
Data availability
The datasets generated and/or analyzed during the current study are not publicly available in order to protect patient privacy but are available from the corresponding author on reasonable request. Data analysis methods have been described thoroughly in the Methods section.
References
Schmid, P., Dent, R. & O’Shaughnessy, J. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 382, e108 (2020).
Schmid, P. et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N. Engl. J. Med. 386, 556–567 (2022).
Schmid, P. et al. Neoadjuvant pembrolizumab or placebo plus chemotherapy followed by adjuvant pembrolizumab or placebo for early-stage triple-negative breast cancer: updated event-free survival results from the phase 3 KEYNOTE-522 study. Presented at the 2023 San Antonio Breast Cancer Symposium; December 5-9, 2023; San Antonio; 2023. p. abstract LBO1-01.
Mittendorf, E. et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet (Lond., Engl.) 396, 1090–1100 (2020).
Gianni, L. et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Ann. Oncol. 33, 534–543 (2022).
Barrios, C. et al. LBA1 Final analysis of the placebo-controlled randomised phase III IMpassion031 trial evaluating neoadjuvant atezolizumab (atezo) plus chemotherapy (CT) followed by open-label adjuvant atezo in patients (pts) with early-stage triple-negative breast cancer (eTNBC). Ann. Oncol. 8, 101220–101220 (2023).
Gianni, L. et al. LBA19 Event-free survival (EFS) analysis of neoadjuvant taxane/carboplatin with or without atezolizumab followed by an adjuvant anthracycline regimen in high-risk triple negative breast cancer (TNBC): NeoTRIP Michelangelo randomized study. Ann. Oncol. 34, S1258–S1259 (2023).
Loibl, S. et al. Durvalumab improves long-term outcome in TNBC: results from the phase II randomized GeparNUEVO study investigating neodjuvant durvalumab in addition to an anthracycline/taxane based neoadjuvant chemotherapy in early triple-negative breast cancer (TNBC). J. Clin. Oncol. 39, 506 (2021).
Cunha, M. T. et al. Long-term outcomes of neoadjuvant immunotherapy plus chemotherapy in patients with early-stage triple-negative breast cancer: an extracted individual patient data and trial-level meta-analysis. Br. J. Cancer 130, 242–250 (2024).
(EBCTCG) EBCTCG. Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet 393, 1440–1452 (2019).
Del Mastro, L. et al. Fluorouracil and dose-dense adjuvant chemotherapy in patients with early-stage breast cancer (GIM2): end-of-study results from a randomised, phase 3 trial. Lancet Oncol. 23, 1571–1582 (2022).
Loibl, S. et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 19, 497–509 (2018).
Mouchemore, K. A. & Anderson, R. L. Immunomodulatory effects of G-CSF in cancer: therapeutic implications. Semin. Immunol. 54, 101512 (2021).
Slingluff, C. L. et al. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin. Cancer Res. 15, 7036–7044 (2009).
Kaisar-Iluz, N. et al. The bilateral interplay between cancer immunotherapies and neutrophils’ phenotypes and sub-populations. Cells 11, 783 (2022).
Popovici, D. et al. Comparative hematological profiles for dose-dense vs. regular anthracycline-based neoadjuvant chemotherapy in non-metastatic breast cancer. Exp. Ther. Med. 22, 747 (2021).
Sharma, P. et al. Clinical and biomarker results of neoadjuvant phase II study of pembrolizumab and carboplatin plus docetaxel in triple-negative breast cancer (TNBC)(NeoPACT). J. Clin. Oncol. 40, 513 (2022).
Pusztai, L. et al. Event-free survival by residual cancer burden with pembrolizumab in early-stage TNBC: exploratory analysis from KEYNOTE-522. Ann. Oncol. 35, 429–436 (2024).
Bonadio, R. C. et al. Management of patients with early-stage triple-negative breast cancer following pembrolizumab-based neoadjuvant therapy: What are the evidences? Cancer Treat. Rev. 110, 102459 (2022).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384, 164–172 (2014).
Acknowledgements
We thank the Information Technology Team from the Instituto D’Or de Pesquisa e Ensino for the assistance with the centralized case report form.
