Abstract
To date, no effective medical approach for the treatment of erectile dysfunction (ED) secondary to ischemic priapism (IP) has been described. The aim of this study was to evaluate the anti-inflammatory, antifibrotic, and antioxidant effects of pirfenidone (PFD) on cavernosal tissue in a rat model of IP. Forty-eight male albino rats aged 8–10 months, with mean weights of 410 ± 18.6 g were randomized into four groups (n = 12 in each group): no IP (group 1); IP for 1 h, followed by intracavernosal pressure (ICP) measurements using electrical cavernous nerve stimulation (CNS) (group 2); IP for 1 h, followed by ICP measurements using electrical CNS 6 weeks later (group 3); and IP for 1 h, oral PFD (30 mg/kg once daily) treatment by oral gavage, followed by ICP measurements using electrical CNS 6 weeks later (group 4). Malondialdehyde (MDA) and reduced glutathione levels were measured spectrophotometrically. In a histological evaluation, cavernosal collagen/smooth muscle ratios were calculated. The intracavernosal pressure values of group 1 were higher than those of groups 2 and 3 (p < 0.05) but similar to those of group 4 (p > 0.05). The mean MDA level was significantly higher in group 3, as compared with that in group 4 (p = 0.004). The mean collagen/smooth muscle ratio in groups 1–4 was 24%, 42%, 65%, and 48%, respectively. Physiological, biochemical, and histopathological evaluations of the PFD effect on cavernosal tissue in a rat model of IP were the strengths and the lack of molecular and immunohistochemical analysis were the limitations of this study. In this study, we examined the effects of PFD on cavernosal tissue in a rat model of IP. We found that PFD reduced cavernosal fibrotic activity and improved erectile function. We conclude that PFD may represent a new treatment option in IP treatment.
Similar content being viewed by others
Introduction
Priapism is defined as a state of persistent penile erection lasting longer than 4 h, unrelated to sexual arousal or desire [1, 2]. It has an incidence of 1/100,000 across age groups and is predominantly found in those aged 20–50 years [1, 2]. Three main subtypes of priapism are recognized: ischemic, nonischemic, and stuttering priapism. Ischemic priapism (IP) is characterized by low flow or veno-occlusion within a closed-compartment syndrome, with acidosis, hypercapnia, and hypoxia in cavernosal blood gas examination [3, 4]. IP is generally painful. In contrast, nonischemic priapism is characterized by high arterial blood flow and is generally nonpainful [3]. Stuttering priapism is an unusual and poorly understood form of low-flow priapism that occurs mostly in sickle-cell disease, and the data obtained from this study may not be applicable to this group. Of these three subtypes, IP is the most common type of priapism (95%) [3, 4]. Previous studies focusing on IP suggested that the presence of a dense fibrotic component secondary to the progression of fibrosis may result in erectile dysfunction (ED) [5, 6]. Research also showed that transforming growth factor-β (TGF-β) played a key role in fibrogenesis and that TGF-β1-neutralizing antibodies decreased the fibrotic effects of IP [7, 8].
Previous animal studies demonstrated that 5-methyl-1-phenyl-2-(1H)-pyridone (PFD), an antifibrotic agent, decreased collagen production and the formation of TGF-β, tumor necrosis factor-α, and platelet-derived growth factor, which are histological markers of progressive fibrosis [9,10,11]. In addition to its antifibrotic effects, anti-inflammatory and antioxidant effects of PFD have been demonstrated [12, 13]. Several studies have investigated the antifibrotic effects of PFD on lung, kidney, skin, and bladder [14,15,16,17]. However, there are no studies in the English literature on the relationship between PFD and cavernosal fibrosis secondary to IP.
In this study, we aimed to investigate the antifibrotic and antioxidant effects of PFD in a rat model of IP and to evaluate its effects on erectile function. To the best of our knowledge, this experimental IP rat model is the first in the literature to evaluate the antifibrotic effect of PFD on prolonged fibrosis and subsequent ED.
