Abstract
To determine if the insulin-like growth factor-1 (IGF-1) pathway is involved in the improvement in erectile function recovery in rats after nerve crush injury treated with pioglitazone (Pio). Sprague-Dawley rats were divided into four groups. The first group received sham operation (n = 5). The second group underwent bilateral cavernous nerve injury (BCNI, n = 7). The third group received BCNI and Pio treatment (BCNI + Pio, n = 7), whereas the fourth group underwent BCNI with Pio treatment and IGF-1 inhibition (BCNI + Pio + JB-1, n = 7). The IGF-1 receptor (IGF-1R) was inhibited by JB-1, a small molecular antagonist of the receptor. After 14 days of treatment, erectile function was measured via intracorporal pressure normalized to mean arterial pressure (ICP/MAP) and the major pelvic ganglion and cavernous nerve harvested for western blot and immunohistochemistry (IHC) of phosphorylated-IGF-1Rβ (p-IGF-1Rβ), phosphorylated-ERK1/2 (p-ERK1/2), and neuronal NOS (nNOS). BCNI + Pio animals exhibited improvements in ICP/MAP, similar to Sham animals, and BCNI + Pio + JB-1 rats demonstrated a reduced ICP/MAP similar to BCNI-only rats at all measured voltages. Western blot results showed upregulation of p-IGF-1Rβ was observed in the BCNI + Pio group. Low levels of p-ERK1/2 were seen in the JB-1-treated animals. The immunoblot results were supported by IHC findings. Intense IHC staining of nNOS was detected in the BCNI + Pio group. The group treated with JB-1 showed minimal protein expression of p-ERK1/2, nNOS, and p-IGF-1Rβ. Pio improves erectile function in rats undergoing BCNI via an IGF-1-mediated pathway.
Similar content being viewed by others
Introduction
Radical prostatectomy, a common treatment for localized prostate cancer, is associated with post-operative erectile dysfunction (ED) in 16–82% of men undergoing the operation [1, 2]. Current treatment options for post-prostatectomy ED include alprostadil injections, vacuum erection devices, and phosphodiesterase-5-inhibitors (PDE5Is) [3]. Success with these therapies, even in combination, is variable; therefore, novel therapies are needed to prevent injury or accelerate recovery of erectile function after pelvic surgery such as prostatectomy. The bilateral cavernous nerve injury (BCNI) procedure in the rat is an accepted animal model to mimic similar injury seen in pelvic surgery [4,5,6].
Previous studies performed by this laboratory have used pioglitazone (Pio) in the BCNI rat model due to its vasculoprotective and neuroprotective effects in cerebrovascular accidents [7]. Aliperti et al. found that Pio improved erectile function recovery after BCNI surgery [8]. Katz et al. determined that Pio promoted neuroregeneration of the cavernous nerve (CN) [9]. However, the mechanism of Pio’s beneficial effects on erectile function recovery has yet to be elucidated.
Pio is part of a class of thiazolidinediones (TZDs) used to treat diabetes. This class of drugs is known to act on the peroxisome proliferator-activated receptor gamma (PPAR-γ) receptor [10]. In diabetes, Pio improves insulin sensitivity in type 2 diabetics and regulates lipid and carbohydrate metabolism through this pathway [11,12,13]. However, Pio is not specific to the PPAR-γ pathway, and more recent studies have demonstrated that some of Pio’s beneficial effects come from activation of the insulin-like growth factor-1 (IGF-1) pro-proliferative pathway of Ras/Raf/MEK (MAPK/ERK kinases)/ERK (extracellular signal-regulated kinases), with Ras being a small G protein and Raf a protein kinase [14, 15]. Furthermore, other laboratories have revealed beneficial effects of IGF-1 administration on peripheral nerve regeneration, including the CN in the rat after cryoablation [16,17,18]. Therefore, the purpose of this experiment was to determine if the IGF-1 pathway had a significant role in the erectile function recovery of rats post-BCNI. In order to evaluate the mechanism of Pio, the IGF-1 receptor (IGF-1R) was competitively antagonized and the effects on erectile function recovery were assessed.
Materials and methods
Animal and experimental design
Male Sprague-Dawley rats (n = 26; Charles River, Wilmington, MA) weighing 350–400 g were evaluated in this study. Rats underwent sham surgery (n = 5), BCNI surgery (n = 7), BCNI + Pio (n = 7), or BCNI + Pio + JB-1 (n = 7). N numbers were selected based on a study by Aliperti et al. [8]. JB-1 trifluoroacetate salt 98% (Sigma-Aldrich, St. Louis, MO) is a competitive antagonist of the IGF-1R. After 14 days of treatment, animals underwent terminal surgery to evaluate erectile function and for tissue harvest. All experiments were conducted in accordance with our University’s guidelines for animal care and use as specified by IACUC.
