Abstract
To investigate the efficacy, tolerability, and patient’s preference of alprostadil cream for topical use administered within the urethral meatus versus the standard administration route, in erectile dysfunction (ED) treatment. Seventy-one patients (mean age 59.7 ± 9.0 years) affected by ED were analyzed in this multicenter, randomized, two-administration routes, cross-over trial. All patients received a single dose of alprostadil cream applying the dispenser to the tip of the penis (without contacting the urethral meatus) (Standard administration route or ST.AR) alternating with a single dose of alprostadil cream applying the dispenser within the urethral meatus (New administration route or NEW.AR) separated by a one-week washout period, according to randomization. The primary objective of the study was to evaluate the change in International Index of Erectile Function (IIEF-5) total score from baseline to the control visit by comparing the ST.AR and NEW.AR. Secondary objectives of the study were to compare the different methods of administration by evaluating the change in the Sexual Encounter Profile (SEP-2 and SEP-3) questionnaire score and the Patient Reported Outcomes (PROs) by scoring the Patient Self-Assessment of Erection (PSAE) questionnaire. The treatment safety profile was assessed by analysis of adverse events (AEs). Based on the study findings it is evident that the NEW.AR is more efficacious than the ST.AR in improving IIEF-5 and SEP scores from baseline to control visit (IIEF-5: +3.8 vs +6.3; p < 0.001; positive response to SEP-2: 10 vs 27; p = 0.002) and in terms of PSAE (a significant improvement from the baseline in 31% of patients; p < 0.001). As regards the safety profile, no difference in terms of local and systemic side effects was found.
Similar content being viewed by others
Introduction
Erectile dysfunction (ED), defined as the consistent inability to achieve or maintain an erection satisfactory for sexual intercourse, is a common male sexual disorder [1,2,3]. The quality of the patient’s and partner’s sex life, as well as the quality of life (QoL) more generally, are strongly affected by the presence of ED.
On the contrary, treating ED is associated with a positive effect on the quality of life and overall satisfaction of both patients and their partners [4]. Four selective phosphodiesterase type 5 inhibitors (PDE5i) have been approved by the European Medicines Agency for the oral treatment of ED, and, according to the Guidelines proposed by the European Association of Urology (EAU), these represent the first line of therapy [5]. Although oral PDE5i are generally safe and effective, they are associated with treatment failure in 11–44% of patients, due to several reasons, such as the effect below their expectations, high cost, loss of interest in sex, and inconvenience of obtaining drugs [6]. On the other hand, injectable drugs showed high efficacy but low patient compliance related to the difficulties due to the method of administration [7]. Based on these considerations, we are still witnessing the constant search for new therapeutic alternatives that can limit the strong psycho-relational impact of ED [8]. For example, one of the most recent therapeutic novelties on the market is the alprostadil cream for topical use. This innovative formulation had been evaluated in several clinical trials and had been considered effective and safe by most patients and their partners, with adverse events (AEs) limited to the site of application [9,10,11,12]. Thanks to this advantageous clinical-therapeutic profile, it has been recently suggested that, after a proper counseling to the patient, the alprostadil cream for topical use can be offered to patients with ED of different etiology and severity as a possible therapeutic alternative to oral drugs [5, 13]. However, in the real-life outpatients setting there are some aspects to clarify in terms of efficacy and patient’s adherence to the treatment. In particular, some doubts have been raised regarding the reliability and efficacy of the method of administration, as initially proposed. The latter, based on keeping the applicator at a distance, albeit minimal, from the urethral meatus, seems related to a lower efficacy and adherence to the treatment. In this sense, the aim of the present study was to investigate, in the context of a real clinical outpatient setting, the efficacy and tolerability of alprostadil cream for topical use, by comparing a new mode of administration based on the introduction of the cream dispenser inside the urethral meatus with the standard use mode.