Materials and methods
Animals
This study complied with the guidelines of the Institutional Animal Care and Ethics Committee of Zonguldak Bulent Ecevit University (Zonguldak, Turkey) (approval no.: 2018/04). In total, 48 healthy Wistar albino rats aged 8–10 months, with mean body weights of 410 ± 18.6 g were included in this study. The animals were housed under standard vivarium conditions in a climate-controlled room (18–22 °C, 40–60% humidity, and 12-h light/dark cycle), with free access to water and standard food. All the procedures were conducted in accordance with the National Institutes of Health guide for the care and use of laboratory animals. To prevent any biases, all the stages of experiments were performed by a neutral crew who were blinded to groups throughout the study.
Groups and experimental design
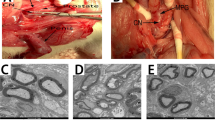
In this experimental study, ICP was preferred as the main marker, both for early-onset and prolonged erectile function, according to experimental groups. Following an adaptation period of 1 week, 48 rats were randomly divided into the following four groups, with 12 animals in each group: a non-IP group and intracavernosal pressure (ICP) measurements using electrical cavernous nerve stimulation (CNS) (group 1); experimental IP for 1 h and subsequent ICP measurements (group 2); experimental IP for 1 h, no PFD treatment, and ICP measurements 6 weeks later (group 3); and experimental IP for 1 h, PFD treatment (30 mg/kg administered orally once daily by oral gavage), and ICP measurements 6 weeks later (group 4). In this study, dosing of the PFD was calculated with reference to a clinical study in patients with idiopathic pulmonary fibrosis [18] and to an experimental rat model of severe pulmonary hypertension [19]. All the surgical procedures were performed under general anesthesia, induced by intraperitoneal ketamine (80 mg/kg). A priapism model was created, using a vacuum device and a constrictor band, as previously described in the literature [20, 21]. A 50-ml plastic syringe was used as a vacuum erection device. The most distal part of the tip of the syringe was cut to create an effective vacuum erection model. A rubber ring (2-mm thick) obtained from a 16 Fr Foley catheter was used to induce and sustain the penile erection. The tip of the device was placed over the penis at the level of the radix penis, and its piston was pulled up slightly, until an adequate erection was achieved. The constrictive ring was then pushed onto the radix penis (Fig. 1a).
a A 1-h ischemic priapism (IP) model was created by placing a constrictive ring (red arrow) on the radix penis. b Transverse section of a corpus cavernosum in group 1 (Masson’s trichrome stain under light microscopy, ×25), with smooth muscle fibers (red color) and collagen contents (blue color). (The yellow arrow denotes the urethra)
Physiological erection model
To produce unilateral CNS, a stainless-steel bipolar-stimulating electrode was attached to the posterolateral edge of the prostate. A 25-gauge needle, which was connected to a heparinized polyethylene tube, was then inserted into the left corpus cavernosum for ICP monitoring, using a data acquisition system (Biopac MP30 System, SantaBarbara, CA, USA). The CNS was administered to all rats, using a 15-Hz, 4-V electrical stimulation at a pulse width of 15 ms for 60 s [22, 23]. The animals were also catheterized intra-aortically for simultaneous intra-aortic blood pressure monitoring (Fig. 2).
Histopathological analysis
Following the ICP measurements, a penectomy was performed, and the cavernosal tissues were separated into two equal parts for further histopathological and spectrophotometric evaluation. Following extraction, the cavernosal tissues were placed in a solution of 10% formaldehyde for fixation and analysis. For the histopathological examination, the cavernosal tissue slices were cut into sections, 4-µm thick, using a fully automated microtome (Fig. 1b). Masson’s trichrome staining was used to measure the ratio of the collagen to the cavernosal smooth muscle. Under light microscopy, quantitative analysis was performed, using the Image J Threshold Color plugin in randomly selected four fields of each slide (×400), as follows: ≤30% collagen = (+), 31–50% collagen = (++), 51–70% collagen = (+++), and >70% collagen = (++++).