Nerve crush surgery
Nerve crush surgery was performed as previously described by the same surgeon [8, 9]. Briefly, rats were anesthetized using intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) and the prostate exposed via midline laparotomy. The major pelvic ganglion (MPG) and CN were identified. In the sham group, no further manipulation was performed. In the BCNI groups, both CNs underwent crush injury by applying a #7 Dumont forceps three times for 15 s each, 3–5 mm distal to the MPG. Adequate crush was confirmed by an observable change in color to gray, with the neurolemma remaining intact. The abdomen was then closed in two layers.
Osmotic mini-pump placement and therapy
Immediately following BCNI, a superficial midscapular incision was made and a 200 µL Alzet osmotic mini-pump (Braintree Scientific, Inc., Braintree, MA) was inserted in the subcutaneous tissue posterior to the scapula, and the wound site was closed with staples (Fig. 1b). Sham, BCNI, and BCNI + Pio rats had the osmotic mini-pumps loaded with saline. BCNI + Pio + JB-1 rats received 100 µg/kg/rat/day JB-1 trifluoroacetate salt 98% continuous infusion based on results from Sumino et al. [19].
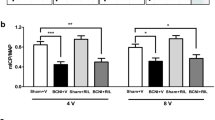
a Intracavernosal pressure/mean arterial pressure (ICP/MAP) results show bilateral cavernous crush injury (BCNI) and BCNI + Pio + JB-1 groups to have significantly lower results than sham at all voltages (2.5 V p = 0.0003; 5 V p = 0.0009, 7.5 V p < 0.0001). The * designates different than sham. b AUC demonstrates decreased duration of erection in bilateral cavernous crush injury (BCNI) and BCNI + Pio + JB-1 groups compared with sham (2.5 V p = 0.0689, 5 V p = 0.0026, 7.5 V p < 0.0001) (* designates different than sham). c Example tracings are shown. The area of electrical stimulation is shown in the black box
Oral therapy
Sham and BCNI rats were treated with saline via oral gavage for 14 days. BCNI + Pio and BCNI + Pio + JB-1 rats were treated with Pio 6.5 mg/kg/rat/day dissolved in phosphate-buffered saline for 14 days. Fourteen days and the dose of Pio were based on previous studies by this group [8, 9].
Erectile response
At 14 days after crush injury, rats were anesthetized using Inactin hydrate 98% (Sigma-Aldrich, Inc., St. Louis, MO) at 100 mg/kg. The right crura was cannulated with a 25-G needle connected to a Namic Perceptor DT pressure transducer (Navilyst Medical, Marlborough, MA) and a data acquisition system (Biopac MP 100A-CE, Santa Barbara, CA) to measure intracorporal pressure (ICP). The right carotid artery was cannulated for continuous measurement of mean arterial pressure (MAP). The CN distal to the crush injury was stimulated with a square pulse stimulator (Grass Instruments SD9 stimulator, West Warwick, RI) at a frequency of 20 Hz, duration 0.5 ms, and pulse width of 0.2 ms at increasing voltages (2.5 V, 5 V, and 7.5 V) for 1 min, with 4-min intervals between stimulations. The ratio between maximal ICP and MAP (ICP/MAP) obtained at the peak of erectile response was calculated to normalize for variations in systemic blood pressure. Total ICP was measured as the area under the curve (AUC) during the time of stimulation. CN and MPG samples were removed and either snap frozen in liquid nitrogen and stored at –80 °C or fixed in formalin for further biochemical and histologic analysis.
Western blot
After homogenization of whole paired MPGs and CNs in RIPA buffer (Santa Cruz Biotechnology, Dallas, TX), the total protein lysates were measured and 20 μg of protein samples were fractionated onto 4–20% gradient sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels. Proteins were transferred onto Hybond ECL nitrocellulose membranes (0.45 μm, Amersham Pharmacia Biotech, Amersham, UK), and incubated with primary antibodies: phosphorylated extracellular signal-regulated kinase 1/2 (p-ERK1/2) (Cell Signaling Technology, Danvers, MA), neuronal nitric oxide synthase (nNOS) (Cell Signaling Technology, Danvers, MA and phosphorylated-IGF-1-recepter β (p-IGF-1Rβ). GAPDH antibody was used as a loading control (Cell Signaling Technology, Danvers, MA). The blots were incubated with horseradish peroxidase secondary antibodies and visualized using the Pierce enhanced chemiluminescence western blot detection system (Life Technologies, Carlsbad, CA). Densitometric analysis for each protein relative to GAPDH was performed using ImageJ software version 1.48 (NIH, Bethesda, MD).
