Abstract
MicroRNAs (miRNAs) are considered important in the pathogenesis of colon cancer. But the mechanism of their role in colon cancer is still largely unknown. Here, we aimed to explore the function of miR-503-5p in the pathogenesis of colon cancer. This study analyzed miRNA microarray of colon cancer. Then, we performed EdU, CCK-8, flow cytometry, Transwell invasion assays and in vivo assays to explore the exact role of miR-503-5p in colon cancer. We observed considerable downregulation of miR-503-5p expression in colon cancer cells and tissues and significant correlation with the TNM stage, differentiation grade and lymph node metastasis of colon cancer. Overexpression of miR-503-5p promoted the apoptosis and G1 arrest of colon cancer cells, and inhibited migration, proliferation, invasion and colony formation. Interestingly, ectopic miR-503-5p overexpression could significantly inhibit vascular endothelial growth factor (VEGF)-A expression and reduce the activity of a luciferase reporter containing the VEGF-A 3′-untranslated region. Furthermore, overexpressed miR-503-5p in human umbilical vein endothelial cells (HUVECs) and colon cancer cells resulted in lower expression levels of VEGFR-2, and subsequently inhibited AKT signaling pathway. Additionally, overexpression of miR-503-5p suppressed both lymphangiogenesis and angiogenesis in vivo and significantly inhibited the tumorigenicity of HT-29 cells in nude mice. In summary, our study shows downregulation of miR-503-5p at least partially contributes to the tumorigenesis of colon cancer through modulating the angiogenesis and lymphangiogenesis by targeting VEGF-A while stimulating AKT signaling pathways. Therapeutic strategies to restore miR-503-5p in colon cancer could be useful to inhibit tumor progression.
Similar content being viewed by others
Introduction
Colon cancer is responsible for the third and fourth cancer incidence and death worldwide, respectively [1, 2]. More than 1,000,000 colon cancer cases are newly diagnosed while ~700,000 patients die of this disease each year [3, 4]. The occurrence and progression of colon cancer involve multiple factors, including activated oncogenes and inactivated tumor suppressors [5, 6]. The mutation of tumor suppressors plays a vital role in the transition from non-invasiveness to invasiveness [7, 8]. Mutations are found in invasive colorectal cancer with an increasing frequency (75%) correlating to the extent of malignance [9]. A lot of research teams have investigated the bio-mechanisms of colon cancer to find various oncogenes and tumor suppressors. Better mastering of the mechanisms of occurrence, progression, migration and resurgence of colon cancer and the exploration of novel molecular biomarkers of colon cancer are greatly beneficial to earlier diagnosis and treatment of this disease.
MicroRNAs (miRNAs) are endogenous noncoding small RNAs with 19–24 nt for mRNA degradation regulation or transcriptional inhibition via targeting the 3-terminal noncoding region of target gene mRNA in plants and animals [10,11,12]. miRNAs are diagnostic markers and therapeutic targets of cancers as supported by rapidly increasing evidences [13,14,15]. The microRNA expression correlated with the prognosis and the therapeutic outcome of colon cancer [16]. On the one hand, some miRNAs were found to be oncogenic in colon cancer, including the miR-92 [17] and miR-135b [18]. On the other hand, some miRNAs were found to be tumor suppressors in colon cancer, including miR-506 [19], miR-219-5p [20], and so on. As vital modulators of angiogenesis and lymphangiogenesis [21, 22], the critical functions of miRNAs in the angiogenesis and lymphangiogenesis of colon cancer should be further studied. In the present study, MiR-503-5p was selected as the object of research. Several studies demonstrate that miR-503-5p exerts inhibiting effects in multiple tumors [23, 24]; however, its mechanism of action in colon cancer should be further explored.
Vascular endothelial growth factors (VEGFs, including VEGF-A, VEGF-B, PlGF, or placental growth factor) and their endothelial tyrosine kinase receptors show central regulating effects on the lymphangiogenesis, angiogenesis and vasculogenesis [25]. VEGF-A binds VEGFR-2, which is primarily expressed on blood ECs, and promotes the angiogenesis and vasopermeability [26]. Even though VEGF-A binding VEGFR-2 on blood ECs primarily leads to angiogenesis, VEGF-A can also mediate inflammation-induced lymphangiogenesis, and VEGF-A produced in the skin can have profound effects at other sites, such as the draining lymph node [27,28,29]. Moreover, in some studies, VEGF-A enhances the tumor progression by multiple mechanisms, and VEGF-A/VEGFR-2 autocrine stimulation mechanisms contribute to the proliferation, invasion and lymphatic metastasis [30, 31]. Thus, these results suggest VEGF-A as a strong therapeutic target to control the tumor growth and metastasis.
In our study, downregulated miR-503-5p was identified in human colon cancer cells and tissues, and miR-503-5p directly targeted VEGF-A as a tumor suppressive miRNA. Ectopic miR-503-5p expression posed induction actions on the apoptosis of colon cancer cells and inhibitory actions on the expression of VEGF-A and VEGFR-2, in vitro invasion, proliferation and migration. Additionally, in a colon cancer xenograft model, the tumor growth, lymphangiogenesis and angiogenesis in vivo were reduced by overexpressed miR-503-5p. In summary, our results uncover a new function of miR-503-5p in colon cancer and may provide a new perspective on the treatment of this disease.