Biochemical analysis
Measurement of lipid peroxidation
Tissue malondialdehyde (MDA) levels were studied as a marker of lipid peroxidation. The cavernosal tissues obtained from the penectomy specimens were homogenized using 10% trichloroacetic acid (Sigma Chemical Co., St. Louis, MO, USA). The homogenates were individually centrifuged at 3000 rpm for 15 min, and the supernatant fractions were added to 1% butylhydroxytoluene (Sigma Chemical Co., St. Louis, MO, USA) and 0.67% thiobarbituric acid (Sigma Chemical Co., St. Louis, MO, USA) and boiled for 15 min. The obtained samples were evaluated spectrophotometrically at 535 nm. The MDA levels in corporal tissue were expressed as nanomoles/gram (nmol/g) [24].
Measurement of antioxidant activity in cavernosal tissue
The level of reduced glutathione (GSH) in cavernosal tissue was studied as an endogenous antioxidant marker. The cavernosal tissues were homogenized using 10% trichloroacetic acid (Sigma Chemical Co., St. Louis, MO, USA), and the homogenate was then centrifuged at 3000 rpm for 15 min. The obtained supernatant was added to 1% butylhydroxytoluene (Sigma Chemical Co., St. Louis, MO, USA), 0.67% thiobarbituric acid (Sigma Chemical Co., St. Louis, MO, USA), and dithiobisnitrobenzoate (Sigma Chemical Co., St. Louis, MO, USA) solution. The tissue absorbance was measured by spectrophotometry at 412 nm. Reduced glutathione levels in corporal tissue were expressed as micromole/gram (μmol/g) [25].
Statistical analysis
The statistical evaluation was performed using the Statistical Package for the Social Sciences for Windows software, version 21.0 (IBM, Chicago, IL, USA). Differences between the groups were assessed using the Student’s T-test, a one-way analysis of variance, and a paired T-test. A p-value of <0.05 was considered statistically significant.
Four animals from the first, second, and third groups and three animals from the fourth group that were not able to create a successful erection model, did not have any signs of ischemia in the pathological examination, or could not be alive during 6 weeks of follow-up, were excluded from the study.
Results
The mean basal ICP value in group 1 (15.0 ± 7.8 mmHg) was higher than that in group 2 (5.1 ± 2.4 mmHg) (p = 0.004) and group 3 (8.4 ± 4.1 mmHg) (p = 0.04) but similar to that in group 4 (12.6 ± 6.4 mmHg) (p = 0.48). The mean maximal ICP (MICP) value in group 1 (51.4 ± 17.6 mmHg) was higher than that in group 2 (16.9 ± 8.3 mmHg) (p = 0.001) and group 3 (31.8 ± 16.2 mmHg) (p = 0.03) but similar to that in group 1 (51.4 ± 17.6 mmHg) and group 4 (53.1 ± 19.1 mmHg) (p = 0.85). The MeICP/mean intra-arterial blood pressure (MIBP) ratio was significantly higher in group 1 (0.42 ± 0.11) as compared with that in group 2 (0.11 ± 0.07) (p = 0.001) but similar to that in group 4 (0.33 ± 0.15) (p = 0.21) (Table 1).
The mean area under curve (AUC) value, which reflected the efficacy of the quality of the ICP response, in group 1 (563.5 ± 353.5), was higher than that in group 2 (253.4 ± 193.6) (p = 0.04), but the mean AUCs were similar in group 1 and group 4 (p = 0.30) (Table 1).
The mean MDA level in group 2 (125.1 ± 22.5 nmol/g) was higher than that in group 1 (64.5 ± 7.2 nmol/g) (p = 0.043). The mean MDA level was significantly higher in group 3 (29.5 ± 5.8 nmol/g) as compared with that in group 4 (6.2 ± 0.8 nmol/g) (p = 0.004) (Table 2).
The mean cavernosal GSH levels were 5.0 ± 1.2, 2.7 ± 0.8, 1.2 ± 0.2, and 2.1 ± 0.9 μmol/g in groups 1–4, respectively. The mean GSH level in group 3 was increased as compared with that in group 4, although the increase was statistically nonsignificant (p = 0.32) (Table 2).
As shown by the histological evaluation, the percentage and grade of cavernosal fibrosis were + and 26%, ++ and 42%, +++ and 65%, and ++ and 48% in groups 1, 2, 3, and 4, respectively (Fig. 3).