Immunohistochemistry (IHC)
Following deparaffinization, sections were dehydrated and treated with 3% hydrogen peroxide for one hour and blocked with 5% goat serum at 1:100 dilution for 1 h. The primary antibodies were p-ERK1/2 (Cell Signaling Technology, Danvers, MA), nNOS rabbit polyclonal antibody (Abcam Inc., Cambridge, MA), and p-IGF-1Rβ (Cell Signaling Technology, Danvers, MA). Following primary antibody incubation, the slide sections were incubated with goat-anti-rabbit secondary antibody Vectastain Elite (Vector Laboratories, Burlingame, CA) at a dilution of 1:50 for 1 h followed by treatment with the ABC Kit for one hour. The color detection was achieved by treatment with DAB (3′-diaminobenzidine tetra-hydrochloride) (Vector Laboratories, Burlingame, CA) for 10 min. Sections were counterstained in hematoxylin for 1 min and dehydrated, cleared, and mounted. Quantitative image analysis (the number of positive-staining cells per high-powered field) of each tissue section was performed using ImageJ software.
Statistical analysis
Data are shown as the mean ± standard error of mean or fold-change difference. Statistical difference between multiple groups was determined using analysis of variance (ANOVA) with the Dunnett multiple comparison test. Statistical analysis was performed using R 3.4.1 (R Foundation for Statistical Computing). p-Values < 0.05 were deemed significant.
Results
Erectile response
Rat erectile function was investigated by determining ICP/MAP (Fig. 1a) and AUC (Fig. 1b). Erectile function was diminished in BCNI and BCNI + Pio + JB-1 groups when compared with sham group for both ICP/MAP at all voltages (2.5 V p = 0.0003; 5 V p = 0.0009, 7.5 V p < 0.0001). AUC results showed similar trends of BCNI and BCNI + Pio + JB-1 groups being lower than sham. However, neither group showed significance at 2.5 V. (2.5 V p = 0.0689, 5 V p = 0.0026, 7.5 V p < 0.0001). The BCNI + Pio group was never significantly different than sham. Representative images are shown in Fig. 1c.
Western blot
The western blot results of the MPG + CN are depicted in Fig. 2. The p-ERK1/2 expression was significantly increased in BCNI + Pio when compared with BCNI or BCNI + Pio + JB-1 (p = 0.0152, p = 0.0230, respectively). Sham was not statistically significant from any other group. Western blot results for nNOS expression showed no statistical significance between groups. The p-IGF-1Rβ levels were significantly higher in BCNI + Pio than sham (p = 0.0002).
a Western blot results for p-ERK1/2 show significantly decreased p-ERK1/2 in bilateral cavernous crush injury (BCNI) (p = 0.0152) and BCNI + Pioglitazone (Pio) + JB-1 (p = 0.0230) when compared with the BCNI + Pio group. The # designates statistical significance when compared with BCNI + Pio. b There was no statistical significance in neuronal nitric oxide synthases (nNOSs) between groups on western blot. c Western blot of phosphorylated insulin-like growth factor-1 receptor beta (p-IGF-1Rβ) shows increased protein levels of the BCNI + Pio group compared with Sham (p = 0.0002) (* designates different than sham). d Western blot images
Immunohistochemistry
IHC analysis results of the MPG + CN are shown in Fig. 3. Higher immunoreactivity to p-ERK1/2 was observed in BCNI and BCNI + Pio + JB-1 in comparison with the sham group (p = 0.0230). The nNOS expression showed significantly higher expression in BCNI and BCNI + Pio groups when compared with sham. In contrast, the expression of nNOS levels was significantly lower in BCNI + Pio + JB-1 compared with Sham groups (p < 0.0001). The p-IGF-1Rβ expression levels were significantly higher in the BCNI + Pio group and significantly lower in the BCNI + Pio + JB-1 group when compared with Sham (p = 0.0002). Figures 3d-e demonstrate representative IHC images, where there is clear upregulation of ERK1/2, IGF-1R, and nNOS protein levels in the BCNI + Pio groups (Fig. 4). Further, nNOS and IGF-1R are located predominantly in the cytoplasm in the BCNI + Pio groups, whereas BCNI and BCNI + Pio + JB-1 groups demonstrate more nuclear staining.
