Abstract
Hyperuricemia has been reported to be a risk factor for hypertension, but this association may be affected by alcohol consumption. This study aimed to investigate whether hyperuricemia remains a risk factor for hypertension after eliminating the effect of alcohol consumption. This study comprised 7848 participants (4247 men and 3601 women) aged 30–74 years without hypertension who had undergone a medical checkup between April 2008 and March 2009 at Saku Central Hospital, Nagano Prefecture, Japan. Hyperuricemia was defined as uric acid >7.0 mg/dl in men, ≥6.0 mg/dl in women, and/or receiving treatment for hyperuricemia or gout. The incidence of hypertension was defined as the first diagnoses of blood pressure ≥140/≥ 90 mmHg and/or initiations of antihypertensive drug treatment. Multivariable-adjusted hazard ratios (HRs) of hyperuricemia for the incidence of hypertension after adjustment for and classification by alcohol consumption were estimated using the Cox proportional hazard model. During a mean of 4.0 years of follow-up, 1679 individuals developed hypertension. After adjustment for alcohol consumption, the HRs (95% confidence interval) associated with hyperuricemia were 1.37 (1.19–1.58) in men and 1.54 (1.14–2.06) in women. Among nondrinkers, the HR was 1.29 (0.94–1.78) in men with hyperuricemia compared with men without, and the corresponding HR was 1.57 (1.11–2.22) in women. The corresponding HR was 1.88 (1.27–2.86) in all participants with baseline blood pressure <120/80 mmHg. The interactions between hyperuricemia and sex (P = 0.534) and between drinking and sex (P = 0.713) were not significant. In conclusion, hyperuricemia predicts the risk for developing hypertension independent of alcohol drinking status.
Similar content being viewed by others
Introduction
Recently, many prospective studies have reported a positive association between hyperuricemia and the incidence of hypertension [1,2,3,4,5]. However, the assessment of uric acid level has not been regarded as important in the prevention of hypertension [6,7,8]. To confirm an independent association of hyperuricemia with hypertension, it is necessary to consider the effect of alcohol consumption, which is associated with both hyperuricemia and hypertension.
Because alcohol drinking leads to a surge in the production of uric acid as a metabolite and a decrease in uric acid excretion, alcohol drinking is an important risk factor for the incidence of hyperuricemia [9,10,11,12]. Alcohol drinking is also strongly associated with hypertension. A meta-analysis of 16 prospective cohort studies reported that alcohol consumption increased the risk of hypertension in men and had a J-shaped relationship with hypertension in women [13]. A meta-analysis of randomized, controlled trials reported that a reduction in alcohol was effective at reducing blood pressure (BP), although most of the subjects were men [14, 15]. Therefore, societies for hypertension worldwide recommended strategies for reducing alcohol consumption to prevent hypertension [6,7,8].
Previous prospective studies that evaluated the relationship between hyperuricemia and the incidence of hypertension adjusted their data for alcohol consumption as a potential confounding factor in middle-aged or older individuals [1,2,3,4,5]. Although only two studies investigated the association between hyperuricemia and the incidence of hypertension in nondrinkers and drinkers, these two studies did not report significant associations in nondrinkers [16, 17]. An independent association between hyperuricemia and hypertension is suggested by previous randomized controlled studies that showed that urate-lowering therapy reduces BP in adolescents who do not drink alcohol [18, 19]. However, whether hyperuricemia is associated with the incidence of hypertension, irrespective of alcohol consumption, remains to be determined in middle-aged or older individuals.
If hyperuricemia remains a risk factor for hypertension after eliminating the effect of alcohol drinking, assessment of uric acid levels will be useful for preventing hypertension. Therefore, we conducted a prospective cohort study to investigate the relationships between hyperuricemia and the incidences of hypertension in Japanese men and women.