Author information
Authors and Affiliations
Contributions
R.C.B., M.C.T., J.B., D.D.R., D.A.S., J.A.P.A., D.M.G., C.H.A., B.M.Z., A.F., M.L.B., P.M.H., L.T., and R.B.S. contributed to the study conception and design. R.C.B., I.M.S., F.C.B., A.C.M.C., M.M.F.M., F.M., R.P.F., C.L.S., Z.S.S., J.A.P.A., D.M.G., C.H.A., B.M.Z., A.F., M.L.B., R.C., M.M.F.M., P.M.H., L.T., and R.B.S. contributed to the conduct or collection, data analysis and interpretation. R.C.B., M.C.T., J.B., D.D.R., D.A.S., J.A.P.A., D.M.G., C.H.A., B.M.Z., A.F., M.L.B., R.C., P.M.H., L.T., and R.B.S. contributed to the drafting of the manuscript and critical revisions. All authors gave their final approval of the manuscript to be submitted.
Corresponding author
Ethics declarations
Competing interests
The Authors declare no Competing Non-Financial Interests but the following Competing Financial Interests: R.C.B.: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo, Nestle Health Science, Addium, Gilead, MSD, BMS, AstraZeneca, Ache, Pfizer. Financial support for educational programs and symposia: AstraZeneca, Daiichi-Sankyo, MSD. Institutional Research grant: Novartis, AstraZeneca. J.B.: Speaker fees and/or honoraria for consulting or advisory functions: AstraZeneca, Daiichi-Sankyo, Lilly, Gilead, Pfizer, Novartis, MSD, Roche, Knight Pharmaceuticals. Financial support for educational programs and symposia: Roche, Daiichi-Sankyo. D.D.R.: Speaker fees and/or honoraria for consulting or advisory functions: AstraZeneca, Daiichi-Sankyo, Lilly, Libbs, Pfizer, Novartis, Roche, GSK, Sanofi, Amgen, Zodiac Pharma. Financial support for educational programs and symposia: Roche. D.A.S.: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo. Financial support for educational programs and symposia: AstraZeneca. J.A.P.A.: Speaker fees and/or honoraria for consulting or advisory functions: Novartis, AstraZeneca, MSD, Lilly. D.M.G.: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo, Teva, Roche, AstraZeneca, Pfizer, Lilly, Novartis. Financial support for educational programs and symposia: AstraZeneca, Libbs, Roche. Research grant: Novartis. B.M.Z.: AstraZeneca, Daiichi-Sankyo, Eli Lilly, Gilead, Pfizer, Novartis, MSD, Roche, Addium. A.F.: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo, Novartis, Gilead, MSD, BMS, AstraZeneca, Pfizer. Financial support for educational programs and symposia: AstraZeneca, Daiichi-Sankyo, MSD, Novartis. C.H.A.: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo, Gilead AstraZeneca, Novartis, MSD. Financial support for educational programs and symposia: AstraZeneca, Daiichi-Sankyo, MSD, Lilly, Rcohe, Novartis, Gilead, Medscape. R.C.: Speaker fees and/or honoraria for consulting or advisory functions: AstraZeneca, Daichii-Sankyo, Eli Lilly, Gilead, MSD, and GSK. M.M.F.M.: Speaker fees and/or honoraria for consulting or advisory functions: AstraZeneca, Daiichi-Sankyo, Eli Lilly, Gilead, Adium, Novartis, MSD, and Roche. Financial support for educational programs and symposia: AstraZeneca, Daiichi-Sankyo, Gilead, Eli Lilly, Roche, MSD and Novartis. P.M.H.: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo. L.T.: Speaker fees and/or honoraria for consulting or advisory functions: Daiichi-Sankyo, MSD, AstraZeneca, Pfizer, Lilly, Novartis. Financial support for educational programs and symposia: AstraZeneca, Roche, Gilead. Institutional Research grant: Novartis. R.B.S.: Speaker fees and/or honoraria for consulting or advisory functions: AstraZeneca, Daiichi-Sankyo, Eli Lilly, Gilead, Libbs, Pfizer, Novartis, MSD, and Roche. Financial support for educational programs and symposia: AstraZeneca, Daiichi-Sankyo, Gilead, Eli Lilly, and MSD. Institutional Research grant: AstraZeneca, Daiichi-Sankyo. I.M.S., F.C.B., A.C.M.C., M.C.T., F.M., R.P.F., C.L.S., and Z.S.S. declare no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bonadio, R.C., de Sousa, I.M., Balint, F.C. et al. Dose dense versus 3 weekly AC during neoadjuvant chemoimmunotherapy for triple negative breast cancer. npj Breast Cancer 10, 73 (2024). https://doi.org/10.1038/s41523-024-00676-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-024-00676-w
- Springer Nature Limited