a Immunohistochemistry (IHC) of p-ERK1/2 shows an upregulation in the bilateral cavernous crush injury + Pio (BCNI + Pio) group and a downregulation in the BCNI + Pio + JB-1 group when compared with sham (p = 0.0230). (* designates different than sham). b IHC of neuronal nitric oxide synthases (nNOSs) BCNI + Pio to have the higher values when compared with sham, with the JB-1 group having significantly lower protein than sham (p < 0.0001). c IHC of phosphorylated insulin-like growth factor-1 receptor beta (p-IGF-1Rβ) similarly shows an increase in the BCNI + Pio group and a decreased protein expression in the BCNI + Pio + JB-1 group (p = 0.0002). d Representative immunohistochemical images (100 × ). e Magnified areas of interest showing clear upregulation of ERK1/2 in the BCNI + Pio and sham groups. nNOS and IGF-1R are located predominantly in the cytoplasm in the BCNI + Pio groups, whereas BCNI and BCNI + Pio + JB-1 groups demonstrate more nuclear staining for nNOS and IGF-1R
Representative image of the IGF-1R cell signaling pathway. IGF-1R shown with an alpha and beta subunit and functions as a receptor tyrosine kinase IGF-1 binds to the extracellular domain of IGF-1R promoting autophosphorylation and subsequent activation. The activated receptor then promotes the phosphorylation of Ras, which promotes Raf phosphorylation. Raf kinases then catalyze the phosphorylation of MEK1/2. MEK1/2 mediates the phosphorylation of tyrosine and threonine in ERK1/2 and its downstream pathway
Discussion
As demonstrated by ICP/MAP and AUC, animals treated with Pio alone resulted in a clear improvement in erectile function recovery when compared with their untreated counterparts [8, 9]. Most importantly, the utility of Pio in the clinical setting is less clear. Pio has a black box warning for use in the setting of congestive heart failure and recently announced that it will update this warning label to include the increased risk of bladder cancer (https://www.fda.gov/Drugs/DrugSafety/ucm519616.htm). Furthermore, data are exceedingly conflicting as to whether Pio is protective, neutral, or detrimental in its role with prostate cancer [20,21,22,23,24,25]. Interestingly, TZDs as a class of medication have shown both upregulation and downregulation of the IGF-1 pathway depending on the type of TZD and the tissue of interest [15]. Therefore, the goal of this study was to further elucidate the mechanism of Pio in erectile function recovery by inhibiting the IGF-1 pathway using a small molecular antagonist of the IGF-1R.
In this study, when JB-1 (the IGF-1R antagonist) was added to treatment, the effects of Pio were negligible. This suggests that Pio improves erectile function recovery by stimulating the IGF-1 pathway shown in Figure 4. This is consistent with other literature that has documented that IGF-1 is beneficial for peripheral nerve regeneration in rat models of nerve injury [16, 17]. The IGF-1R is known to have two major downstream pathways, one for metabolism and one for proliferation. It was hypothesized that if Pio were to stimulate the IGF-1 pathway, the proliferation pathway of Ras/Raf/MEK/ERK was the determining factor. Therefore, p-ERK1/2 protein, the active form of ERK1/2, was quantified using western blot and IHC. As the IGF-1R was antagonized, it is expected that the levels of ERK1/2 would vary accordingly. Western blot and IHC results showed that Pio increased the active form of ERK1/2, whereas the addition of JB-1 was not associated with an upregulation of the ERK1/2 protein. ERK1/2 is a serine/threonine kinase, activated by MEK, which has over 50 substrates and a significant but blurred role in many types of cell proliferation [26, 27]. ERK1/2 is known to be upregulated in nerve regeneration and differentiation [27, 28]. These data give further evidence that the IGF-1 pathway is necessary for Pio’s beneficial effect on neuroprotection and thus, erectile functional recovery.