Materials and methods
Study design and ethical considerations
The present multicenter, randomized, two-administration routes, cross-over study was carried out at ten institutions in Italy and included Italian patients affected by ED. The study was carried out in accordance with the Declaration of Helsinki and GCP. Furthermore, this study was conducted in line with the Consolidated Standards of Reporting Trials statement (The Ottawa Hospital Research Institute, Ottawa, ON, Canada). All patients provided written informed consent. After a 4-week screening period, all eligible patients were randomized (1:1) to one of the two study arms, which provided a different sequence of the two modes of administration, according to the cross-over scheme. The CONSORT statement had been consulted (http://www.consort-statement.org) and applied whenever possible.
Study protocol
After a screening visit (Visit 1) to assess baseline erectile function (EF) by using dedicated questionnaires and safety measures (physical exams, laboratory tests, urine analysis, and vital signs), all patients were randomly assigned by a computer generated randomization protocol to receive a single dose of 300 µg alprostadil cream (VITAROS®, manufactured by Ferring GmbH, Germany) applied according to the standard method (Standard administration route [ST.AR] which provides for the application with the tip of the dispenser near the urethral meatus, although without direct contact with it) or as an alternative applied according to the experimental method (New administration route [NEW.AR] which provides for the application with the tip of the dispenser that is inserted directly inside the urethral meatus, generating a direct contact with this) (Visit 2). The next day, all patients underwent follow-up visit with assessment of EF by using dedicated questionnaires [International Index of Erectile Function – 5 (IIEF-5) [14], Successful vaginal penetrations based on Sexual Encounter Profile (SEP-2 and SEP-3) [15] and Patient Self-Assessment of Erection (PSAE) [16] and safety measures (physical exams, laboratory tests, urine analysis, and vital signs) (Visit 3). After 7 days the patients returned to receive the alprostadil administration alternative to the first receipt (Visit 4), with a new follow-up visit the next day (Visit 5). Figure 1 summarizes the different modalities of drug application other than the study flow-chart.
Application routes
Standard application route (ST.AR)
The medication was applied using a single-unit dose dispenser to the tip of the penis. The dispenser (without any direct contact with the urethral meatus) accurately dispensed a single dose of 300 µg alprostadil cream onto the external opening of the meatus. Subsequently the flaccid penis was held upright for 30 s waiting for the absorption of the drug.
New application route (NEW.AR)
The medication was applied using a single-unit dose dispenser within the urethral meatus. The tip of the dispenser was inserted directly inside the urethral meatus, generating a direct contact with this, and dispensed a single dose of 300 µg alprostadil cream. Subsequently the flaccid penis was held upright for 30 s waiting for the absorption of the drug.
Below are the details of the individual visits provided by the study.
Visit 1: Patients’ enrolment
After a 4 week wash-out period all patients were assessed for inclusion and exclusion criteria; all recruited patients signed the informed consent and completed the following questionnaires:
-
IIEF-5
-
SEP-2 and SEP-3
-
PSAE
All patients were then randomized to ST.AR or NEW.AR to be administered on Visit 2.
Visit 2: Study treatment (according to randomization)
The patients received the drug according to the modality foreseen by the randomization process. Immediately after the application of the drug, all patients were asked to report local and systemic side effects and to define the degree of pain at the genital level according to the Visual Analogue Scale (VAS) scale.
Visit 3: Patients’ outcome assessment
The next day, all patients underwent follow-up visit with assessment of EF by using dedicated questionnaires (IIEF-5, SEP-2, SEP-3, PSAE) and safety measures (physical exams, laboratory tests, urine analysis, and vital signs). In addition, all patients were asked to report new onset local and systemic side effects the day after the application of the drug. Furthermore, patients’ reported outcomes (PROs) had been collected by scoring the PSAE questionnaire.
Visit 4: Patients’ assessment and study treatment (according to cross-over design)
Seven days after Visit 3, all patients completed the study questionnaires (IIEF-5, SEP-2, SEP-3, PSAE). Subsequently they received a new dose of drug administered with the method other than the previous one, according to the study’s cross-over scheme.