Materials and methods
Human colon cancer tissues
Forty pairs of colon cancer tissues and corresponding para-carcinoma tissues were harvested from the patients of our hospital diagnosed from August 2014 to September 2017. All the patients with their tissues sampled were diagnosed by pathological examinations and underwent no preoperative chemotherapy or radiotherapy. They provided their informed consent voluntarily. The Ethics Committee of Cancer Hospital of China Medical University, Liaoning Cancer Hospital & Institute approved all experiments. All tissues collected were preserved in liquid nitrogen.
Cell culture
The Chinese Academy of Sciences Cell Bank (Shanghai, China) supplied five authenticated colon cancer cell lines (HT-29, LoVo, HCT116, RKO, and SW620), a authenticated normal human colon epithelial cell line (NCM460) and human umbilical vein endothelial cells (HUVECs). The short tandem repeat analysis confirmed that each cell line was the authentic. In a 5% CO2 humidified incubator, Roswell Park Memorial Institute 1640 medium (RPMI-1640; Gibco, BRL, Carlsbad, CA, USA) was used for cell culture at 37 °C, together with 10% fetal bovine serum (FBS; Gibco), 100 mg/mL streptomycin and 100 U/mL penicillin (Sigma-Aldrich, St. Louis, MO, USA).
Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR)
MirVANA RNA isolation Kit (Ambion) was used for the extraction of total RNAs from colon cancer cells and tissues as per kit instructions at the extraction concentration confirmed using a NanoDrop spectrophotometer. The standby solution was maintained at −80 °C. For qRT-PCR, RiboLock nucleic acid enzyme inhibitor and M-MLV reverse transcriptase (Applied Biosystems) were utilized for reverse transcription of RNAs into cDNA. For real-time PCR, the GenePharma SYBR Green method was used with Bio-Rad IQ-5 to react for a 10-min hot cycle at 95 °C, followed by 50 cycles of 15 s at 94 °C, 30 s at 55 °C and 30 s at 70 °C. Quantitative analysis of miRNAs was performed using 2-ΔΔCt method. The following design of PCR primers for mature miR-503-5p or U6 was used: miR-503-5p sense, 5′-ACACTCCAGCTGGGGACGTCTTGACAAGGGC-3′ and reverse, 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCGCTGCTA-3′; U6 sense, 5′-GTGCTCGCTTCGGCAGCACAT-3′ and reverse, 5′-TACCTTGCGAAGTGCTTAAAC-3′.
Western blot analysis
After the preparation of protein lysates using RIPA buffer (Thermo Scientific Inc., Waltham, MA, USA) with 1% protease inhibitor, Western blotting was conducted for separate three times. Subsequently, 4% sodium dodecyl sulfate polyacrylamide gel electrophoresis was used for the electrophoresis of protein lysates, with proteins electrotransferred onto polyvinylidene fluoride membrane (Millipore, USA). The membrane was blocked using Bovine Serum Albumin (BSA) in TBST buffer and probed using anti-VEGFR-2 and anti-VEGF-A (sc-6251 and sc-507, respectively; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-pAKT, anti-AKT and anti-b-actin (#4056, #9272, #4970, respectively; Cell Signaling Technology, USA) in TBST (containing 0.1% Tween 20). The detection of immunoreactive proteins was conducted using horseradish peroxidase-conjugated anti-rabbit or mouse IgG (Cell Signaling Technology, USA) by chemiluminescence (Pierce ECL kit).
Cell proliferation assay
Cell Counting Kit-8 assay (CCK-8, Dojindo, Japan) was utilized for cell proliferation assay as per the instructions. During 1, 2, 3, 4, and 5 days, 1 × 103 cells/well cells were seeded into 96-well plates for cell growth. After adding 10 μl of CCK-8 solution into each well, 1 h of sample incubation was performed at 37 °C. A microplate reader (MultiSkan Spectrum) was used for reading the absorbance at a wavelength of 450 nm. Each experiment was performed for three separate times, with their results averaged.
Colony formation assay
For the digestion of transfected colon cancer cells into single cell suspension, these cells were processed by trypsin and collagenase, followed by seeding into 12-well plate at 400 cells/well and 14 d of culture in an incubator with 5% CO2 at 37 °C. Then, cells were subject to two runs of PBS washing and 15 min of 2% crystal violet staining. After drying the plate at room temperature, the clones formed were counted. At least three independent experiments were performed.
Flow cytometry analysis of cell cycle
Cells were seeded into six-well plate for incubation in complete media until 80%-90% confluence was observed. After washing the collected cells twice using cold PBS, overnight fixation of cells began in 70% cold ethanol at 4 °C. After incubation in 1 μg/ml RNase A at 37°C for 30 min, the cells were stained with 50 μg/ml propidium iodide. FACSCalibur flow cytometer (BD Biosciences, USA) was applied for flow cytometry analysis. Three experiments were conducted independently.