nNOS is well known to be a major player involved in erection. There was no significant difference in nNOS between groups using western blot. However, IHC showed a significant increase in BCNI + Pio and a significant decrease in BCNI + Pio + JB-1. Although there is a discrepancy between the two protein analyses, the same trend was shown in a study by Katz et al. In this study, western blot also showed no statistical significance of nNOS protein in the MPG + CN after Pio treatment, but there was a detectable increase in nNOS in the MPG + CN when IHC was performed [9]. As western blot examines overall protein content, while IHC demonstrates location, it is possible that the amount of protein is the same between groups, but the protein is more concentrated in the cytoplasm of nerve terminals and Schwann cells, rather than located near the nucleus or extracytoplasmic [29]. This is consistent with literature that states nNOS activity is dependent on its subcellular location [30]. Similar to our previous experiment, this provides evidence that Pio has a neuroprotective effect on the MPG + CN after injury, and that inhibition of the IGF-1 pathway negates this protective effect. More experimentation can be done to determine whether nNOS was increased due to an increase in neuron number after treatment or a compensatory increase in production of nNOS by the surviving neurons.
The last outcome measure of interest was the active form of the IGF-1R. Western blot results showed an increase when Pio was administered, with JB-1 inhibiting this increase in p-IGF-1Rβ. IHC similarly showed an increase in p-IGF-1Rβ with Pio and a decrease in p-IGF-1Rβ when the IGF-1R was inhibited by JB-1. As only the MPG + CN tissue was analyzed in this study, it is unknown whether Pio improved erectile function recovery only through its neuroprotective effects or if it affects the downstream corporal tissue, as well. For example, it is possible that downstream corporal tissue may utilize the PPAR-γ pathway, whereas the upstream MPG + CN may be activated by the IGF-1 pathway. Pio is known to upregulate distinct pathways depending on the type of tissue and to have cross-talk between the PPAR-γ and IGF-1 pathways [15]. Therefore, this possibility cannot be excluded. In the study by Aliperti et al., anti-smooth muscle actin immunofluorescence showed increased staining intensity after addition of Pio at 2 weeks. However, it is not known in this study whether the corporal tissue was affected by the inhibition of IGF-1R by JB-1. Future work will need to be performed to further elucidate the difference in the Pio mechanism at the level of the nerve compared with the level of the erectile tissue microarchitecture.
It is also possible that JB-1, the antagonist, could have had a direct effect on erectile function. Future studies should examine the effects of JB-1 alone on ICP/MAP in the rat both with and without CN injury. Another option to examine this potential confounder could be a washout period of JB-1 before end point analysis.
Another limitation is that the dose of Pio used in this study is nearly 10-fold more than the Food and Drug Administration recommends [9]. Again, the clinical utility of using Pio is a real area of concern. However, the short duration of Pio needed for neuroprotection post-operatively may make the harmful associations with chronic use negligible. What may be more clinically relevant is to determine the mechanism associated with neuroprotection from Pio. As Pio has multiple effects that vary based on the type of tissue, isolating the mechanism needed for neuroprotection may yield a more meaningful treatment or prevention measure against post-prostatectomy ED. Other downstream markers of the IGF-1 pathway were not analyzed in this study, but may provide useful mechanistic information and should be determined in the future.
Conclusion
Overall, these data suggest that Pio improves erectile function recovery after BCNI in the rat via an IGF-1-mediated mechanism. Further work should be performed to further elucidate the mechanism and improve the clinical utility of pharmacotherapy for post-prostatectomy ED.
References
Kendirci M, Hellstrom WJ. Current concepts in the management of erectile dysfunction in men with prostate cancer. Clin Prostate Cancer. 2004;3:87–92.
Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 2016;197:S165–70.
Schauer I, Keller E, Muller A, Madersbacher S. Have rates of erectile dysfunction improved within the past 17 years after radical prostatectomy? A systematic analysis of the control arms of prospective randomized trials on penile rehabilitation. Andrology. 2015;3:661–5.
Weyne E, Mulhall J, Albersen M. Molecular pathophysiology of cavernous nerve injury and identification of strategies for nerve function recovery after radical prostatectomy. Curr Drug Targets. 2015;16:459–73.
Chung E, De Young L, Brock GB. Investigative models in erectile dysfunction: a state-of-the-art review of current animal models. J Sex Med. 2011;8:3291–305.
Quinlan DM, Nelson RJ, Partin AW, Mostwin JL, Walsh PC. The rat as a model for the study of penile erection. J Urol. 1989;141:656–61.
Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130:685–96.
Aliperti LA, Lasker GF, Hagan SS, Hellstrom JA, Gokce A, Trost LW et al. Efficacy of pioglitazone on erectile function recovery in a rat model of cavernous nerve injury. Urology. 2014;84:1122–7.