Methods
Study participants
The participants were 12,356 individuals aged 30–74 years who had undergone comprehensive medical checkups between April 2008 and March 2009 (baseline) at Saku Central Hospital. The details of this study have been described previously [20,21,22]. Of these individuals, 3443 were excluded for having hypertension at baseline (systolic BP ≥ 140 mmHg, diastolic BP ≥ 90 mmHg, and/or were receiving antihypertensive treatment). A total of 8807 did not have missing data of variables used in this study. After excluding 959 participants who did not undergo at least one follow-up examination before the end of March 2014, data from 7848 remained for analysis. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Saku Central Hospital (committee approval number: R201404-02) and the Ethics Committee of Teikyo University (committee approval number: 17-040). An opt-out consent procedure was implemented, and the standard questionnaires included opt-out information.
Data collection and definitions at baseline
Participants completed a questionnaire that included demographic characteristics, medical history, alcohol consumption, physical activity (0, 1–59, or 60 min/week), and smoking habits (never, current, or quit). Alcohol consumption was calculated as the amount of intake per time multiplied by frequency and was categorized according to the recommended values of the Ministry of Health, Labour and Welfare of Japan (<140 g/week in men and <70 g/week in women) and twice the recommended value only in men [19]. Twice the recommended value was set as 275 g/week, which was less than 280 g/week because the calculated alcohol consumption in the present study was discrete. Data in women were not divided by twice the recommended value because of the small sample size.
All participants fasted overnight and had a blood test in the morning. Hyperuricemia was defined as uric acid >7.0 mg/dl in men, ≥6.0 mg/dl in women, and/or receiving treatment for hyperuricemia or gout according to a previous study [5]. The cut-off point of uric acid for women is 7.0 mg/dl in Japan [23]. However, we used the cut-off point of 6.0 mg/dl because the number of women with uric acid >7.0 mg/dl was small (n = 27). The estimated glomerular filtration rate (eGFR) was calculated by the following formula: 194 × creatinine−1.094 × age−0.287 for men and 194 × creatinine−1.094 × age−0.287 × 0.739 for women [24]. The cut-off point of eGFR was 60 ml/min/1.73 m2 [25]. HbA1c (%) is presented as National Glycohemoglobin Standardization Program equivalent values (%) and was calculated using the formula HbA1c (%) = 1.02 × HbA1c (Japan Diabetes Society, %) + 0.25% [26]. Diabetes was defined by either fasting blood glucose ≥126 mg/dl, HbA1c ≥6.5%, and/or the use of medication for diabetes [27]. Dyslipidemia was defined as having either high-density lipoprotein cholesterol <40 mg/dl, low-density lipoprotein cholesterol ≥140 mg/dl, triglycerides ≥150 mg/dl and/or the use of anti-dyslipidemic medication [28]. BP was measured twice by trained nurses using the ES-H55 device with a standard arm cuff (Terumo Corporation, Tokyo, Japan) [29], which is a semiautomatic device based on the cuff-oscillometric method. Participants were in a seated position after at least a 5-min rest. The mean of the two BP measurement values was used. Height and weight were measured while participants wore a hospital gown, and body mass index (BMI) was calculated as weight (kg) divided by the square of height (m). Overweight was defined as BMI ≥ 25.0 kg/m2.
Incidence of hypertension and follow-up
All participants underwent annual follow-up exams at Saku Central Hospital. The end of the follow-up period was March 2014. Of 7848 participants, 49.7%, 16.9%, 10.0%, 12.4%, and 11.0% underwent medical check-ups five, four, three, two, and one time, respectively, from baseline to March 2014. The incidence of hypertension was defined as a first diagnosis of systolic BP ≥ 140 mmHg, diastolic BP ≥ 90 mmHg, and/or receipt of antihypertensive treatment [6]. Individuals who did not develop hypertension during the follow-up period were censored on the date of their last examination.
Statistical analysis
The differences in the characteristics of participants who were or were not followed up were analyzed using the Student’s t test or the χ2 test.
