Abstract
Short periods of testosterone suppression have been shown to reduce trabecular smooth muscle content and increase interstitial connective tissue accumulation in animal models. However, the long-term effects of testosterone suppression remain unclear. The aim of this study was to evaluate the long-term effects of testosterone suppression on penile structure and erectile function in rats. Subjects were divided into two groups by observation period (short-period group (group I), 12 weeks; long-period group (group II), 20 weeks). Each group comprised three different subgroups (10 rats each): sham-operated control, surgical castration, and testosterone replacement (4 weeks after an 8-week castration period). Group II subgroups included a sham control, surgical castration, and testosterone replacement (4 weeks after a 16-week castration period). Erectile function was assessed by measuring intracavernosal pressure in response to cavernous nerve stimulation, and expression of the endothelial nitric oxide synthase (eNOS) protein was determined by western blot analysis. Serum testosterone values were measured via radioimmunoassay. The results indicated that serum testosterone level, penile length and girth, cavernosal smooth muscle content, and eNOS activity decreased significantly in castrated animals. These effects were rescued by testosterone undecanoate injection. Erectile function was normalized over 4 weeks in rats that received androgen replacement. Expression of eNOS was decreased in the corpus cavernosum of castrated animals compared with controls, while androgen replacement normalized the expression of eNOS. These results were consistently observed regardless of the duration of androgen deprivation. Thus, these data suggest that androgen regulates the expression of eNOS in the rat penile corpus cavernosum and confirm the importance of androgens in the maintenance of erectile function. Additionally, long-term androgen deprivation does not induce irreversible structural or erectile functional changes in sexually mature adult male rats.
Similar content being viewed by others
Introduction
Erectile function is a complex neurovascular process modulated by multiple biochemical and physiologic factors. Normal erectile function in the penis depends on adequate vascular flow and intact blood-holding structures to create venous occlusion. Holding structures include hard coporal body and thickened cavernosal smooth muscles, which are modulated by testosterone [1]. Many studies have explored the role of serum androgen level maintenance in adult men. In order to evaluate erectile function, these studies used a model with short-term testosterone deprivation and replacement. However, there have been fewer studies on functional and structural penile changes in patients under the influence of long-term castration. Patients with prostate cancer continue to get older every year after definitive treatment, and many older men suffer from erectile dysfunction due to late-onset hypogonadism. Therefore, we believe that research on the reversibility of penile function and structure after long-term deprivation of testosterone is needed.
We previously reported that continuous testosterone suppression shortens the length of the penis in patients who undergo continuous androgen suppression therapy (ADT) for prostate cancer [2]. Short periods of testosterone suppression have been shown to reduce trabecular smooth muscle content and increase interstitial connective tissue accumulation in animal models [3, 4]. These results indicate that androgen affects growth and maintenance of the penis.
The goal of the present study was to evaluate the effects of long-term testosterone deprivation on penile structure and erection in a rat model. We further aimed to determine whether changes in erectile tissue and hemodynamic parameters of the cavernosum caused by ADT could be reversed by administration of testosterone.
Materials and methods
Animal subjects and treatment protocol
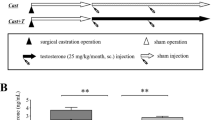
Sixty adult male Sprague-Dawley rats (11 weeks old) were used in the current study. Subjects were randomly divided into two groups by observation period (group I (n = 30), 12 weeks; group II (n = 30), 20 weeks). Each group comprised three different subgroups (10 rats each): sham-operated control, surgical castration, and testosterone replacement (4 weeks after an 8-week castration period). Group II subgroups included sham control, surgical castration, and testosterone replacement (4 weeks after a 16-week castration period).
Rats were castrated with a vertical scrotal incision under ketamine anesthesia (90 mg/kg intramuscular (IM)) and xylazine analgesic (10 mg/kg IM). Testosterone replacement was achieved via monthly intramuscular injection of 100 mg/kg of testosterone undecanoate (TU). After 4 weeks of androgen treatment, erectile function was assessed, and blood was collected from the heart to determine circulating androgen levels via radioimmunoassay. Penile tissue was harvested for biochemical and histological analyses, as described below. This study was approved by the Institutional Animal Care and Use Committee of Jeju National University (20130007) and complied with ethical regulations for animal study.