Discussion
Previous studies of PFD investigated its effects on idiopathic—or drug-induced—lung inflammation and fibrosis [18, 26], renal fibrosis [15], skin scarring [16], and bladder fibrosis [17]. This study is the first to investigate the effect of PFD on cavernosal fibrosis and erectile function.
Cavernosal, neuronal, and vascular integrity are the main components necessary for initiating and maintaining an adequate erection. IP is a clinical condition that causes ED by affecting at least one of these components. Several experimental animal models mimicking a human erection have been described in the literature to evaluate erectile function [8, 20,21,22,23]. Among these models, electrical rhythmic stimulation of the cavernous nerve is one of the most widely applied techniques for simulating a human erection, with subsequent monitorization of the ICP/intra-aortic pressure ratio. Using this technique, we compared different ICP fractions, such as the basal ICP, mean MICP, MeICP, and MeICP/MIBP ratio, to shed light on erectile function among the different groups (Fig. 2, Table 1). As shown by the results, these ICP fractions were significantly higher in group 1, as compared with those in groups 2 and 3. This finding can be explained by increased fibrotic activity secondary to cavernosal ischemia in groups 2 and 3. The histological evaluation of the cavernosal specimens in groups 2 and 3 was consistent with increased fibrotic activity, as compared with that in group 1 (Fig. 3). As the priapism process progressed, an increased rate of fibrosis was observed in group 3 (mean collagen/smooth muscle ratio: 65%) as compared with that in group 1 (mean collagen/smooth muscle ratio: 24%). PFD given orally once a day by oral gavage for 6 weeks led to a decrease in cavernosal fibrosis in group 4 (48%). At the end of the 6-week period, the mean amount of collagen had regressed in group 4, and the level of fibrotic activity was similar to that in group 2 (42%) (Fig. 3). Furthermore, statistically significant improvements in ICP measurements were observed in group 4 (PFD-treated group) as compared with the same parameters in groups 2 and 3 (Table 1). In addition, the MICP, PICP, and AUC values in group 4 were higher than those in group 3, demonstrating that erectile function in this group 6 weeks after the PFD treatment was better than that in group 3 in this rat model of IP. In terms of cavernosal histology and ICP variables, the findings showed that PFD effectively prevented the formation of fibrosis and improved erectile function in this present IP rat model. Considering the effect of PFD on erectile function in rats, despite its speculative potential, clinical implications of this agent can be reflected in human model of delayed ischemic priapism.
Nonenzymatic lipid peroxidation is a harmful chain reaction, which directly damages the cellular membrane and indirectly damages other cell components via reactive aldehydes. The levels of MDA are commonly used as a marker of lipid peroxidation at the cellular level [21, 27]. In this study, cavernosal MDA levels were significantly increased in group 2 after IP for 1 h, as compared with those in group 1. This finding reflected the increase in lipid peroxidation, due to cavernosal tissue ischemia, and was consistent with that of previous reports in the literature [21, 27]. The levels of MDA decreased in group 3 after 6 weeks. The lowest MDA levels were recorded in group 4 after 6 weeks of the PFD treatment. The decrease in MDA levels in group 4 as compared with those in group 3 supports the antioxidant activity of PFD. Previous rat model studies on the effect of priapism on MDA levels investigated only its short-term effects [21, 27,28,29,30]. To the best of our knowledge, this study is the first to evaluate the long-term changes in MDA levels in cavernosal tissue. We believe that the lack of data in the literature on the long-term effect of IP on MDA levels increases the value of our study.
Although there was no statistically significant difference in the mean GSH levels of the groups, it is noteworthy that the level of GSH progressively decreased from group 1 to 3. The mean GSH level, which was slightly increased in group 4 (PFD-treated group) (Table 2) was similar to that in group 2. This finding pointed to the recovery of oxidant activity in the PFD-treated group within 6 weeks. Given the observed increase in the GSH level in the PFD group, we will investigate the antioxidant response to different doses of PFD in the future. The increase in the GSH level in the PFD group has encouraged us to investigate the antioxidant response to different doses of PFD in the future.
Limitations of the study
The PFD effect on penile erectile function was analyzed using physiological, biochemical, and histopathological parameters in a rat model of IP. However, this study has some limitations including
- 1.