Baseline characteristics, classified according to presence or absence of hyperuricemia, were analyzed using the Student’s t test or the χ2 test. Furthermore, to investigate the association between hyperuricemia and alcohol consumption at baseline, logistic regression analyses were used to estimate the multivariable-adjusted odds ratios for hyperuricemia according to alcohol consumption. The confounders were age (per 10 years), eGFR (≥60 ml/min/1.73 m2 or <60 ml/min/1.73 m2), diabetes, dyslipidemia, BMI (<25 kg/m2 or ≥ 25 kg/m2), smoking status (never, current, and quit), physical activity (0, 1–59, ≥ 60 min/week), family history of hypertension, and systolic BP (per 10 mmHg).
To investigate the dose-response effects of uric acid level and alcohol consumption level on the incidence of hypertension, Cox proportional hazard models were used, which generated hazard ratios (HRs) and 95% confidence intervals (CIs) for hypertension per 1.0 mg/dl increment in uric acid level and alcohol consumption (1–139, 140–274, or ≥275 g/week in men, and 1–69 or ≥70 g/week in women) compared with nondrinkers, classified according to sex. Model 1 included uric acid level, absence or presence of medical treatment for hyperuricemia or gout, and confounders. The confounders were age (per 10 years), eGFR (≥60 ml/min/1.73 m2 or <60 ml/min/1.73 m2), diabetes, dyslipidemia, BMI (<25 kg/m2 or ≥25 kg/m2), smoking status (never, current, quit), physical activity (0, 1–59, ≥60 min/week), family history of hypertension, and systolic BP (per 10 mmHg) at baseline. Model 2 included the variables in model 1 and alcohol consumption (0, 1–139, 140–274, and ≥275 g/week in men and 0, 1–69, and ≥70 g/week in women). Because there was a strong correlation between systolic and diastolic BP (r = 0.74), diastolic BP was not included in the multivariable models.
Participants were classified as normouricemia or hyperuricemia and nondrinkers (0 g/week) or drinkers (≥1 g/week). The HRs of those with hyperuricemia in comparison to normouricemia and drinkers in reference to nondrinkers were estimated. Model 1 included the presence or absence of hyperuricemia and the above-described confounders. Model 2 included model 1 variables and alcohol drinking. Interactions between hyperuricemia and sex and between alcohol drinking and sex with regard to the incidence of hypertension were assessed in a model that included the model 2 variables and sex.
Furthermore, participants were classified into four categories by normouricemia or hyperuricemia and nondrinkers or drinkers. The HRs for participants who had hyperuricemia and/or were drinkers were estimated in comparison to normouricemic nondrinkers, according to sex and whether they had normal BP (systolic/diastolic BP < 120/80 mmHg) or not (systolic/diastolic BP ≥ 120/80 mmHg), using the cut-off defined in the 2019 Japan Society of Hypertension guidelines [30]. The confounders were the same as described above. The interactions between hyperuricemia and drinking were assessed. As a sensitivity analysis, the same analysis was conducted, but participants with diabetes were excluded because high blood glucose concentration is associated with uric acid excretion [31].
All reported p values are two-tailed, and those <0.05 were considered statistically significant. All data were analyzed using STATA release 14 (StataCorp LP, College Station, TX, USA).
Results
The differences between the characteristics of participants who were and were not followed up are shown in Supplementary Table 1. Participants who were not followed up were significantly younger and had a higher prevalence of current smoking and physical activity 0 min/week than participants who were followed up. In addition, men who were not followed up had a significantly lower prevalence of a family history of hypertension.
The baseline characteristics according to the presence or absence of hyperuricemia are shown in Table 1. All the variables, except for physical activity level and family history of hypertension in both sexes and diabetes in women, significantly differed between participants with hyperuricemia and those with normouricemia. Participants of both sexes with hyperuricemia had higher systolic and diastolic BP and higher prevalences of eGFR <60 ml/min/1.73 m2, dyslipidemia, overweight, heavy alcohol drinking and past smoking. Logistic regression analyses generated multivariable-adjusted odds ratios (95% CIs) for hypertension of 1.24 (1.00–1.54) in men who drank 1–139 g alcohol/week, 1.74 (1.40–2.24) in men who drank 140–274 g/week and 2.37 (1.81–3.11) in men who drank ≥274 g/week, compared with nondrinkers (p for linear trend < 0.001). The corresponding odds ratios were 0.95 (0.63–1.41) for women who drank 1–69 g alcohol/week and 2.10 (1.27–3.47) for women who drank ≥70 g/week, compared with nondrinkers (p for linear trend 0.037).
