Abstract
Hypertension and type 2 diabetes frequently coexist, suggesting that the two diseases have common pathophysiological bases. This review describes the pathophysiological mechanisms of how type 2 diabetes is frequently associated with hypertension. Multiple common factors mediate between both diseases. Factors that induce both type 2 diabetes and hypertension include obesity-induced hyperinsulinemia, activation of the sympathetic nervous system, chronic inflammation, and changes in adipokines. Vascular complications resulting from type 2 diabetes and hypertension include endothelial dysfunction, vasodilation/constriction dysfunction of peripheral vessels and increased peripheral vascular resistance, arteriosclerosis, and chronic kidney disease. While many of these vascular complications are caused by hypertension, they also exacerbate the pathology of hypertension. In addition, insulin resistance in the vasculature blunts insulin-induced vasodilation and blood flow to skeletal muscle, which contributes to impaired glucose uptake to skeletal muscle and glucose intolerance. In obese and insulin-resistant patients, increase in the circulating fluid volume forms the major pathophysiology of elevated blood pressure. On the other hand, in non-obese and/or insulin-deficient patients, especially those in the middle- or later stages of diabetes, peripheral vascular resistance is the major pathophysiology of hypertension.

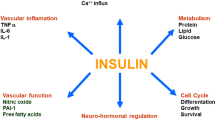
The relationship between various factors involved in the pathogenesis of type 2 diabetes and hypertension. It should be noted that all the factors shown in the figure are not necessarily present simultaneously in every patient.
Similar content being viewed by others
Introduction
Type 2 diabetes and hypertension are both common diseases. For example, according to the 2019 National Health and Nutrition Survey Report in Japan, 19.7% of men and 10.8% of women were strongly suspected of having diabetes (defined as hemoglobin A1c greater than 6.5%) and 29.9% of men and 24.9% of women had a systolic blood pressure of 140 mm Hg or higher. Furthermore, diabetes and hypertension frequently coexist. The rate of hypertension in patients with diabetes is ~50%, which is about twice that in non-diabetic patients [1,2,3,4]. Conversely, the rate of diabetes in patients with hypertension is two to three times higher than in non-hypertensive individuals [1, 4,5,6]. In addition, the prevalence of concomitant diabetes and hypertension is even higher in patients with factors such as older age, obesity, and renal dysfunction. The frequent coexistence of diabetes and hypertension suggests not only that they are common diseases but also that they have common pathophysiological bases. Genetic predisposition is one such common base [7], and patients with type 2 diabetes have characteristics that enhance the risk of developing hypertension. For example, a recent study identified a combination of 14 single nucleotide polymorphisms (SNPs) for hypertension in patients with type 2 diabetes and found that the number of SNPs was dose-dependently associated with the risk of hypertension [8]. However, acquired and modifiable factors such as excessive energy intake, alcohol consumption, smoking, and lack of physical activity are considered to be more strongly involved in the development and coexistence of both diseases. In particular, when obesity occurs as a result of these genetic and/or acquired factors, the risk of developing diabetes and hypertension increases further, as evidenced by their inclusion as components of metabolic syndrome [9].
Another important implication regarding the coexistence of diabetes and hypertension is that their coexistence further increases the risk of micro- and macrovascular complications [3, 10,11,12,13], e.g., the risk for cardiovascular diseases is about fourfold higher in individuals with both diabetes and hypertension than in those without either disease [3, 10]. Consequently, it is recommended that blood pressure should be strictly controlled in patients with diabetes [13, 14]. In this review, we first outline the pathologies of type 2 diabetes and hypertension, which are closely related as mentioned above. I would like to review the relationship between these factors in pathology and consider how diabetes and hypertension coexist and whether there is a causal relationship between them.