Apoptosis assays
During cell apoptosis assays, the staining of cells collected was performed using an Annexin V-Fluorescein Isothiocyanate (FITC) Apoptosis Detection Kit II (BD Biosciences, Pharmingen, CA, USA) following its instructions. In brief, the collected cells were washed twice using 5 ml cold PBS. After the resuspension of 1.0 × 105 cells in 100 µl of binding buffer, these cells were mixed with 5 µl of propidium iodide and 5 µl of FITC-labeled Annexin V for 20 min in dark place at room temperature, and 400 µl of binding buffer was added post incubation. Cell apoptosis analysis was performed using CellQuestTM Pro software (ver. 4.0.2, Becton Dickinson) through flow cytometry (FACSCalibur, BD, USA).
Wound-healing assay
Cells were seeded into six-well plate for culture to confluence. A p-200 µl pipette tip (Qiagen, Valencia, CA, USA) was used to scratch confluent monolayer of cells. Subsequently, PBS was used for three runs of cell washing to remove suspension cells and cell debris. After adding fresh serum-free medium, 24 h was reserved for wound closing by the cells under normal conditions. The wound photographs were obtained using a computer-assisted microscope (Nikon).
Cell invasion assays
The QCMTM 24-well Fluorimetric Cell Invasion Assay kit (ECM554, Chemicon International, Temecula, CA, USA) was utilized for cell invasion assays according to its instructions. The kit uses an insert polycarbonate membrane with an 8-lm pore size. The insert was coated with a thin layer of ECMatrixTM that occluded the membrane pores and blocked migration of noninvasive cells. With 500 µl culture medium with 10% FBS as the chemoattractant, 4% paraformaldehyde was utilized for fixing the cells migrating and invading the underside membrane. The fluorescence method was used for counting the invaded cells.
Tumor xenograft treatment model
Animal experimental procedures were approved by the Institutional Animal Care and Use Committee of China Medical University with the animal ethics number KT2019124. The left flank of BALB/c nude mice (5–6 weeks, 20–22 g, n = 3 each group) was administered with 1 × 107 HT-29 cell suspension by subcutaneous injection. All the mice were acquired from the Laboratory Animal Center (Shanghai, China) and housed in a sterile room under a 12-h light/dark cycle at ~23 °C and 50% humidity, with ad libitum access to food and water. Seven days post implantation when tumors formed, mice were randomly stratified into two treatment groups: negative control lentivirus treated Lv-NC group; lentivirus encoding miR-503-5p treated Lv-miR-503-5p group. In treatment groups, the tail vein of a mouse was intravenously with 250 µl lentivirus every 24 h for 3 weeks. After treatment, the tumor volume was measured with calipers two major axes every 7 days and calculated as: V = 0.5 × L (length) × W2 (width). All surgeries were performed under sodium pentobarbital anesthesia via intraperitoneal injection (40 mg/kg) and all efforts were made to minimize suffering. At 4 weeks post-injection, the mice were anaesthetized with 40 mg/kg sodium pentobarbital and then sacrificed by 10% formalin perfusion fixation of central nervous system; death was confirmed by completely stopping of the heartbeat and breathing, as well as disappearance of the foot withdrawal reflex. The tumor tissues were isolated and weighed.
Generation of stable cell lines expressing miR-503-5p
A hsa-miR-503-5p contained DNA fragment was amplified from normal human colon epithelial cell line (NCM460) genomic DNA and then cloned into the pcDNA-copGFP vector (System Biosciences, USA). The lentivirus vector expressing miR-503-5p was referred to as LV-miR-503-5p. Lentiviral vectors LV-miR-503-5p or LV-miR-NC (which was used as a negative control) and Lentiviral packaging plasmids were co-transfected in 293FT packaging cells using Lipofectamine2000 (Invitrogen, CA, USA) according to the manufacturer’s instruction. After 48 h, the lentivirus collected from the supernatant was filtered and utilized for infecting the colon cancer cells SW620, HT-29 and HUVECs. After 2 weeks of antibiotic selection, stable clones were collected and qRT-PCR was utilized for confirming the expression level of mature miR-503-5p.
Dual-luciferase reporter gene assay
During the construction of VEGF-A-3′-UTR plasmid, PCR was used for amplifying the 3′-UTR region of human VEGF-A mRNA containing a seed sequence of mature miR-503-5p-binding sites which was then cloned into the pGL3-basic vector (Promega, Madison, WI, USA) downstream of luciferase reporter gene. This construct was engineered as wild-type (WT) and referred to as VEGF-A 3′-UTR-WT. A site-directed mutagenesis kit (Takara) was used for generating the mutated 3′-UTR and PCR amplified WT 3′-UTR was used as the template. Additionally, mutated sequence inserted into the luciferase reporter was called VEGF-A-3′-UTR-MUT. The co-transfection of Human HEK293T cells was conducted using mock miR-NC or LV-miR-503-5p vector, firefly luciferase reporter with mutant or WT 3′-UTR of VEGF-A. After 48 h, cells were collected for the assay with the Dual Luciferase Assay kit (Promega, Madison, WI, USA) as per its instructions.
In vitro angiogenesis assays
One of essential angiogenic properties of HUVECs was manifested as capillary tube formation on Matrigel, thus tube formation assay was performed to determine in vitro angiogenic activity of HUVECs. Transfected HUVECs were exposed to 24 h of serum starvation by using endothelial basal medium (Clonetics, CA, USA) containing 0.2% BSA for incubation. Subsequently, serum-starved HUVECs were collected, with 8 × 104 cells seeded into Matrigel-coated 12-well plates (basement membrane matrix, BD Biosciences, USA). The tube formation which defined as a tubular structure whose length was four times longer than the width was observed under a computer-assisted microscope (Nikon) after 8 h of incubation. The tubes were counted in three fields with LAS software (Leica), while images of tube morphology were obtained in ten randomly selected microscopic fields for each sample at 100× magnification.