During a mean follow-up of 3.99 years (median, 25–75th percentile: 4.75, 3.04–5.01), 1679 participants developed hypertension. Table 2 shows the multivariable-adjusted HRs and 95% CIs per 1.0 mg/dl increment in uric acid level for the incidence of hypertension. The HRs per 1.0 mg/dl increment in uric acid were 1.14 (p < 0.001) in men and 1.17 (p < 0.001) in women, and these were not altered by adjustment for alcohol consumption. Alcohol consumption was also positively associated with the incidence of hypertension in both sexes. Because of the small number of women undergoing medical treatment for hyperuricemia or gout, the same analysis was conducted using model 2, but excluding women undergoing medical treatment. This generated similar results: the HR was 1.16 (1.07–1.27) per 1.0 mg/dl increment in uric acid concentration. The HRs for the confounders are shown in Supplementary Table 2. Age, systolic BP, eGFR, and family history of hypertension were significantly associated with the incidence of hypertension in both sexes.
The risk of hyperuricemia compared with normouricemia is shown in Table 3. After adjustment for alcohol consumption, men with hyperuricemia had a 1.37-fold higher risk of hypertension, and women with hyperuricemia had a 1.54-fold higher risk of hypertension. Alcohol drinkers of both sexes also had significantly higher risks of hypertension when compared with nondrinkers. There were no interactions between hyperuricemia and sex (P = 0.534) or between drinking and sex (P = 0.713).
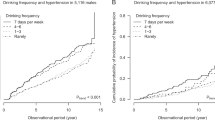
Figure 1 shows the multivariable-adjusted HRs and 95% CIs for the incidence of hypertension when participants were classified according to categories of both uricemic and alcohol drinking status and by sex. In women, compared with nondrinkers who had normouricemia, participants in all of the other three categories were at significantly higher risk of hypertension. In men, the risk of nondrinkers with hyperuricemia was 1.29-fold higher compared with nondrinkers with normouricemia, although the risk did not have significance. Because of the small sample size, the following additional analyses were conducted without categorizing the participants according to sex. The sensitivity analyzes that excluded participants with diabetes generated HRs of 1.47 (1.16–1.88) in nondrinkers with hyperuricemia, 1.26 (1.11–1.43) in drinkers with normouricemia, and 1.76 (1.48–2.10) in drinkers with hyperuricemia. After classification according to having normal BP (BP < 120/80 mmHg) or not (BP ≥ 120/80 mmHg), the risk of hyperuricemia compared to normouricemia among nondrinkers was significantly higher in the normal BP group. The HRs were 1.88 (1.24–2.86) in nondrinkers with hyperuricemia, 1.56 (1.24–1.95) in drinkers with normouricemia, and 3.30 (2.44–4.49) in drinkers with hyperuricemia among participants with normal BP. The corresponding HRs were 1.21 (0.92–1.61), 1.12 (0.97–1.29), and 1.33 (1.09–1.63) among participants with BP ≥ 120/80 mmHg.
Multivariable-adjusted hazard ratios for incidence of hypertension according to the combined variable of hyperuricemia and alcohol drinking status in (a) men and (b) women. Numbers on the cylinders indicate the hazard ratio (95% confidence interval). Numbers in the cylinders indicate case number. Confounding factors were age (per 10 years), estimated glomerular filtration rate (≥60 ml/min/1.73 m2 or <60 ml/min/1.73 m2), diabetes, dyslipidemia, BMI (<25 kg/m2 or ≥25 kg/m2), smoking status (never, current, quit), physical activity (0, 1–59, ≥60 min/week), family history of hypertension, and systolic blood pressure (per 10 mmHg) at baseline. CI confidence interval, HR hazard ratio, Ref reference
Discussion
This prospective cohort study aimed to determine whether hyperuricemia remains a risk factor for hypertension after eliminating the effect of alcohol consumption. Although alcohol drinking was positively associated with the presence of hyperuricemia at baseline, the significant dose-response effect of uric acid concentration on the incidence of hypertension during the follow-up period remained after adjustment for alcohol consumption in both sexes. Furthermore, the risk of hyperuricemia was significantly higher after adjustment for alcohol drinking status in both sexes. Finally, nondrinkers with hyperuricemia were at higher risk of hypertension compared to nondrinkers with normouricemia, although the significance was observed only in women. The interactions between hyperuricemia and sex and between drinking and sex were not significant. These findings suggest that hyperuricemia is a risk factor for hypertension, independent of alcohol consumption.