Classification and pathophysiology of type 2 diabetes
All cases of type 2 diabetes have chronic hyperglycemia as a common feature, but the etiology and pathophysiology of the disease are diverse. The American Diabetes Association classifies the etiology into type 1 diabetes, type 2 diabetes, specific types of diabetes due to other causes, and gestational diabetes [15]. In addition, type 2 diabetes is often divided into a type in which obesity and insulin resistance are the main pathologies and another type in which obesity is not present and insulin secretion deficiency is the main pathology. This review explains the typical features of these two types below. However, the actual pathophysiologies of individual patients with type 2 diabetes cannot be explained only by being obese with insulin resistance or non-obese with insulin secretory deficiency, and most patients exhibit varying proportions of both features and are somewhere between these pathological classifications [15].
Type 2 diabetes with obesity and insulin resistance as the main pathology
As obesity progresses, adipocytes become larger and adipose tissue becomes hypertrophied. Such adipose tissue, especially visceral adipose tissue, undergoes histological and functional changes, referred to as “pathological expansion” or “unhealthy expansion”, which include chronic inflammation, increased oxidative stress, hypoxia, tissue fibrosis, and alterations in adipocyte-derived bioactive substances called adipokines in both human and rodent [16,17,18,19]. Among the features of pathological expansion, in particular a decrease in adiponectin and increase in the production of inflammatory cytokines such as tumor necrosis factor alpha (TNFα) and interleukin-6 (IL-6) are involved in the induction of whole-body insulin resistance. Hypertrophic adipose tissue is infiltrated by inflammatory M1 macrophages, which produce more inflammatory cytokines than enlarged adipocytes do [16, 19,20,21].
In non-obese individuals, fat is found mainly in subcutaneous adipose tissue, and adipocytes exert physiological functions such as producing and secreting adiponectin. However, as people gain weight, fat ectopically accumulates in visceral adipose tissue, liver (resulting in fatty liver), skeletal muscle, heart, kidney, perivascular, and pericardial spaces. This so-called ectopic fat accumulation impairs physiological tissue function. In the pathogenesis of obesity-associated glucose intolerance, some types of ectopic fat, such as visceral and hepatic fat, are involved in the induction of insulin resistance [21]. In addition, ectopic fat is also seen in the pancreas as interlobular or intralobular infiltration of adipocytes and accumulation of intracellular lipid droplets. The effect of pancreatic fat accumulation on glucose metabolism is still controversial, but research has suggested that fat-cell infiltration is involved in the deterioration of the insulin-secreting capacity through islet inflammation [22, 23].
The type of diabetes with strong insulin resistance is predominant in Western countries, and its etiology is related to lifestyle habits and environmental factors. In this context, it is important that although East Asian people, including Japanese, and Asian American people are less obese than people of other ethnicities, most of them also have varying degrees of insulin resistance before the onset of type 2 diabetes [15, 24]. Hyperinsulinemia occurs to compensate for insulin resistance, especially around the time of onset of type 2 diabetes. After long-lasting insulin resistance, the compensatory hypersecretion of insulin fails to match the resistance. As a result, insulin action is relatively deficient, blood glucose levels rise, and type 2 diabetes develops [15]. In addition, when insulin resistance is the underlying pathology and glucose intolerance is accompanied by metabolic abnormalities such as hypertension and dyslipidemia, metabolic syndrome or insulin resistance syndrome is diagnosed.
Type 2 diabetes with insulin deficiency as the main pathology
The mean body mass index (BMI) of Japanese patients with type 2 diabetes is about 25 to 26 kg/m2 [25], which is clearly lower than the mean BMI of patients with type 2 diabetes in Western countries (30–32 kg/m2) [26, 27]. The Japanese Society for the Study of Obesity defines obesity as a BMI of 25 or higher (in contrast, the WHO defines obesity as a BMI of 30 or higher), but one in two Japanese people with type 2 diabetes does not have obesity as defined by this criterion. On the other hand, the prevalence of diabetes is fairly similar in Japan and the United States, which is related to the fact that people in East Asian countries, including Japan, have a genetically lower ability to secrete insulin than people in Western countries [28]. Type 2 diabetes is generally caused by genetic and environmental factors. Whereas insulin resistance is associated more strongly with lifestyle and environmental factors, insulin secretion is more closely related to a genetic predisposition or family history in first-degree relatives (even more so than type 1 diabetes). However, the genetics of type 2 diabetes are not fully clarified, and they are under intense investigation in the context of precision medicine [9].