Visit 5: Patients’ outcome assessment
On the occasion of this visit, the same procedures as for visit 3 were repeated.
Inclusion and exclusion criteria
Patients eligible for this study were aged between 18 and 75 years, had a diagnosis of ED lasting more than 3 months, and were classified as mild-to-moderate ED based on the IIEF-5 score of 21 or less. Patients were excluded from participation in this study if they were affected with ED caused by untreated endocrine disease, clinically significant penile pathology (implant, excessive curvature, fibrosis, and sexually transmitted disease), clinically significant renal or hepatic disease as determined via laboratory assay, and the use of prescribed or over the counter ED medication, supplements, or devices. Patients were required to discontinue the use of ED treatments prior to entry into the study.
Outcome measurements
The primary objective of the study was to evaluate the change in IIEF-5 total score from baseline to the control visit by comparing the ST.AR and NEW.AR. Secondary objectives of the study were to evaluate the change over the treatment course and to compare the different methods of administration in terms of SEP-2/SEP-3 scores and the PROs by scoring the PSAE questionnaire. All patients and their partners were asked to answer the following question for each application modality: “What do you think about the drug administration route in terms of feasibility and satisfaction?”, and then indicating their preferred mode of administration. Safety was assessed by analysis of AEs, changes in laboratory test results, and physical examination findings.
In order to evaluate the efficacy and safety of the each route of administration, we considered and analyzed questionnaires and PROs data from each route of administration independently from the randomization sequence.
Statistical analysis
In order to obtain significant results to analyze, the required sample size for the present study was calculated under the following conditions: Difference between the groups, 3 (SD = 1) score points in the IIEF-5 questionnaire (20% of improvement should be considered as significant); α error level, 0.05 two-sided; statistical power, 80%; and anticipated effect size, Cohen’s d = 0.5 [17]. The calculation yielded 2 × 41 measurements; moreover, considering a dropout of 10% the final count should be set to 90 measurements: at least 45 patients to enroll due to the cross-over design. Data were analyzed based on the per-protocol (PP) approach. This approach is due to the nature of the design, cross-over study. The use of a intention-to-treat (ITT) approach is generally a recommended method in superiority trials to avoid any bias; however, in the cross-over study the risk of bias due to the PP approach is low. The change from baseline in IIEF-5 score, the PSAE and SEP questions were analyzed using a one-way analysis of variance with treatment as a factor. Efficacy and safety analyses were carried out for the patients who were randomized and received the two doses of the study drug. Demographic and baseline characteristics, study medication use, and changes in vital signs after administration of the test dose were analyzed using either analysis of variance models (continuous variables) or the Cochran-Mantel-Haenszel test (categorical variables). Randomization based on a single sequence of random assignments (simple randomization) was performed using a pseudo-random number generator software (Research Randomizer Version 4.0, Social Psychology Network, Wesleyan University, Middletown, CT, USA).
Results
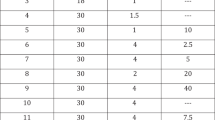
Eighty-three patients were screened and of these 80 were enrolled and randomized. Finally, 71 patients (mean age 59.7 ± 9.0 years) completed the study and were included in the final analysis. Nine patients did not complete the study and were lost during the follow-up. Table 1 details all demographic, clinical and laboratory data at the baseline assessment.
Figure 2 shows the histogram comparing the results after the therapy crossover. A statistically significant difference had been reported between the baseline and the switch therapy time in terms of IIEF-5 [ST.AR: IIEF-5 15.0 (SD 3.9) vs 17.1 (SD 3.5); p = 0.02); NEW.AR: IIEF-5 15.0 (SD 3.9) vs 20.7 (SD 3.6); p < 0.001)].
Table 2 reports the data concerning all the parameters under study based on the different method of administration used.