Hyperuricemia was associated with the incidence of hypertension without a drinking habit in our study. The mechanisms whereby hypertension develops in patients with hyperuricemia may include endothelial dysfunction, vascular smooth muscle cell proliferation, insulin resistance, and impaired endothelial nitric oxide production [32]. A prospective study in the USA investigated the association between uric acid level and the incidence of hypertension among 398 nondrinking men and women [16]. This previous study reported that those with the highest quartile of uric acid level had a 1.72-fold (95% CI, 0.99–2.99) higher relative risk compared with those with the lowest quartile. This finding in a previous study supports the present findings, although the risk was not significant because of the small sample size in the previous study. In addition, a cohort study on Japanese men reported that an increment of 1 mg/dl in uric acid slightly, but not significantly, increased the risk of hypertension among nondrinkers [17]. In the present study, even among individuals with a baseline BP < 120/80 mmHg, nondrinkers with hyperuricemia had a significant, 1.88-fold higher risk of hypertension than nondrinkers with normouricemia, implying a causal relationship between hyperuricemia and hypertension. Therefore, the present study is the first to report a significant association between uric acid level or hyperuricemia and the incidence of hypertension among nondrinkers. In addition, the corresponding risk was 1.21 in individuals with BP ≥ 120–139/80–89 mmHg at baseline. A recent Japanese prospective cohort study has shown that hyperuricemia is associated with a 1.35-fold higher risk of hypertension than normouricemia in individuals with BP 120–139/80–89 mmHg [5]. Although this previous study did not stratify the participants according to their alcohol consumption, its findings are also consistent with those of the present study. Thus, it is suggested that hyperuricemia predict the risk of hypertension, independent of alcohol drinking status.
We have also shown that alcohol intake is associated with hypertension without the involvement of hyperuricemia. Previous epidemiological studies that focused on estimating the risk of alcohol drinking for hypertension did not adjust for uric acid level or hyperuricemia [13]. In the present study, we found that alcohol drinkers without hyperuricemia were at high risk for hypertension. Although alcohol drinking is already a well-known risk factor for hypertension [6,7,8], evidence of the risk of alcohol drinking for hypertension is scarce among Asian women. Because alcohol consumption in Asian women is small, only a few epidemiological studies could classify women into three or more quantitatively defined categories of alcohol consumption to estimate the risk of hypertension [33, 34]. However, these studies did not report a significantly higher risk of heavy alcohol consumption for hypertension. Therefore, the present study has also provided evidence of a significant risk of high alcohol consumption for hypertension among Asian women.
A strength of the present study was the high follow-up rate for annual health check-ups in a community-based population. However, this study also has some limitations. First, dietary information regarding salt intake was not available. Although the estimated risks may be attenuated after adjustment for salt intake, the effect may not be larger than that of BMI or alcohol consumption [35]. Second, heart rate data were not available. An increase in sympathetic activity increases BP and reduces uric acid excretion. Therefore, a confounding effect of sympathetic activity could have been adjusted for if heart rate had been adjusted for as an index of sympathetic activity [36]. Third, selection bias was possible because study participants were individuals who underwent routine comprehensive medical check-ups. The participants who were excluded from the study because they did not undergo a follow-up examination were relatively young, were more likely to be current smokers and to engage in no physical activity, and had a lower prevalence of a family history of hypertension (men only). These participants may have been less health-conscious than those who underwent follow-up assessments. As reported by the National Health and Nutrition Survey in Japan in 2008, the prevalence of hypertension, defined as BP ≥ 140/90 mmHg and/or receiving medical treatment for hypertension, was 44.6% among Japanese adults aged 30–74 years [37]. The prevalence of hypertension was 27.9% among those aged 30–74 years who had undergone medical check-ups at Saku Central Hospital in 2008. Thus, participants in the present study were healthier than the general population in Japan. Therefore, the interpretation of the present study findings should be applied to the general population with caution.