The lack of molecular and immunohistochemical analysis
- 2.
The lack of clarification of PFD dosing in a rat model of ischemic priapism
- 3.
The lack of data regarding dose adjustment in human beings
- 4.
Because all the animals were killed at the end of the experiments, the lack of observational data on sexual life among the groups following PFD use
- 5.
The lack of data for stuttering priapism and nonischemic priapism
Conclusions
In this study, PFD treatment reduced cavernosal fibrosis, and the treatment was associated with an improvement in erectile function and histology in an experimentally induced rat model of IP. The results suggest that PFD may represent a new treatment option in the management of ED secondary to IP. Although the findings are promising and point to a possible oral medical treatment for IP, further studies are needed.
References
Salonia A, Eardley I, Giuliano F, Hatzichristou D, Moncada I, Vardi Y, et al. European Association of Urology guidelines on priapism. Eur Urol. 2014;65:480–9.
Cherian J, Rao AR, Thwaini A, Kapasi F, Shergill IS, Samman R. Medical and surgical management of priapism. Post Med J. 2006;82:89–94.
Broderick GA, Kadioglu A, Bivalacqua TJ, Ghanem H, Nehra A, Shamloul R. Priapism: pathogenesis, epidemiology, and management. J Sex Med. 2010;7:476–500.
Zacharakis E, Garaffa G, Raheem AA, Christopher AN, Muneer A, Ralph DJ. Penile prosthesis insertion in patients with refractory ischaemic priapism: early vs delayed implantation. BJU Int. 2014;114:576–81.
Yuan J, Desouza R, Westney OL, Wang R. Insights of priapism mechanism and rationale treatment for recurrent priapism. Asian J Androl. 2008;10:88–101.
Pautler SE, Brock GB. Priapism. From priapus to the present time. Urol Clin North Am. 2001;28:391–403.
Kinashi H, Ito Y, Sun T, Katsuno T, Takei Y. Roles of the TGF-β VEGF-C pathway in fibrosis-related lymphangiogenesis. Int J Mol Sci. 2018;19:2487.
Sanli O, Armagan A, Kandirali E, Ozerman B, Ahmedov I, Solakoglu S, et al. TGF-beta1 neutralizing antibodies decrease the fibrotic effects of ischemic priapism. Int J Impot Res. 2004;16:492–7.
Zanotti S, Bragato C, Zucchella A, Maggi L, Mantegazza R, Morandi L, et al. Anti-fibrotic effect of pirfenidone in muscle derived-fibroblasts from Duchenne muscular dystrophy patients. Life Sci. 2016;15:127–36.
Stahnke T, Kowtharapu BS, Stachs O, Schmitz KP, Wurm J, Wree A, et al. Suppression of TGF-β pathway by pirfenidone decreases extracellular matrix deposition in ocular fibroblasts in vitro. PLoS ONE. 2017;23(12):e0172592. eCollection 2017.
Yu W, Guo F, Song X. Effects and mechanisms of pirfenidone, prednisone and acetylcysteine on pulmonary fibrosis in rat idiopathic pulmonary fibrosis models. Pharm Biol. 2017;55:450–5.
Zhao XY, Zeng X, Li XM, Wang TL, Wang BE. Pirfenidone inhibits carbon tetrachloride and albumin complex induced liver fibrosis in rodents by preventing activation of hepatic stellate cells. Clin Exp Pharm Physiol. 2009;36:963–8.
Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on transforming growth factor beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharm Exp Ther. 1999;291:367–73.
Oku H, Shimizu T, Kawabata T, Nagira M, Hikita I, Ueyama A, et al. Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharm. 2008;590:400–8.
Chen JF, Ni HF, Pan MM, Liu H, Xu M, Zhang MH, et al. Pirfenidone inhibits macrophage infiltration in 5/6 nephrectomized rats. Am J Physiol Ren Physiol. 2013;304:F67–85. Epub 2012 Nov 14.
Armaendariz Borunda J, Lyra Gonzalez I, Medina Preciado D, Gonzalez-García I, Martinez-Fong D, Miranda RA, et al. A controlled clinical trial with pirfenidone in the treatment of pathological skin scarring caused by burns in pediatric patients. Ann Plast Surg. 2012;68:22–8.