A statistically significant difference between baseline and the final evaluation visit of the study (Visit 5) had been detected in terms of IIEF-5 score for both the administration routes, thus confirming the efficacy of the topical alprostadil cream regardless of how it was administered [ST.AR: IIEF 15.0 (SD 3.9) vs 18.9 (SD 3.6); p < 0.001); NEW.AR: IIEF 15.0 (SD 3.9) vs 21.4 (SD 3.4); p < 0.001)]. However, evaluating the extent of the increase of the single study parameters it is evident the possibility of obtaining a further improvement of the EF by using the NEW.AR rather than the ST.AR. (IIEF-5 mean change from baseline to Visit 3: ST.AR + 3.8; NEW.AR + 6.3; p < 0.001). Similarly, an advantage has been found in favor of NEW.AR in terms of SEP-2 (p = 0.002) and SEP-3 (<0.001). It is important to specify that the ST.AR was not able to achieve a statistically significant efficacy in terms of SEP-3 when compared to the baseline.
Patients Reported Outcomes (PROs)
All patients were asked about the efficacy and feasibility of the two routes of administration and which was their preferred mode altogether. Overall, both patients and their partners showed a preference for NEW.AR compared to ST.AR (PSAE score: p < 0.001), reporting it as their preferred mode of administration and motivating it with the lowest risk of producing drug dispersion.
Adverse events (AEs)
Table 3 reports the incidence of AEs according to the different administration route applied.
No difference in terms of both local and/or systemic AEs had been detected. The most frequently reported AEs resulted: moderate erythema (66 patients) and urethral burning (57 patients) that reduced in intensity within one minute from application, without any impact on sexual intercourse or patient’s quality of life.
Discussion
Main findings
Based on the results of the present study, we showed that the administration of alprostadil cream for topical use directly applying the tip of the dispenser inside the urethral meatus allows to increase the efficacy of the drug without greater incidence of side effects. At the same time this new mode of administration is preferred by patients, thus increasing their adherence to the treatment.
Results in the context of previous studies
The efficacy of topical application of alprostadil cream compared to placebo had been extensively proven in several phase I, RCTs studies and in one meta-analysis [9,10,11,12,13, 18,19,20]. A phase II study, showed a percentage of patients reporting improved erections during the 12 weeks of treatment of 52% [10]. In our study we obtained a percentage of patients who reported an improvement in their erections in 71% of cases at the first follow-up and of 76% at the second follow-up. Interestingly, we observed that the higher percentage of patients reporting improved erections was mostly related to the use of the new method of administration. In fact, when we asked to the patients to deliver the drug by using the dispenser within the urethra meatus, the percentage of patients reporting improved erections is higher, in particular at the 6 months follow-up evaluation. Probably this advantage can be related to the fact that the new mode of application is also easier to perform in the patient’s perspective, with a minimum risk of wasting the drug. So much so that in the common clinical practice a dispersion of the drug was often reported with a consequent reduction of its efficacy.
The distinguishing feature of our study consists precisely in the fact that in the face of a general confirmation of the effectiveness of topical therapy with alprostadil, there is a real advantage deriving from the intra-meatal application of the active ingredient.
Consistent with the common thought, the majority of patients in the present study demonstrated a time-dependent overall improvement in EF, especially in the new administration route. All patients reported a durable improvement in EF at the second follow-up evaluation. From the partner’s point of view, as reported in other trials [9,10,11,12,13], the rate of AEs in very low in the two administration routes. On the other hand, the partners confirmed the preference for the new administration route highlighting that this new route is more natural and easier to perform. Finally, the present study population has not peculiar characteristics but is very similar to the real-life clinical practice. We failed to perform a patient’s stratification with different levels of disease severity and comorbidities in order to identify the characteristics of the ideal patient that could benefit most from the use of the new method of drug administration.