In conclusion, hyperuricemia is a risk factor for hypertension, and it is independent of alcohol drinking. Assessment for uric acid levels independent of drinking habits may be useful for strategies of preventing hypertension among Japanese men and women.
References
Zheng R, Yang T, Chen Q, Chen C, Mao Y. Serum uric acid concentrations can predict hypertension: a longitudinal population-based epidemiological study. Horm Metab Res. 2017;49:873–9.
Kuwabara M, Kuwabara R, Hisatome I, Niwa K, Roncal-Jimenez CA, Bjornstad P, et al. “Metabolically healthy” obesity and hyperuricemia increase risk for hypertension and diabetes: 5-year Japanese cohort study. Obesity. 2017;25:1997–2008.
Wang J, Qin T, Chen J, Li Y, Wang L, Huang H, et al. Hyperuricemia and risk of incident hypertension: a systematic review and meta-analysis of observational studies. PLoS ONE. 2014;9:e114259.
Chen Q, Yin YJ, Chen WY, Wu JN, Huang X. Assessment of the association between serum uric acid levels and the incidence of hypertension in nonmetabolic syndrome subjects: a prospective observational study. Medicine. 2018;97:e9765.
Kuwabara M, Hisatome I, Niwa K, Hara S, Roncal-Jimenez CA, Bjornstad P, et al. Uric acid is a strong risk marker for developing hypertension from prehypertension: a 5-year Japanese cohort study. Hypertension. 2018;71:78–86.
Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, et al. Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014;37:253–390.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 Practice Guidelines for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC). Blood Press. 2018;27:314–40.
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248.
Choi HK1, Atkinson K, Karlson EW, Willett W, Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004;363:1277–81.
Li Z1, Guo X, Liu Y, Chang Y, Sun Y, Zhu G, et al. The relation of moderate alcohol consumption to hyperuricemia in a rural general population. Int J Environ Res Public Health. 2016;13:E732.
Makinouchi T, Sakata K, Oishi M, Tanaka K, Nogawa K, Watanabe M, et al. Benchmark dose of alcohol consumption for development of hyperuricemia in Japanese male workers: An 8-year cohort study. Alcohol. 2016;56:9–14.
Shiraishi H, Une H. The effect of the interaction between obesity and drinking on hyperuricemia in Japanese male office workers. J Epidemiol. 2009;19:12–16.
Roerecke M, Tobe SW, Kaczorowski J, Bacon SL, Vafaei A, Hasan OSM, et al. Sex-specific associations between alcohol consumption and incidence of hypertension: a systematic review and meta-analysis of cohort studies. J Am Heart Assoc. 2018;7:e008202.
Xin X, He J, Frontini MG, Ogden LG, Motsamai OI, Whelton PK. Effects of alcohol reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2001;38:1112–7.
Dickinson HO, Mason JM, Nicolson DJ, Campbell F, Beyer FR, Cook JV, et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens. 2006;24:215–33.
Shankar A, Klein R, Klein BE, Nieto FJ. The association between serum uric acid level and long-term incidence of hypertension: population-based cohort study. J Hum Hypertens. 2006;20:937–45.
Kansui Y, Matsumura K, Morinaga Y, Inoue M, Kiyohara K, Ohta Y, et al. Impact of serum uric acid on incident hypertension in a worksite population of Japanese men. J Hypertens. 2018;36:1499–505.
Soletsky B, Feig DI. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension. 2012;60:1148–56.
Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924–32.