Duan LJ, Qi J, Huang T, Gu X, Xu D, Kong XJ, et al. Pirfenidone attenuates bladder fibrosis and mitigates deterioration of bladder function in a rat model of partial bladder outlet obstruction. Mol Med Rep. 2015;12:3639–47.
Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, et al. Double-blind, placebo controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–7.
Poble PB, Phan C, Quatremare T, Bordenave J, Thuillet R, Cumont A, et al. Therapeutic effect of pirfenidone in the sugen/hypoxia rat model of severe pulmonary hypertension. FASEB J. 2019;33:3670–9.
Jin YC, Gam SC, Jung JH, Hyun JS, Chang KC, Hyun JS. Expression and activity of heme oxygenase-1 in artificially induced low-flow priapism in rat penile tissues. J Sex Med. 2008;5:1876–82.
Uluocak N, Atilgan D, Erdemir F, Parlaktas BS, Yasar A, Erkorkmaz U, et al. An animal model of ischemic priapism and the effects of melatonin on antioxidant enzymes and oxidative injury parameters in rat penis. Int Urol Nephrol. 2010;42:889–95. Epub 2010 Jan 30.
Zhao S, Kang R, Deng T, Luo L, Wang J, Li E, et al. Comparison of two cannulation methods for assessment of intracavernosal pressure in a rat model. PLoS ONE. 2018;13:e0193543. https://doi.org/10.1371/journal.pone.0193543. eCollection 2018.
La Favor JD, Fu Z, Venkatraman V, Bivalacqua TJ, Van Eyk JE, Burnett AL. Molecular profile of priapism associated with low nitric oxide bioavailability. J Proteome Res. 2018;17:1031–40.
Casini A, Ferrali M, Pampella A, Maellaro E, Comporti M. Lipid peroxidation and cellular damage in extrahepatic tissues of bromobenzene-intoxicated mice. Am J Pathol. 1986;123:520–31.
Aykaç G, Uysal M, Yalçin AS, Koçak-Toker N, Sivas A, Oz H. The effect of chronic ethanol ingestion on hepatic lipid peroxide, glutathione, glutathione peroxidase and glutathione transferase in rats. Toxicology. 1985;36:71–6.
Kehrer JP, Margolin SB. Pirfenidone diminishes cyclophosphamide-induced lung fibrosis in mice. Toxicol Lett. 19977;90:125–32.
Cevik O, Cadirci S, Sener TE, Tinay I, Akbal C, Tavukçu HH, et al. Quercetin treatment against ischemia/reperfusion injury in rat corpus cavernosum tissue: a role on apoptosis and oxidative stress. Free Radic Res. 2013;47:683–91.
Kucukdurmaz F, Kucukgergin C, Akman T, Salabas E, Armagan A, Seckin S, et al. Duration of priapism is associated with increased corporal oxidative stress and antioxidant enzymes in a rat model. Andrologia. 2016;48:374–9.
Karaguzel E, Bayraktar C, Kutlu O, Yulug E, Mentese A, Okatan AE, et al. The possible protective effects of dipyridamole on ischemic reperfusion injury of priapism. Int Braz J Urol. 2016;42:146–53.
Evliyaoglu Y, Kayrin L, Kaya B. Effect of allopurinol on lipid peroxidation induced in corporeal tissue by veno-occlusive priapism in a rat model. Br J Urol. 1997;80:476–9.
Acknowledgements
We would like to thank Meryem Akpolat Ferah, due to her contribution to staining of slides with Masson’s trichrome stain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cinar, O., Bolat, M.S., Erdem, S. et al. The effect of an antifibrotic agent, pirfenidone, on penile erectile function in an experimental rat model of ischemic priapism. Int J Impot Res 32, 232–238 (2020). https://doi.org/10.1038/s41443-019-0152-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41443-019-0152-9
- Springer Nature Limited
This article is cited by
-
Pirfenidone targeted mechanisms for alleviating methotrexate-induced testiculopathy in Wistar rats
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)