In vivo Matrigel plug assay
Matrigel plug assay was conducted to measure the angiogenesis as previously described [32]. Nude mice were purchased from the National Laboratory Animal Center (Beijing, China). 5-week-old BALB/c athymic nude mice were fed with autoclaved food and water during the experiment. All the experiments with nude mice were performed strictly in accordance with the protocol approved by the Institutional Animal Care and Use Committee of China Medical University. In brief, HT-29-Lv-NC and HT-29-Lv-miR-503-5p cells re-suspended in 400 mL of solution containing 80% Matrigel at a density of 1 × 106 were subcutaneously injected. After 7 days, plugs were removed, photographed, and collected.
Statistical analysis
SPSS 16.0 statistical software was utilized for all statistical analyses. For normally distributed data with equal variance, the difference was evaluated by two-tailed Student t test (two‐group comparisons) or ANOVA followed by the post hoc Bonferroni test (multigroup comparisons) as appropriate. For nonnormally distributed data or data with unequal variances, the difference was evaluated by a nonparametric Mann–Whitney U test (two‐group comparisons) or the Kruskal–Wallis test followed by the post hoc Bonferroni test (multigroup comparisons). Data are presented as the mean ± standard deviation. P < 0.05 was taken to represent a statistically significant difference.
Results
miR-503-5p expression was downregulated in human colon cancer and cell lines
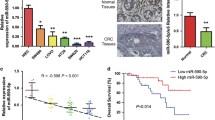
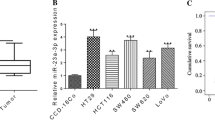
The analysis of GSE48267 microarray revealed lowly expressed miR-503-5p in colon cancer tissues relative to para-carcinoma tissues. The criteria of log2FC > 2 or < −2 and P value < 0.05 were utilized for identifying differential expression of miRNAs (including miR-503-5p) in colon cancer tissues (Fig. 1a). To explore whether miR-503-5p regulated the colon cancer tumorigenesis in human, miR-503-5p expression was assessed in 40 paired colon cancer samples and non-tumorous tissues through qRT-PCR. Table 1 provided the clinical and pathological characteristics of 40 colon cancer cases. Notably downregulated miR-503-5p was observed in tumor tissues versus non-tumorous tissues (Fig. 1b). The potential association of miR-503-5p expression with clinical and pathological characteristics was also explored, indicating that downregulated miR-503-5p correlated with lymphatic metastasis in colon cancer (Fig. 1c). Moreover, miR-503-5p expression was also investigated in human colon cancer cells, including HT-29, LoVo, HCT116, RKO, and SW620. A considerable decrease in the expression of miR-503-5p was identified in all human colon cancer cell lines versus the normal human colon epithelial cell line NCM460 (Fig. 1d). Therefore, these findings suggested that downregulated miR-503-5p might be crucial in colon carcinogenesis and its progression, and that miR-503-5p might contribute to the carcinogenesis of this disease.
a Microarray analysis of GSE48267 from platform GPL10850. Heatmap for the dysregulation of 27 miRNAs (including 11 downregulated miRNAs and 16 upregulated miRNAs) in colon cancer tissues versus non-tumorous tissues (log2FC > 2 or log2FC < −2, P < 0.05). b Relative miR-503-5p expression in colon cancer tissues versus non-tumorous tissues. c Significant decrease in miR-503-5p expression in patients with stronger lymphatic metastasis than in those without lymphatic metastasis. d Relative miR-503-5p expression in colon cancer cell lines relative to normal human colon epithelial cell line NCM460. Data represented three separate experiments. Data were presented as the means ± SEM of three experiments. **P < 0.01, ***P < 0.001.
miR-503-5p triggered apoptosis and suppressed proliferation of colon cancer cells
To investigate the effects of miR-503-5p overexpression and knockdown on colon cancer cells, SW620 and HT-29 cells were used. Transfection efficiency of miR-503-5p overexpression, inhibitor, and negative control was shown in Fig. 2a and Supplementary Fig. 1A.
a QRT-PCR for examining stable cell lines expressing miR-503-5p. b CCK8 assay for cell proliferation determination on day 1, 2, 3, 4, and 5 after transfection. c Typical results of EdU for colon cancer cells post stable transfection with miR-NC or miR-503-5p (bar = 50 μm). d Typical results of colony formation for colon cancer cells post stable transfection with miR-NC or miR-503-5p (bar = 500 mm). e Flow cytometry analysis of cell cycle. f Annexin V/PI assay for cell apoptosis detection. Data represented three separate experiments. Data were presented as the means ± SEM of three experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
To identify the potential of miR-503-5p as a tumor suppressor gene, the roles of overexpressed miR-503-5p in colon cancer cell proliferation were examined in vitro. The growth rate of SW620 and HT-29 cells was notably reduced by overexpressed miR-503-5p (Fig. 2b–d) and it was stimulated by miR-503-5p inhibitor (Supplementary Fig. 1B, 1C and 1D). Additionally, to analyze the mechanisms underlying the influence of miR-503-5p on cell proliferation, flow cytometry was applied to assess the cycle distribution of miR-503-5p transfected colon cancer cells. The cell proportion in S phase was notably abated by ectopic miR-503-5p expression (Fig. 2e). Collectively, overexpressed miR-503-5p implicated in the G1/G0 arrest of colon cancer cells and retarded in vitro colon cancer cell proliferation from these results.