Type 2 diabetes in which the main pathology is hyposecretion of insulin is often not accompanied by obesity from the early stage of diabetes onset. Furthermore, even in patients with type 2 diabetes whose main pathologies were initially obesity and insulin resistance/hyperinsulinemia, over time pancreatic β-cell function decreases (so-called exhaustion of pancreatic β-cells), as do blood insulin levels. The ultimate result may be insulin dependence, in which patients require insulin injections to survive [15, 29].
It should be noted that the two typical features described above not only represent the different types of patients with type 2 diabetes but also may change over the course of an individual’s medical history.
Pathophysiology of hypertension
The pathology of hypertension is primarily explained by increased circulatory fluid volume and increased peripheral vascular resistance [30]. The former is related to body fluid volume and cardiac contractile force. Body fluid volume is determined to a large extent by the balance between sodium excretion and reabsorption in the kidney, and this balance is affected by the renin-angiotensin-aldosterone system (RAS), salt intake, and salt sensitivity, among other things. Salt is excreted into the urine when blood pressure exceeds a certain threshold as classically described by Guyton’s pressure–natriuresis relationship curve [31]. When the threshold is elevated, i.e., when increased blood pressure is required for urinary sodium excretion, the condition is defined as being strongly salt sensitive. Salt sensitivity is influenced by impaired sodium excretion from the kidneys, as is seen in older people and patients with chronic kidney disease, and excess sodium accumulation, as is seen in people with obesity and diabetes. Excess salt intake combined with salt sensitivity leads to hypertension by increasing the volume of body fluid.
On the other hand, peripheral vascular resistance is related to vasoconstriction, vasodilation, and vascular remodeling. Vascular smooth muscle is the main effector of vascular constriction and dilation responses. The vasoconstrictive response is regulated by humoral factors such as RAS, catecholamines, and endothelin-1 and by neural regulation via sympathetic nerve activity, whereas the vasodilatory response is regulated by the release of vasodilators from vascular endothelial cells and responses to exogenous vasodilators such as nitric oxide (NO).
Insulin also has a vasodilatory effect that increases the delivery of insulin and glucose to target tissues, such as skeletal muscle [32]. In isolated arteries, the vasomotor effects of insulin are dependent on the balance between endothelial-derived NO-mediated vasodilation and endothelin-1-mediated vasoconstriction [33, 34]. The net effect of insulin is typically vasodilation in healthy subjects [35, 36]. Importantly, insulin also has effects that centrally activate the sympathetic nervous system and adrenergic receptor-mediated vasoconstriction, resulting in the limitation of NO-mediated vasodilation [32, 37, 38].
Vascular remodeling of resistance vessels (arterioles) increases peripheral vascular resistance because it constricts the lumen, and these changes increase intra-arterial blood pressure. Conversely, hypertension itself also induces several functional vessel abnormalities, such as impaired vasodilation due to endothelial dysfunction, remodeling of resistance vessels, and atherosclerosis of arteries. When hypertension coexists with other metabolic disorders, such as diabetes and dyslipidemia (e.g., in metabolic syndrome or insulin resistance syndrome), these changes progress further. Thus, hypertension and vascular functional and morphological abnormalities are mutually facilitative and lead to a vicious circle [30].
Pathophysiology of hypertension complicated with diabetes
Patients with diabetes have a high rate of comorbidity with hypertension, and vice versa. One reason for this relationship is that the two diseases have some common background factors and some disease mechanisms promote each other. The relationship between various factors involved in the pathogenesis of diabetes and hypertension is summarized in Fig. 1. However, it should be noted that all the factors shown in the figure are not necessarily present simultaneously in one patient. For example, among patients with type 2 diabetes, the major factors leading to hypertension are different in obese/insulin-resistant and non-obese/insulin-deficient patients and in patients in different disease stages, as discussed below.