Measurement of erectile function
Rats from each subgroup and their age-matched controls were anesthetized with IM ketamine (90 mg/kg) and xylazine (10 mg/kg). All measurements were blinded. With the animal placed in the supine position, the penis was denuded of overlying skin, and the stretched penile length (between base and tip) and girth (at mid-shaft) were measured with steel and paper rulers, respectively. The bladder and prostate were exposed through a midline abdominal incision. The major pelvic ganglion and cavernosal nerve were identified posterolaterally to the prostate on one side. Bipolar platinum wire electrodes were placed around the cavernous nerve. The penis was denuded of skin, and a 24-gauge needle filled with 250 U/ml of heparin was inserted into one side of the corpus cavernous in order to monitor intracavernous pressure. The carotid artery was cannulated for measurement of systemic arterial pressure. Systemic arterial and intracavernosal blood pressure were measured with pressure transducers connected to a computerized system for data acquisition and analysis. Stimulation parameters were 10 V at a frequency of 12 Hz, a pulse width of 1 ms, and a duration of 1 min. During tumescence, the maximal intracavernous pressure (ICP) was recorded. The total ICP was determined by the area under the curve from the beginning of cavernous nerve stimulation to a point 20 s after stimulus termination. The ratios of maximal ICP and total ICP to mean arterial pressure (MAP) were calculated to adjust for variation in systemic blood pressure.
Tissue harvesting and immunohistochemical staining
After the functional study was completed, rats were killed through bilateral thoracotomy. A mid-portion of the penile segment was harvested for histological assessment. Tissue samples were fixed in cold 2% formaldehyde and 0.002% picric acid in 0.1 M phosphate buffer at pH 8.0 for 4 h, followed by overnight immersion in buffer containing 30% sucrose. The specimens were then embedded in OCT Compound (American Master Tech Scientific, Lodi, CA, USA) and stored at −70 °C until use. Sections were cut at 5 μm, mounted onto charged slides, and air dried for 5 min. Representative slides were stained with Masson’s trichrome for connective tissue and smooth muscle histology.
Western blot analysis
A portion of each penis was frozen, processed into tissue powder, and homogenized in phosphate buffer (10 mM phosphate (pH 7.4) and 150 mM NaCl) containing protease inhibitors (Thermo Fisher Scientific Inc., Waltham, MA, USA). The homogenates were centrifuged at 12,000 rpm for 20 min. The total protein concentration in the supernatant was measured using Lowry protein assay. Equal amounts of protein (50 μg) were electrophoresed through 16.5% polyacrylamide gels under denaturing conditions (12% sodium dodecyl sulfate (SDS)) and transferred onto a nitrocellulose membrane. The membrane was blocked in 20 mM Tris-saline buffer (pH 7.2) containing 0.1% Tween 20 and 0.2% casein for 1 h and incubated overnight with antibodies against endothelial nitric oxide synthase (eNOS) (Santa Cruz Biotechnology, Dallas, TX, USA) or β-actin (Abcam, Cambridge, UK). Before re-probing with anti-actin antibody, the membrane was stripped in 62.5 mM Tris-HCl, pH 6.7, 2% SDS, 10 mM 2-mercaptoethanol at 56 °C for 30 min and then washed four times in 1× Tris-buffered saline. Results were quantified by densitometry.
Statistical analysis
For calculating the number of animals for experiments evaluated statistically, it was hypothesized that the changes introduced by androgen deprivation and replacement of androgen would produce ±30% minimum changes in the different endpoints to be studied, and that the endpoint with the least expected change would determine the number of animals. Assuming a variance of 8–10%, it was calculated that 10 animals per group should allow to detect a 40% change in ICP or protein with a 85% power, at alpha = 0.05.