Strength and limitations of the study
This represents the first prospective, randomized, cross-over study aimed to compare in terms of efficacy and safety two different application modalities for alprostadil cream. The use of the cross-over evaluation methodology should be considered a strength of the study due to the fact that all patients could try both methods of administration, and therefore provide clinical comparison results, overcoming any differences in the general characteristics of the patients enrolled in the study and randomized to two different administration sequences. The study, however, shows some limitations to take into account. Firstly, the un-blinded fashion, which is related to the design of the study. Moreover, the lack of a placebo group could be considered a limitation of the study. However, as the benefit of alprostadil vs placebo had already been extensively demonstrated in previous phase 3 clinical trials, this objective was not specifically in the design of this study. Lastly, another limitation of the present study was the non-registration of the trial and the request for authorization to the ethics committee. However, it should be noted that according to the study design there was no randomization to different treatments nor a comparison with placebo, but all the patients enrolled in the study received both treatments even if with different sequence.
Clinical applicability
This study provides important implications to be applied in everyday clinical practice.
In fact, making use of the new method of administration of the drug is shown a significant increase in its effectiveness as well as adherence to therapy thanks to a higher rate of approval by patients, without increasing the risk of complications.
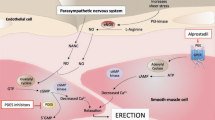
Of greatest clinical importance, the incidence of uro-genital collateral effects remains very low, and far less (65% less) than alternative methods of use of alprostadil (intra-cavernous or intra-urethral), as reported by literature [21]. Moreover, the absence of systemic side effects is already known for the intrinsic properties of the drug. Various studies on pharmacokinetic properties have amply demonstrated the absence of systemic absorption as well as rapid metabolization, which are the main reasons for almost non-existent systemic toxicity [22].
Conclusions
The application of the single dose dispenser of alprostadil cream for topical use within the urethral meatus is able to implement the therapeutic efficacy, without altering the tolerability profile of the drug. This new administration route should be taken into account in everyday clinical practice in order to improve the patient’s adherence to the treatment and treatment-related efficacy.
References
Braun M, Wassmer G, Klotz T, Reifenrath B, Mathers M, Engelmann U. Epidemiology of erectile dysfunction: results of the Cologne Male Survey. Int J Impot Res. 2000;12:305–11.
Liu LH, Zhang T, Zhang YR, Liu TS, Zhang HB, Chen FZ, et al. Metabolic syndrome and risk for ED: a meta-analysis. Int J Impot Res. 2014;26:196–200.
NIH Consensus Development Panel on Impotence. JAMA.1993;270:83–90.
Latini DM, Penson DF, Lubeck DP, Wallace KL, Henning JM, Lue TF. Longitudinal differences in disease specific quality of life in men with erectile dysfunction: results from the exploratory comprehensive evaluation of erectile dysfunction study. J Urol. 2003;169:1437–42.
Hatzimouratidis K (Chair), Giuliano F, Moncada I, Muneer A, Salonia A (Vice-chair), Verze P. EAU Guidelines on Erectile Dysfunction, Premature Ejaculation, Penile Curvature and Priapism http://uroweb.org/guideline/male-sexual-dysfunction/ UPDATE MARCH 2018
Carvalheira AA, Pereira NM, Maroco J, Forjaz V. Dropout in the treatment of erectile dysfunction with PDE5: a study on predictors and a qualitative analysis of reasons for discontinuation. J Sex Med. 2012;9:2361–9.
El-Sakka AI. Intracavernosal prostaglandin E1 self vs office injection therapy in patients with erectile dysfunction. Int J Impot Res. 2006;18:180–5.
Jannini EA, Sternbach N, Limoncin E, Ciocca G, Gravina GL, Tripodi F, et al. Health-related characteristics and unmet needs of men with erectile dysfunction: a survey in five European countries. J Sex Med. 2014;11:40–50.