Most patients with type 2 diabetes have insulin resistance before plasma glucose levels become elevated. Compensatory hypersecretion of insulin by pancreatic β-cells can lead to hyperinsulinemia, especially before and after the onset of diabetes [15]. Hyperinsulinemia contributes to elevated blood pressure through several mechanisms; for example, insulin promotes sodium reabsorption and increases body fluid volume by opening the Na+/H+ transporter expressed in renal tubular cells [39, 40]. In addition, hyperinsulinemia activates the sympathetic nervous system and increases renin secretion, which elevates blood pressure by increasing peripheral vascular resistance and cardiac output [41]. Hyperinsulinemia is also involved in a mechanism for hypertension via sympathetic nerve activation in obesity even in the absence of diabetes. Furthermore, accelerated arteriosclerosis due to hyperinsulinemia also contributes to elevated blood pressure [21, 42].
Similar to insulin resistance, the pathological expansion of adipose tissue can predate the onset of type 2 diabetes [17, 18]. Changes in adipokines and increases in inflammatory cytokines in hypertrophied adipose tissue are also involved in the pathology of hypertension and cause glucose intolerance. In particular, the adipokine angiotensinogen, which is produced and released from enlarged adipocytes, activates RAS and enhances both body fluid retention and vasoconstriction, causing an elevation of blood pressure [43]. Although insulin acts to promote vascular remodeling, this process takes time and is milder in the earlier stages of diabetes than in the middle and later stages. Thus, during the earlier stages of diabetes, when insulin resistance is the predominant pathology, an increase in the circulating fluid volume is considered to be the major pathophysiology leading to hypertension [29].
As diabetes progresses, insulin secretion decreases because of the exhaustion of pancreatic β-cells (not because of an improvement in insulin resistance), and as a result, the direct effects of hyperinsulinemia are weakened. On the other hand, persistent hyperglycemia and oxidative stress impair vascular endothelial function, resulting in reduced production of endogenous vasodilators, such as NO and prostaglandin I2, and decreased reactivity to vasodilators, such as exogenous NO, and a reduced vasodilatory response. Vasoconstriction is also enhanced through the diabetes-associated enhancement of the sympathetic nervous system and tissue RAS [41]. Thus, increased peripheral vascular resistance is considered to be the major pathophysiology of hypertension in the middle and later stages of diabetes [29].
Furthermore, the development of chronic kidney disease because of diabetic kidney disease decreases the glomerular filtration rate and sodium excretion from the kidney, further elevating blood pressure [44]. Concomitant arteriosclerosis also promotes an increase in blood pressure (Fig. 1) [21, 42]. In this review, detailed explanation about the involvement of these well-documented vascular complications in pathophysiology of hypertension will be avoided.
Conclusion
In this review, the pathophysiological mechanisms of how type 2 diabetes is frequently associated with hypertension were described. As described above, multiple common factors mediate between both diseases. They are not involved in the development of hypertension in all diabetic patients, and the factors involved differ for each patient, such as the presence or absence of obesity and the disease stage of diabetes. That is, in patients with obesity/insulin resistance as the main pathologies and in the early stages of diabetes, retention of body fluid forms the main pathology of hypertension. On the other hand, in non-obesity patients whose main pathology is insulin secretion deficiency or in the middle stage of diabetes or later, increased vascular resistance forms the main pathology of hypertension. These are not completely dichotomous, and there is a gradation between the two typical mechanisms (Fig. 1).
In addition, the causal relationships of these multiple factors are simplified and shown in Fig. 2. Factors that induce both type 2 diabetes and hypertension include obesity-induced hyperinsulinemia, activation of the sympathetic nervous system, and changes in adipokines (Fig. 2a). Vascular complications resulting from type 2 diabetes and hypertension, either alone or coexisting, include endothelial dysfunction, vasodilation/constriction dysfunction of peripheral blood vessels and increased peripheral vascular resistance, arteriosclerosis, and chronic kidney disease. (Fig. 2b). Many of these vascular complications are caused by hypertension, but they also work to exacerbate the pathology of hypertension (Fig. 2c).