Differences between subjects were evaluated using various statistical methods. Unpaired two-sided Student’s t-tests were used for comparison of means of normally distributed parameters. In all other cases, Mann–Whitney U-tests were used for comparisons between groups (GraphPad Prism ver. 5.00, GraphPad Software, San Diego, CA, USA). P-values less than 0.05 were considered statistically significant.
Results
Serum testosterone
Serum testosterone significantly decreased in both groups after castration (group I: 1.30 ± 0.35; group II: 0.75 ± 0.23 ng/mL) and returned to control levels (group I: 4.48 ± 0.76; group II: 4.2 ± 0.87 ng/mL) after testosterone replacement (P = 0.005). Mean body weights did not differ significantly among any of the treatment groups (Table 1).
Gross appearance of penis and erectile function study
Mean penile length and girth of group I were 4.20 ± 0.55 and 1.50 ± 0.15 cm, respectively, while in group II they were 4.30 ± 0.43 and 1.40 ± 0.21 cm, respectively, There were no significant differences in penile length or girth based on castration period (P = 0.871). Penile length and girth were significantly reduced after castration (group I: 3.30 ± 0.35; group II: 0.75 ± 0.23 ng/mL), but returned to control levels (group I: 4.48 ± 0.76; group II: 4.2 ± 0.87 ng/mL) after testosterone replacement (P = 0.005). Castration-induced gross changes were not significantly different between groups I and II (P = 0.792). Mean ICP/MAP and total ICP/MAP were significantly lower in the castrated groups; after 4 weeks of testosterone replacement, these parameters significantly improved regardless of the period of castration (Figs. 1 and 2).
Testosterone replacement restores intracavernosal pressure in castrated rats despite prolonged castration. Lower solid bar indicates the electro-stimulus time. The x-axis indicates the elapsed time. ICP intracavernous pressure, TU testosterone undecanoate. ‘A’ indicates that ICP was restored after 4 weeks of testosterone replacement. Graph B also shows restoration of ICP despite a longer period of androgen deprivation
Testosterone replacement restores intracavernous pressure and gross morphology of the penis in castrated rats despite prolonged castration. The 12-week group was harvested and measured after 12 weeks, the 20-week group was done after 20 weeks. Asterisks indicate a significant decrease compared to the control. Sharp mark indicates a significant increase compared to the castrated group. ICP intracavernous pressure, MAP mean arterial pressure (2/3 diastolic blood pressure +1/3 systolic blood pressure), TU testosterone undecanoate, Tx treatment
Western blot analysis
Protein expression of eNOS significantly decreased after castration and was rescued after 4 weeks of testosterone replacement in both groups. However, there were no differences between castration and replacement groups according to castration period (Fig. 3).
Western blot of neuronal nitric oxide synthase (eNOS). Results from the 12-week group indicate that eNOS activity in rats that underwent 8 weeks of castration was restored after 4 weeks of replacement of testosterone. Results for the 20-week group were similar despite the longer castration period (20 weeks). Asterisks indicate a significant decrease compared to the control. TU castration and replacement with testosterone undecanoate for 4 weeks, Cx castration without treatment
Histochemical assessment of tissue structure
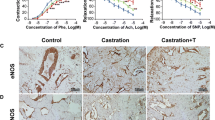
Masson’s trichrome staining demonstrated a significantly higher smooth muscle to collagen ratio in control and testosterone-replaced animals compared to the 8- and 16-week castration groups (Fig. 4).
Smooth muscle contents in the corpus cavernosum Representative images from rats in the control, castration, and castration with testosterone replacement groups. a The 12-week group, b The 20-week group. Smooth muscle and connective tissue are stained with red and blue, respectively, using the trichrome method. The smooth muscle content was higher in the control and replacement groups than the castration groups. Arrow bars indicate cavernosal smooth muscle. Bar scale is 100 µm. Magnification ×100
Discussion
In this study, we found that testosterone replacement could improve erectile function in castrated adult male rats, irrespective of the period of testosterone deprivation. In line with this observation, we found no irreversible penile changes on histological analysis. Additionally, of the many parameters used to characterize penis erectability, all those assessed in the current study—penile length and girth, ICP, eNOS activity, cavernosal smooth muscle content related to connective tissue, and endothelial tissue—were sufficiently rescued by short-term (i.e., 4 weeks) testosterone replacement.