To identify whether the apoptosis enhanced the growth inhibition, flow cytometry analysis was made for colon cancer cells after transfection with miR-503-5p or miR-NC vector. The findings suggested that ectopic miR-503-5p expression induced more colon cancer cell apoptosis (Fig. 2f).
miR-503-5p inhibited migration and invasion of colon cancer cells
As shown by our findings, considerable downregulation of miR-503-5p was identified in specimens from colon cancer patients and colon cancer cell lines. A hypothesis was proposed that overexpressed miR-503-5p implicated in the suppression of colon cancer cell migration and invasion, accordingly. For hypothesis test, wound-healing assay was conducted to assess the roles of miR-503-5p in cell migration. The migration of cells with overexpressed miR-503-5p was strikingly slower than miR-NC cells (Fig. 3a). In addition, as shown by invasion assays, the invasion of cells with overexpressed miR-503-5p was remarkably decreased and the invasion of cells transfected with miR-503-5p inhibitor was remarkably increased relative to control cells (Fig. 3b, Supplementary Fig. 1E). In summary, miR-503-5p strikingly repressed the colon cancer cell migration and invasion from these results.
a Wound-healing assay for determining the effects of miR-503-5p on cell migration (bar = 100 μm). b Invasion of colon cancer cells post stable transfection with miR-NC or miR-503-5p (bar = 100 μm). Data represented three separate experiments. Data were presented as the means ± SEM of three experiments. *P < 0.05, **P < 0.01.
miR-503-5p directly targeted VEGF-A gene via interaction with the 3′-UTRs
The target gene candidates of miR-503-5p were searched using TargetScan 3.1, and then VEGF-A was considered as a potential target site of miR-503-5p. To verify this site, miR-503-5p binding sequences in the 3′-UTR of VEGF-A mRNA (VEGF-A WT) or mutated sequence (VEGF-A MUT) were sub-cloned downstream of firefly luciferase reporter gene in pGL3 vector (Fig. 4a). The conservation of miR-503-5p (seed sequence) and VEGF-A 3′UTR (binding site) across species are shown in the Supplementary Fig. 2. The construct was subject to co-transfection with pcDNA/miR-503-5p or pcDNA/miR-NC into HEK293T cells. The reporter with WT 3′-UTR showed significant decrease in elative luciferase activity post co-transfection of pcDNA/miR-503-5p. However, concurrent transfection of pcDNA/miR-503-5p had no influence on the luciferase activity of mutant reporters (Fig. 4b), indicating that miR-503-5p might repress VEGF-A expression via miR-503-5p-binding sequence in 3′-UTR of VEGF-A.
a Sequence of miR-503-5p matching with VEGF-A 3′-UTR. b Dual-luciferase reporter gene assay of HEK293T cells post co-transfection with miR-503-5p/miR-NC and wild-type/mutant-type VEGF-A. c Western blot for the expression levels of VEGF-A and VEGFR-2 in cells. d Western blotting for total and phosphorylated AKT. Data represented three separate experiments. Data were presented as the means ± SEM of three experiments. **P < 0.01.
The levels of VEGF-A protein expression in colon cancer cells with overexpressed miR-503-5p were notably repressed as shown by Western blot analysis (Fig. 4c). These findings uncovered that miR-503-5p directly targeted VEGF-A in colon cancer cells through interacting with the 3′-UTR in VEGF-A gene.
VEGF-A binds VEGFR-2 to promote angiogenesis and vascular permeability, and mediate inflammation-induced lymphangiogenesis [30, 31, 33]. Therefore, an analysis was made to analyze whether the protein level of VEGFR-2 was affected by miR-503-5p-mediated VEGF-A suppression. Colon cancer cells with overexpressed miR-503-5p also presented with significant decrease in the protein level of VEGFR-2 relative to miR-NC cells (Fig. 4c). An active phosphatidylinositol 3-kinase-AKT pathway by VEGFRs was vital in the growth and survival of endothelial and cancer cells. Thus, another analysis was also conducted to determine whether this pathway was implicated in miR-503-5p-induced apoptosis enhancement and growth suppression. The phosphorylation of signaling molecules AKT in colon cancer cells was considerably retarded by ectopic miR-503-5p expression (Fig. 4d). Taken together, this indicated that miR-503-5p interfered with the activation of AKT stimulated by VEGFR-2-dependent signaling pathway through targeting the VEGF-A.
miR-503-5p-VEGF-A regulatory loop plays a vital role in cellular functions
We next examined whether VEGF-A affected cell proliferation and invasion in colon cancer cells. The expression levels of VEGF-A were elevated by using VEGF-A overexpression vector (Supplementary Fig. 3A). Compared with negative controls, over-expression of miR-503-5p obviously inhibited capability of cell proliferation and invasion, and VEGF-A upregulation could reverse miR-503-5p suppression effect (Supplementary Fig. 3B,C).