Causal relationship of the factors between hypertension and type 2 diabetes. The causal relationship between various factors involved in the pathogenesis of diabetes and hypertension is summarized. a common factors that induce both type 2 diabetes and hypertension, b vascular complications resulting from type 2 diabetes and hypertension, c vascular complications which exacerbate hypertension, d vascular dysfunction which deteriorate glucose metabolism. It should be noted that all factors in this figure do not necessarily apply simultaneously to every patient
Finally, I will discuss only one point about whether vascular dysfunction is involved in the deterioration of glucose metabolism (Fig. 2d). Insulin signaling in vascular endothelial cells dilates blood vessels through the production of NO [32,33,34,35,36, 45]. Insulin resistance in the vasculature, which is observed in obesity and type 2 diabetes, blunts insulin-induced dilation in resistance arteries across vascular beds. Reduced insulin-stimulated vasodilation and blood flow to skeletal muscle limits glucose uptake and contributes to impaired glucose control in type 2 diabetes [32].
References
Iimura O. Insulin resistance and hypertension in Japanese. Hypertens Res. 1996;19:S1–8.
Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37:1053–9.
Lastra G, Syed S, Kurukulasuriya LR, Manrique C, Sowers JR. Type 2 diabetes mellitus and hypertension: an update. Endocrinol Metab Clin North Am. 2014;43:103–22.
Das UN. Risk of type 2 diabetes mellitus in those with hypertension. Eur Heart J. 2008;29:952–3. author reply 953-4
Conen D, Ridker PM, Mora S, Buring JE, Glynn RJ. Blood pressure and risk of developing type 2 diabetes mellitus: the Women’s Health Study. Eur Heart J. 2007;28:2937–43.
Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–7.
Shalimova A, Fadieienko G, Kolesnikova O, Isayeva A, Zlatkina V, Nemtsova V, et al. The role of genetic polymorphism in the formation of arterial hypertension, type 2 diabetes and their comorbidity. Curr Pharm Des. 2019;25:218–27.
Cheng CF, Hsieh AR, Liang WM, Chen CC, Chen CH, Wu JY, et al. Genome-wide and candidate gene association analyses identify a 14-SNP combination for hypertension in patients with type 2 diabetes. Am J Hypertens. 2021;34:651–61.
Chung WK, Erion K, Florez JC, Hattersley AT, Hivert MF, Lee CG, et al. Precision medicine in diabetes: a consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43:1617–35.
Hu G, Jousilahti P, Tuomilehto J. Joint effects of history of hypertension at baseline and type 2 diabetes at baseline and during follow-up on the risk of coronary heart disease. Eur Heart J. 2007;28:3059–66.
Ninomiya T, Kubo M, Doi Y, Yonemoto K, Tanizaki Y, Rahman M, et al. Impact of metabolic syndrome on the development of cardiovascular disease in a general Japanese population: the Hisayama study. Stroke. 2007;38:2063–9.
Kushiro T, Kario K, Saito I, Teramukai S, Sato Y, Okuda Y, et al. Increased cardiovascular risk of treated white coat and masked hypertension in patients with diabetes and chronic kidney disease: the HONEST Study. Hypertens Res. 2017;40:87–95.
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S158–90.
Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481.
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S19–S40.
Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58:2574–82.
Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Investig. 2011;121:2094–101.
Rutkowski JM, Stern JH, Scherer PE. The cell biology of fat expansion. J Cell Biol. 2015;208:501–12.
Takikawa A, Mahmood A, Nawaz A, Kado T, Okabe K, Yamamoto S, et al. HIF-1alpha in myeloid cells promotes adipose tissue remodeling toward insulin resistance. Diabetes. 2016;65:3649–59.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Investig. 2003;112:1796–808.
Neeland IJ, Ross R, Despres JP, Matsuzawa Y, Yamashita S, Shai I, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7:715–25.
Horii T, Fujita Y, Ishibashi C, Fukui K, Eguchi H, Kozawa J, et al., Islet inflammation is associated with pancreatic fatty infiltration and hyperglycemia in type 2 diabetes. BMJ Open Diabetes Res Care. 2020;8:e001508.
Kozawa J, Shimomura I. Ectopic Fat Accumulation in Pancreas and Heart. J Clin Med. 2021;10:1326.