After adult development, the penis still requires androgen to maintain its structure [1]. Structural, hormonal, neural, and metabolic elements interact to maintain erectile function. Of these components, structural and neural integrity are mediated by androgen. Lu et al. [5] previously reported that endothelial structures can be damaged as a result of low testosterone levels. Keast et al. [6] showed the critical role of testosterone in maturation and maintenance of terminal axon density and neuropeptide expression. In a recent study, Hwang et al. [7] demonstrated that androgen supports maintenance of the cavernosal endothelium and regulation of vascular endothelial growth factor.
Several other studies indicated that androgen deprivation results in decreased levels of testosterone and ICP, which in turn lead to reduced venous occlusion due to changes in cavernosal smooth muscle and connective tissue [3, 8]. Indeed, Traish et al. [8] demonstrated with electron microscopy that castrated rats exhibit disorganized myocyte arrangement. This disarrangement can also be restored via replacement of testosterone. Miranda et al. [9] reported reversible structural changes related to periods of androgen deprivation. After 2 months castrated, adult male rats showed significantly decreased smooth muscle content, sinusoidal space, and total cavernous area; however, these structural changes were restored with 1 month of androgen replacement [9]. These results are similar to what we observed in group I in the present study (castration for 8 weeks and 4 weeks of androgen replacement). Because these study periods may be too short to detect irreversible changes, we also included a group with a longer castration period (i.e., 16 weeks). However, despite this longer period of androgen deprivation, we still observed restoration of penile erection and structure with replacement of testosterone. Thus, the minimal castration period necessary to observe irreversible changes in penile structure cannot be determined from this study. After penile changes induced by 8 weeks of testosterone deprivation, we observed no significant penile changes up to 16 weeks. It is possible that meaningful permanent penile changes do not develop in sexually mature male rats after more than 16 weeks of testosterone deprivation.
Penile changes after prostate cancer treatment are a concern for both clinicians and patients. Prostatectomy can induce significant penile changes [10,11,12]. Haliloglu et al. [13] reported shortened penile length after neoadjuvant ADT and external beam radiotherapy. Additionally, we previously reported changes in human penile length from a long-term study of androgen deprivation without radiation therapy. In that study, the mean follow-up period was approximately 24 months, and penile length decreased until 15 months after ADT. Later, there were no significant changes in length despite this period of prolonged ADT [2]. This study also showed the failure to maintain penile function and structure without testosterone. Therefore, we would suggest that once matured penile tissues are always restored with androgen replacement in healthy men.
In the current study, there were no significant differences in penile changes between rats that underwent 8 weeks of ADT and those that underwent 16 weeks. Our current results demonstrate that rats have an earlier latency of penile changes than humans. These differences can be explained by the human adrenal gland, which produces dehydroepiandrosterone (DHEA) and 4-androstenedione (A-dione), as opposed to rats. DHEA and A-dione might contribute to maintenance of penile structure via peripheral conversion to androgen [14]. This would allow humans to sustain penile length for a longer period of testosterone deprivation than rats. Finally, we suggest that adult impotence caused by testosterone deprivation can be restored by testosterone replacement, regardless of the deprivation period. We believe that these results may be applicable to men undergoing radical prostatectomy. A study by Ory et al. [15] enrolled 82 men with prostate cancer treated with various modalities who took testosterone replacement therapy for a mean of 41 months. There was no meaningful biochemical recurrence during the follow-up period in patients who underwent radical prostatectomy, despite significant increases in serum testosterone and PSA [15]. For hypogonadal men seeking to restore erectile function after definitive treatment, replacement of testosterone should be suggested.