Tatsumi Y, Morimoto A, Asayama K, Sonoda N, Miyamatsu N, Ohno Y, et al. Risk of developing type 2 diabetes according to blood pressure levels and presence or absence of hypertensive treatment: the Saku study. Hypertens Res. 2019;42:105–13.
Tatsumi Y, Morimoto A, Asayama K, Sonoda N, Miyamatsu N, Ohno Y, et al. Association between alcohol consumption and incidence of impaired insulin secretion and insulin resistance in Japanese: The Saku study. Diabetes Res Clin Pract. 2018;135:11–17.
Sonoda N, Morimoto A, Tatsumi Y, Asayama K, Ohkubo T, Izawa S, et al. A prospective study of the impact of diabetes mellitus on restrictive and obstructive lung function impairment: The Saku study. Metabolism. 2018;82:58–64.
Yamanaka H, Japanese society of gout and nucleic acid metabolism. Japanese guideline for the management of hyperuricemia and gout: second edition. Nucleosides Nucleotides Nucleic Acids. 2011;30:1018–29.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150.
Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, et al. International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. Diabetol Int. 2012;3:8–10.
Haneda M, Noda M, Origasa H, Noto H, Yabe D, Fujita Y. Japanese clinical practice guideline for diabetes 2016. Diabetol Int. 2018;9:1–45.
Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, et al. Committee for epidemiology and clinical management of atherosclerosis. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb. 2018;25:846–984.
Tochikubo O, Nishijima K, Ohshige K, Kimura K. Accuracy and applicability of the Terumo ES-H55 double-cuff sphygmomanometer for hospital use. Blood Press Monit. 2003;8:203–9.
Hirawa N, Umemura S, Ito S. Viewpoint on guidelines for treatment of hypertension in Japan. Circ Res. 2019;124:981–3.
Chen J, Qiu SH, Guo HJ, Li W, Sun ZL. Increased urinary glucose excretion is associated with a reduced risk of hyperuricaemia. Diabet Med. 2019;36:902–7.
Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N. Engl J Med. 2008;359:1811–21.
Bai G, Zhang J, Zhao C, Wang Y, Qi Y, Zhang B. Adherence to a healthy lifestyle and a DASH-style diet and risk of hypertension in Chinese individuals. Hypertens Res. 2017;40:196–202.
Okubo Y, Sairenchi T, Irie F, Yamagishi K, Iso H, Watanabe H, et al. Association of alcohol consumption with incident hypertension among middle-aged and older Japanese population: the Ibarakai Prefectural Health Study (IPHS). Hypertension. 2014;63:41–47.
Intersalt Cooperative Research Group. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 h urinary sodium and potassium excretion. BMJ. 1988;297:319–28.
Johnson RJ, Rodriguez-Iturbe B, Kang DH, Feig DI, Herrera-Acosta J. A unifying pathway for essential hypertension. Am J Hypertens. 2005;18:431–40.
Ministry of Health, Labour and Welfare. https://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h20-houkoku-03.pdf (in Japanese). Accessed 17 June 2019.
Acknowledgements
We thank all of the researchers and coworkers at Saku Central Hospital for their excellent medical examinations and follow-up surveys.
Funding
This work was supported by a grant-in-aid for Young Scientists from the Japan Society for the Promotion of Science (18K17396).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tatsumi, Y., Asayama, K., Morimoto, A. et al. Hyperuricemia predicts the risk for developing hypertension independent of alcohol drinking status in men and women: the Saku study. Hypertens Res 43, 442–449 (2020). https://doi.org/10.1038/s41440-019-0361-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-019-0361-0
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
Development of a risk prediction score for hypertension incidence using Japanese health checkup data
Hypertension Research (2022)
-
Impact of hyperuricemia on chronic kidney disease and atherosclerotic cardiovascular disease
Hypertension Research (2022)
-
Association of childhood anthropometric measurements and laboratory parameters with high blood pressure in young adults
Hypertension Research (2021)
-
J-shaped curve for the association between serum uric acid levels and the prevalence of blood pressure abnormalities
Hypertension Research (2021)
-
The interaction on hypertension between family history and diabetes and other risk factors
Scientific Reports (2021)