Katz EG, Moustafa AA, Heidenberg D, Haney N, Peak T, Lasker GF, et al. Pioglitazone enhances survival and regeneration of pelvic ganglion neurons after cavernosal nerve injury. Urology. 2016;89:76–82.
Smith U. Pioglitazone: mechanism of action. Int J Clin Pract Suppl. 2001;121:13–8.
Kintscher U, Law RE. PPARgamma-mediated insulin sensitization: the importance of fat versus muscle. Am J Physiol Endocrinol Metab. 2005;288:E287–91.
Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–88.
Escher P, Wahli W. Peroxisome proliferator-activated receptors: insight into multiple cellular functions. Mutat Res. 2000;448:121–38.
Higashi Y, Holder K, Delafontaine P. Thiazolidinediones upregulate insulin-like growth factor-1 receptor via a peroxisome proliferator-activated receptor gamma-independent pathway. J Biol Chem. 2010;285:36361–8.
Mughal A, Kumar D, Vikram A. Effects of thiazolidinediones on metabolism and cancer: relative influence of PPARgamma and IGF-1 signaling. Eur J Pharmacol. 2015;768:217–25.
Bochinski D, Hsieh PS, Nunes L, Lin GT, Lin CS, Spencer EM, et al. Effect of insulin-like growth factor-1 and insulin-like growth factor binding protein-3 complex in cavernous nerve cryoablation. Int J Impot Res. 2004;16:418–23.
Sjoberg J, Kanje M. Insulin-like growth factor (IGF-1) as a stimulator of regeneration in the freeze-injured rat sciatic nerve. Brain Res. 1989;485:102–8.
Apel PJ, Ma J, Callahan M, Northam CN, Alton TB, Sonntag WE, et al. Effect of locally delivered IGF-1 on nerve regeneration during aging: an experimental study in rats. Muscle Nerve. 2010;41:335–41.
Sumino Y, Yoshikawa S, Mori K, Mimata H, Yoshimura N. IGF-1 as an important endogenous growth factor for recovery from impaired urethral continence function in rats with simulated childbirth injury. J Urol. 2016;195:1927–35.
Lewis JD, Habel LA, Quesenberry CP, Strom BL, Peng T, Hedderson MM, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA. 2015;314:265–77.
Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, Kim L, et al. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol. 2007;25:1476–81.
Koro C, Barrett S, Qizilbash N. Cancer risks in thiazolidinedione users compared to other anti-diabetic agents. Pharmacoepidemiol Drug Saf. 2007;16:485–92.
Boxall N, Bennett D, Hunger M, Dolin P, Thompson PL. Evaluation of exposure to pioglitazone and risk of prostate cancer: a nested case-control study. BMJ Open Diabetes Res Care. 2016;4:e000303.
Suzuki S, Mori Y, Nagano A, Naiki-Ito A, Kato H, Nagayasu Y, et al. Pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, suppresses rat prostate carcinogenesis. Int J Mol Sci. 2016;17:2027.
Erdmann E, Harding S, Lam H, Perez A. Ten-year observational follow-up of PROactive: a randomized cardiovascular outcomes trial evaluating pioglitazone in type 2 diabetes. Diabetes Obes Metab. 2016;18:266–73.
Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139.
Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305.
Chattopadhyay S, Shubayev VI. MMP-9 controls Schwann cell proliferation and phenotypic remodeling via IGF-1 and ErbB receptor-mediated activation of MEK/ERK pathway. Glia. 2009;57:1316–25.
Rothe F, Langnaese K, Wolf G. New aspects of the location of neuronal nitric oxide synthase in the skeletal muscle: a light and electron microscopic study. Nitric Oxide. 2005;13:21–35.
Carnicer R, Suffredini S, Liu X, Reilly S, Simon JN, Surdo NC, et al. The subcellular localization of neuronal nitric oxide synthase determines the downstream effects of NO on myocardial function. Cardiovasc Res. 2017;113:321–31.
Funding
Funding for this project was provided by the Sexual Medicine Society of North America.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Heidenberg, D., Haney, N., Rezk, B.M. et al. Pioglitazone’s beneficial effects on erectile function preservation after cavernosal nerve injury in the rat are negated by inhibition of the insulin-like growth factor-1 receptor: a preclinical study. Int J Impot Res 31, 1–8 (2019). https://doi.org/10.1038/s41443-018-0054-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41443-018-0054-2
- Springer Nature Limited
This article is cited by
-
A sugar sweet new treatment for erectile dysfunction after radical prostatectomy
International Journal of Impotence Research (2019)