Consultation, W.H.O.E. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63.
Takeuchi, M, Horikawa C, Hatta M, Takeda Y, Nedachi R, Ikeda I, et al., Secular trends in dietary intake over a 20-year period in people with type 2 diabetes in Japan: a comparative study of two nationwide registries; Japan Diabetes Complications Study (JDCS) and Japan Diabetes Clinical Data Management Study (JDDM). Nutrients. 2021;13:3428.
Group, UPDS. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
Xie J, Zhang X, Shao H, Jing S, Shan T, Shi Y, et al. An affordable approach to classifying type 2 diabetes based on fasting plasma glucose, TyG index and BMI: a retrospective cohort study of NHANES Data from 1988 to 2014. Diabetol Metab Syndr. 2022;14:113.
Fukushima M, Usami M, Ikeda M, Nakai Y, Taniguchi A, Matsuura T, et al. Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross-sectional study of Japanese type 2 diabetes. Metabolism. 2004;53:831–5.
Ohishi M. Hypertension with diabetes mellitus: physiology and pathology. Hypertens Res. 2018;41:389–93.
Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res. 2014;37:253–390.
Guyton AC, Manning RD Jr, Hall JE, Norman RA Jr, Young DB, Pan YJ. The pathogenic role of the kidney. J Cardiovasc Pharm. 1984;6:S151–61.
Padilla J, Manrique-Acevedo C, Martinez-Lemus LA. New insights into mechanisms of endothelial insulin resistance in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2022;323:H1231–8.
Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94:1172–9.
Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W, et al. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia. 2009;52:752–64.
Eringa EC, Stehouwer CD, Merlijn T, Westerhof N, Sipkema P. Physiological concentrations of insulin induce endothelin-mediated vasoconstriction during inhibition of NOS or PI3-kinase in skeletal muscle arterioles. Cardiovasc Res. 2002;56:464–71.
Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–904.
Vollenweider L, Tappy L, Owlya R, Jéquier E, Nicod P, Scherrer U. Insulin-induced sympathetic activation and vasodilation in skeletal muscle. Effects of insulin resistance in lean subjects. Diabetes. 1995;44:641–5.
Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Investig. 1991;87:2246–52.
Sweeney G, Klip A. Regulation of the Na+/K+-ATPase by insulin: why and how? Mol Cell Biochem. 1998;182:121–33.
Martinez FJ, Sancho-Rof JM. Epidemiology of high blood pressure and obesity. Drugs. 1993;46:160–4.
Seravalle G, Grassi G. Sympathetic nervous system, hypertension, obesity and metabolic syndrome. High Blood Press Cardiovasc Prev. 2016;23:175–9.
Love KM, Liu Z. DPP4 activity, hyperinsulinemia, and atherosclerosis. J Clin Endocrinol Metab. 2021;106:1553–65.
Karlsson C, Lindell K, Ottosson M, Sjöström L, Carlsson B, Carlsson LM. Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II. J Clin Endocrinol Metab. 1998;83:3925–9.
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. Chronic kidney disease and risk management: standards of care in diabetes-2023. Diabetes Care. 2023;46:S191–S202.
Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 2011;13:294–307.
Acknowledgements
This study did not receive any financial support. IU has received honoraria from Sumitomo Dainippon Pharma as lecture fees.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Usui, I. Common metabolic features of hypertension and type 2 diabetes. Hypertens Res 46, 1227–1233 (2023). https://doi.org/10.1038/s41440-023-01233-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01233-x
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
The association between index-year, average, and variability of the triglyceride-glucose index with health outcomes: more than a decade of follow-up in Tehran lipid and glucose study
Cardiovascular Diabetology (2024)
-
Patients with diabetes and obesity exhibit characteristics in eating and coping behaviors and personality traits
Diabetology International (2024)
-
The vascular influence of melatonin on endothelial response to angiotensin II in diabetic rat aorta
Journal of Bioenergetics and Biomembranes (2024)
-
Pretreatment body mass index affects achievement of target blood pressure with sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes mellitus and chronic kidney disease
Hypertension Research (2024)