miR-503-5p inhibited tumor growth, lymphangiogenesis and angiogenesis in vivo
To determine whether miR-503-5p expression affected in vivo tumor growth, xenograft experiment was conducted using HT-29 cells to explain the therapeutic roles of miR-503-5p in tumor cell growth. Figure 5a showed the xenograft tumors. After the experiment, the tumor volume and weight of Lv-miR-503-5p treated mice were repressed relative to Lv-miR-NC treated mice (Fig. 5b). Pulmonary metastasis images and slices were showed in Fig. 5c. Subsequently, resected tumor tissues were exposed to qRT-PCR of miR-503-5p expression, Western blot and immunohistochemical staining of VEGF-A. Lv-miR-503-5p treated groups presented with significant enhancement of miR-503-5p expression (Fig. 5d), markedly lower VEGF-A, VEGFR-2, p-AKT, and AKT protein levels (Fig. 5e, f) versus control groups. Tumor tissues were analyzed through immunohistochemical staining using anti-LYVE-1 and anti-CD34 antibodies with respect to the angiogenesis and lymphangiogenesis of tumors. Quantitative analysis revealed pronounced decreases in lymph and blood vessels in Lv-miR-503-5p treated groups relative to control groups (Fig. 5g). To further confirm the inhibitory role of miR-503-5p on tumor angiogenesis in colon cancer, the HT-29 cell-based Matrigel plug assay was performed. Our results showed that overexpression of miR-503-5p inhibited tumor angiogenesis in HT-29 Matrigel plug tissues (Fig. 5h). In summary, these findings unveiled that the miR-503-5p expression greatly inhibited the in vivo tumor progression, and miR-503-5p had regulating actions on tumorigenesis by the inhibition of VEGF-A-mediated lymphangiogenesis and angiogenesis.
a Xenograft tumors. b Measurement of tumor volume and weight. c Lung metastasis in nude mice (bar = 100 μm). d Higher miR-503-5p expression in miR-503-5p-treated tumors relative to control groups. e Western blotting for the detection of VEGF-A, VEGFR-2, p-AKT and AKT protein levels. f Notable decrease in miR-503-5p treated groups versus control groups based on immunohistochemical staining for VEGF-A (bar = 50 μm). g Considerable reduction of lymphatic microvessel densities (LMVD) and microvessel densities (MVD) in tumor tissues from miR-503-5p-treated mice based on immunohistochemical analysis (bar = 20 μm). h The effect of miR-503-5p on the angiogenesis in vivo was evaluated by Matrigel plug assay (bar = 1 cm). Data were presented as the means ± SEM of three experiments. *P < 0.05, **P < 0.01.
Overexpression of miR-503-5p inhibited tube formation in vitro
One of essential angiogenic properties of HUVECs was the capillary tube formation on Matrigel, thus we explored whether tube formation was affected by the miR-503-5p induced downregulation of VEGF-A and his receptors. 48 h post transfection with LV-miR-NC or LV-miR-503-5p, HUVECs were subject to 24 h of serum starvation. In the next day, 8 h of cell culture was performed on a Matrigel-coated 12-well plate. Well-organized capillary-like structures were formed in LV-miR-NC-transfected HUVECs as shown in Fig. 6a. However, the tube-forming activity was obviously impaired by transfection with miR-503-5p. Therefore, our data suggested that miR-503-5p posed negative effects on the angiogenesis of HUVECs. Since AKT is an important downstream pathway of VEGF/VEGFR signals, and negative regulation of VEGF-A and his receptors (VEGFR-2) was identified in miR-503-5p-overexpressing HUVECs (Fig. 6b), this study also explored whether miR-503-5p affected the AKT phosphorylation. Notable decrease in AKT phosphorylation was observed in miR-503-5p-transfected HUVECs relative to miR-503-5p-NC-transfected HUVECs in Fig. 6c. These results indicated that lower VEGF-A protein by miR-503-5p retarded the activation of AKT signaling pathways induced by VEGFR.
a Micrographs of HUVECs formed capillary-like structure post transfection with miR-NC or miR-503-5p. Measurement of tube numbers in three fields with LAS software (Leica) (bar = 100 μm). b Western blot for VEGF-A and VEGFR-2 expression in HUVEC cells with overexpressed miR-503-5p. c Western blotting for total and phospho-AKT in HUVEC cells with overexpressed miR-503-5p. Data represented three separate experiments. Data were presented as the means ± SEM of three experiments. ***P < 0.001.
Discussion
Based on increasing evidences recently, miRNAs are considered as the regulators of tumor phenotype through regulation of the expression levels of key genes and signaling pathways implicated in the tumorigenesis and downstream malignant progression [34, 35]. Park and Kim proved that miR-503-5p blocked colony-forming activity and metastatic activity of ovarian cancer cells [36]. The research of Sun et al. showed that miR-503-3p induces apoptosis of lung cancer cells by regulating p21 and CDK4 expression [24]. In our study, pronounced downregulation of mature miR-503-5p expression was identified in colon cancer tissues relative to non-tumorous tissues. Furthermore, a correlation of relative miR-503-5p expression in colon cancer was observed with tumor differentiation, pathological stage and lymphatic metastasis. Additionally, the miR-503-5p expression in colon cancer cell lines was strikingly decreased relative to the normal human colon epithelial cell line.