This study had some limitations. We did not find any evidence of irreversible changes of penile function and structure, perhaps due to the relatively short period of androgen deprivation. Alternatively, castration might not result in irreversible changes in penis size in sexually mature male rats. Further long-term studies will be needed to confirm our findings.
Conclusions
Androgen regulates the expression of eNOS in the rat penile corpus cavernosum. The importance of androgens in the maintenance of erectile function was confirmed in this study. Long-term androgen deprivation did not induce irreversible structural or erectile functional changes in adult male rats.
References
Traish AM. Androgens play a pivotal role in maintaining penile tissue architecture and erection: a review. J Androl 2009;30:363–9.
Park KKK, Lee SHS, Chung BHB. The effects of long-term androgen deprivation therapy on penile length in patients with prostate cancer: a single-center, prospective, open-label, observational study. J Sex Med 2011;8:3214–9.
Traish AM, Park K, Dhir V, Kim NN, Moreland RB, Goldstein I. Effects of castration and androgen replacement on erectile function in a rabbit model. Endocrinology 1999;140:1861–8.
Traish A, Munarriz R, O’Connell L, Choi S, Kim S Effects of medical or surgical castration on erectile function in an animal model. J Androl 2003;24:381–384
Lu Y-L, Kuang L, Zhu H, Wu H, Wang X-F, Pang Y-P, et al Changes in aortic endothelium ultrastructure in male rats following castration, replacement with testosterone and administration of 5alpha-reductase inhibitor. Asian J Androl 2007;9:843–7.
Keast JR, Gleeson RJ, Shulkes A, Morris MJ. Maturational and maintenance effects of testosterone on terminal axon density and neuropeptide expression in the rat vas deferens. Neuroscience 2002;112:391–8.
Hwang EC, Oh KJ, Jung SI, Kim NN, Ahn KY, Park K. Effects of androgen on the expression of vascular endothelial growth factor in the penile corpus cavernosum. Urology 2011;77:1381–1386
Traish A, Kim N. The physiological role of androgens in penile erection: regulation of corpus cavernosum structure and function. J Sex Med 2005;2:759–70.
Miranda AF, Gallo CB, De Souza DB, Costa WS, Sampaio FJ. Effects of castration and late hormonal replacement in the structure of rat corpora cavernosa. J Androl 2012;33:1224–1232
Vasconcelos JS, Figueiredo RT, Nascimento FLB, Damião R, da Silva EA. The natural history of penile length after radical prostatectomy: a long-term prospective study. Urology 2012;80:1293–7.
Fraiman M, Lepor H, McCullough A. Changes in penile morphometrics in men with erectile dysfunction after nerve-sparing radical retropubic prostatectomy. Mol Urol 1999;3:109–15.
Munding MD, Wessells HB, Dalkin BL. Pilot study of changes in stretched penile length 3 months after radical retropubic prostatectomy. Urology 2001;58:567–569
Haliloglu A, Baltaci S, Yaman O. Penile length changes in men treated with androgen suppression plus radiation therapy for local or locally advanced prostate cancer. J Urol 2007;177:128–30.
Traish AM, Guay AT. Are androgens critical for penile erections in humans? Examining the clinical and preclinical evidence. J Sex Med 2006;3:382–407.
Ory J, Flannigan R, Lundeen C, Huang JG, Pommerville P, Goldenberg SL. Testosterone therapy in patients with treated and untreated prostate cancer: impact on oncologic outcomes. J Urol 2016;196:1082–9.
Acknowledgements
This work was supported by the research grant of the Jeju National University Hospital in 2014. * Jung Sik Huh and Byung Ha Chung, both author equally contributed to complete this study as first author
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Huh, J.S., Chung, B.H., Hong, C.H. et al. The effects of testosterone replacement on penile structure and erectile function after long-term castration in adult male rats. Int J Impot Res 30, 122–128 (2018). https://doi.org/10.1038/s41443-017-0010-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41443-017-0010-6
- Springer Nature Limited