Rooney M, Pfister W, Mahoney M, Nelson M, Yeager J, Steidle C. Long-term, Multicenter Study of the Safety and Efficacy of Topical Alprostadil Cream in Male Patients with Erectile Dysfunction. J Sex Med. 2009;6:520–34.
Padma-Nathan H, Steidle C, Salem S, Tayse N, Yeager J, Harning R. The efficacy and safety of a topical alprostadil cream, Alprox-TD, for the treatment of erectile dysfunction: two phase 2 studies in mild-to-moderate and severe ED. Int J Impot Res. 2003;15:10–17.
Steidle C, Padma-Nathan H, Salem S, Tayse N, Thwing D, Fendl J, et al. Topical alprostadil cream for the treatment of erectile dysfunction: a combined analysis of the phase II program. Urology. 2002;60:1077–82.
Padma-Nathan H, Yeager JL. An integrated analysis of alprostadil topical cream for the treatment of erectile dysfunction in 1732 patients. Urology. 2006;68:386–91.
Moncada I, Cuzin B. Clinical efficacy and safety of Vitaros©/Virirec© (Alprostadil cream) for the treatment of erectile dysfunction. Urologia. 2015;82:84–92.
D’Elia C, Cerruto MA, Cavicchioli FM, Cardarelli S, Molinari A, Artibani W. Critical points in understanding the Italian version of the IIEF 5 questionnaire. Arch Ital Urol Androl. 2012;84:197–201.
Araujo AB, Allen KR, Ni X, Rosen RC. Minimal clinically important differences in the vaginal insertion and successful intercourse items of the sexual encounter profile. J Sex Med. 2012;9:169–79.
Cappelleri JC, Stecher VJ. An assessment of patient-reported outcomes for men with erectile dysfunction: Pfizer’s perspective. Int J Impot Res. 2008;20:343–57.
Machin D, Campbell MJ, Fayers P, Pinol A. Statistical tables for the design of clinical studies. edn. 3. Oxford, UK: Blackwell; 1998.
Kim ED, McVary KT. Topical prostaglandin-E1 for the treatment of erectile dysfunction. J Urol. 1995;153:1828–30.
Montorsi F, Guazzoni C, Barbieri L, et al. Clinical and hemodynamic effects of transdermal alprostadil for mild arteriogenic impotence: a double-blind placebo controlled study. Int J Impot Res. 1995;7:10–11.
Goldstein I, Payton TR, Schechter PJ. A double-blind, placebo-controlled, efficacy and safety study of topical gel formulation of 1% alprostadil (Topiglan) for the in-office treatment of erectile dysfunction. Urology. 2001;57:301–5.
Campbell HE. Clinical monograph for drug formulary review: erectile dysfunction agents. J Manag Care Pharm. 2005;11:151–71.
Hanchanale V, Eardley I. Alprostadil for the treatment of impotence. Expert Opin Pharmacother. 2014;15:421–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cai, T., Palumbo, F., Liguori, G. et al. The intra-meatal application of alprostadil cream (Vitaros®) improves drug efficacy and patient’s satisfaction: results from a randomized, two-administration route, cross-over clinical trial. Int J Impot Res 31, 119–125 (2019). https://doi.org/10.1038/s41443-018-0087-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41443-018-0087-6
- Springer Nature Limited
This article is cited by
-
Conservative Non-surgical Options for Erectile Dysfunction
Current Urology Reports (2023)
-
The Italian Society of Andrology and Sexual Medicine (SIAMS), along with ten other Italian Scientific Societies, guidelines on the diagnosis and management of erectile dysfunction
Journal of Endocrinological Investigation (2023)
-
Combination therapy with topical alprostadil and phosphodiesterase-5 inhibitors after failure of oral therapy in patients with erectile dysfunction: a prospective, two-arm, open-label, non-randomized study
International Journal of Impotence Research (2022)
-
Hard flaccid syndrome: initial report of four cases
International Journal of Impotence Research (2020)