In vitro experiments were carried out using constructed lentivirus-mediated miR-503-5p-overexpression SW620 and HT-29 colon cancer cells to determine the effects of miR-503-5p in colon cancer. Our results clearly indicated that miR-503-5p showed significant suppression effects on the proliferation, migration, invasion, and colony formation of colon cancer cells, and induction effects on apoptosis, and G1 arrest in vitro. Furthermore, our study indicated that overexpression of miR-503-5p suppressed both lymphangiogenesis and angiogenesis in vivo and significantly inhibited the tumorigenicity of HT-29 cells in nude mice. These findings signify that miR-503-5p is an inhibitor of colon cancer tumorigenesis. MiR-503-5p may serve as a vital marker for the diagnosis of colon cancer, and also an effective target for the treatment of this disease. In addition to the roles in cancer cells, such roles of miR-503-5p may also participate in other tumor cells like endothelial cells, and further study is warranted.
As seen in Fig. 7, the findings revealed the important regulation roles of miR-503-5p in VEGF-A expression, and laid the foundation for developing new anti-colon cancer treatments, especially for angiogenesis and lymphangiogenesis resistance. Many current studies have clearly indicated that one miRNA could control multiple oncogenes [37,38,39], so re-expression of miRNAs had been suggested to hold considerable potential in tumor gene therapy [40]. Here, our in vivo colon cancer xenograft treatment model data provide strong evidence that restoration of miR-503-5p expression may have considerable therapeutic significance for colon cancer.
miR-503-5p was downregulated in colon cancer. VEGF-A and VEGFR-2 upregulation were observed when miR-503-5p directly targeted them in endothelial and cancer cells. Thereby the downstream VEGFR signaling pathways AKT was stimulated to ultimately promote the progression, angiogenesis, and lymphangiogenesis of colon cancer.
This research has a limitation. Our study only included a small size of patient tissues, and further investigation of a larger patient population is necessary to confirm the clinical significance of miR-503-5p in colon cancer.
Taken together, the present study reveals downregulation of miR-503-5p in colon cancer and considers miR-503-5p as a tumor suppressor through directly targeting VEGF-A. Ectopic miR-503-5p expression shows inhibiting effects on the tumor progression, angiogenesis and lymphangiogenesis while blocking AKT signal pathways. Together these data may provide a strategy for targeting the miR-503-5p/VEGF-A/VEGFR2 axis as a new therapy to treat colon cancer.
Data availability
Upon reasonable request, the datasets used and analyzed during the present study are available from the corresponding author.
Change history
06 September 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41434-024-00486-6
References
Zeng M, Zhu L, Li L, Kang C. miR-378 suppresses the proliferation, migration and invasion of colon cancer cells by inhibiting SDAD1. Cell Mol Biol Lett. 2017;22:12.
Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476–87.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Zhao J, Chen Y, Liu F, Yin M. Overexpression of miRNA-143 inhibits colon cancer cell proliferation by inhibiting glucose uptake. Arch Med Res. 2018;49:497–503.
Uppada SB, Gowrikumar S, Ahmad R, Kumar B, Szeglin B, Chen X, et al. MASTL induces colon cancer progression and chemoresistance by promoting Wnt/beta-catenin signaling. Mol Cancer. 2018;17:111.
Steck SE, Butler LM, Keku T, Antwi S, Galanko J, Sandler RS, et al. Nucleotide excision repair gene polymorphisms, meat intake and colon cancer risk. Mutat Res. 2014;762:24–31.
Canote R, Du Y, Carling T, Tian F, Peng Z, Huang S. The tumor suppressor gene RIZ in cancer gene therapy (review). Oncol Rep. 2002;9:57–60.
Wang LH, Wu CF, Rajasekaran N, Shin YK. Loss of tumor suppressor gene function in human cancer: an overview. Cell Physiol Biochem. 2018;51:2647–93.
Bultman SJ. Interplay between diet, gut microbiota, epigenetic events, and colorectal cancer. Mol Nutr Food Res. 2017;61:1500902.
Menzel P, McCorkindale AL, Stefanov SR, Zinzen RP, Meyer IM. Transcriptional dynamics of microRNAs and their targets during Drosophila neurogenesis. RNA Biol. 2019;16:69–81.
Tarallo A, Carissimo A, Gatto F, Nusco E, Toscano A, Musumeci O, et al. microRNAs as biomarkers in Pompe disease. Genet Med. 2019;21:591–600.
Chen X, Xie D, Zhao Q, You ZH. MicroRNAs and complex diseases: from experimental results to computational models. Brief Bioinform. 2019;20:515–39.
Hibner G, Kimsa-Furdzik M, Francuz T. Relevance of microRNAs as potential diagnostic and prognostic markers in colorectal cancer. Int J Mol Sci. 2018;19:2944.
Qadir MI, Faheem A. miRNA: a diagnostic and therapeutic tool for pancreatic cancer. Crit Rev Eukaryot Gene Expr. 2017;27:197–204.
Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12.
Xu Q, Tong JL, Zhang CP, Xiao Q, Lin XL, Xiao XY. miR-27a induced by colon cancer cells in HLECs promotes lymphangiogenesis by targeting SMAD4. PLoS ONE. 2017;12:e0186718.
Tsuchida A, Ohno S, Wu W, Borjigin N, Fujita K, Aoki T, et al. miR-92 is a key oncogenic component of the miR-17-92 cluster in colon cancer. Cancer Sci. 2011;102:2264–71.
Valeri N, Braconi C, Gasparini P, Murgia C, Lampis A, Paulus-Hock V, et al. MicroRNA-135b promotes cancer progression by acting as a downstream effector of oncogenic pathways in colon cancer. Cancer Cell. 2014;25:469–83.
Zhang Y, Lin C, Liao G, Liu S, Ding J, Tang F, et al. MicroRNA-506 suppresses tumor proliferation and metastasis in colon cancer by directly targeting the oncogene EZH2. Oncotarget. 2015;6:32586–601.
Cheng J, Deng R, Zhang P, Wu C, Wu K, Shi L, et al. miR-219-5p plays a tumor suppressive role in colon cancer by targeting oncogene Sall4. Oncol Rep. 2015;34:1923–32.
Bao L, Chau C, Bao J, Tsoukas MM, Chan LS. IL-4 dysregulates microRNAs involved in inflammation, angiogenesis and apoptosis in epidermal keratinocytes. Microbiol Immunol. 2018;62:732–6.
Hunter S, Nault B, Ugwuagbo KC, Maiti S, Majumder M. Mir526b and Mir655 promote tumour associated angiogenesis and lymphangiogenesis in breast cancer. Cancers. 2019;11:938.
Li X, Han X, Yang J, Sun J, Wei P. miR-503-5p inhibits the proliferation of T24 and EJ bladder cancer cells by interfering with the Rb/E2F signaling pathway. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017;33:1360–4.
Sun Y, Li L, Xing S, Pan Y, Shi Y, Zhang L, et al. miR-503-3p induces apoptosis of lung cancer cells by regulating p21 and CDK4 expression. Cancer Biomark. 2017;20:597–608.
Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154–65.
Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76.
Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Investig. 2004;113:1040–50.
Halin C, Tobler NE, Vigl B, Brown LF, Detmar M. VEGF-A produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood. 2007;110:3158–67.
Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Detmar MJ, et al. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med. 2002;196:1497–506.
Matsumoto K, Ema M. Roles of VEGF-A signalling in development, regeneration, and tumours. J Biochem. 2014;156:1–10.
Roskoski R Jr. Vascular endothelial growth factor (VEGF) and VEGF receptor inhibitors in the treatment of renal cell carcinomas. Pharmacol Res. 2017;120:116–32.
Malinda KM. In vivo matrigel migration and angiogenesis assay. Methods Mol Biol. 2009;467:287–94.
Feng Y, Hu J, Ma J, Feng K, Zhang X, Yang S, et al. RNAi-mediated silencing of VEGF-C inhibits non-small cell lung cancer progression by simultaneously down-regulating the CXCR4, CCR7, VEGFR-2 and VEGFR-3-dependent axes-induced ERK, p38 and AKT signalling pathways. Eur J Cancer. 2011;47:2353–63.
Minna E, Romeo P, Dugo M, De Cecco L, Todoerti K, Pilotti S, et al. miR-451a is underexpressed and targets AKT/mTOR pathway in papillary thyroid carcinoma. Oncotarget. 2016;7:12731–47.
Aghaee-Bakhtiari SH, Arefian E, Naderi M, Noorbakhsh F, Nodouzi V, Asgari M, et al. MAPK and JAK/STAT pathways targeted by miR-23a and miR-23b in prostate cancer: computational and in vitro approaches. Tumour Biol. 2015;36:4203–12.
Park GB, Kim D. MicroRNA-503-5p inhibits the CD97-mediated JAK2/STAT3 pathway in metastatic or paclitaxel-resistant ovarian cancer cells. Neoplasia. 2019;21:206–15.
Jiao LR, Frampton AE, Jacob J, Pellegrino L, Krell J, Giamas G, et al. MicroRNAs targeting oncogenes are down-regulated in pancreatic malignant transformation from benign tumors. PLoS ONE. 2012;7:e32068.
Murugan AK, Munirajan AK, Alzahrani AS. MicroRNAs: modulators of the Ras oncogenes in oral cancer. J Cell Physiol. 2016;231:1424–31.
Barisciano G, Colangelo T, Rosato V, Muccillo L, Letizia Taddei M, Ippolito L, et al. miR-27a is a master regulator of metabolic reprogramming and chemoresistance in colorectal cancer. Br J Cancer. 2020;122:1354–66.
Yamamoto H, Mori M. MicroRNAs as therapeutic targets and colorectal cancer therapeutics. Adv Exp Med Biol. 2016;937:239–47.
Acknowledgements
I extend my sincere gratitude to my administrator Na Zhang, for her instructive advice and useful suggestion on my thesis. Specially thanks to Shenglong Li, for his valuable suggestions, guidance, and help in the completion of this article. Without his consistent and illuminating instructions, this thesis could not have reached its present form.
Author information
Authors and Affiliations
Contributions
LW designed the study. CS and SS performed the experiments. YZ analyzed the data. NH drafted this manuscript. LW reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41434-024-00486-6"
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, L., Sun, C., Zhang, Y. et al. RETRACTED ARTICLE: miR-503-5p inhibits colon cancer tumorigenesis, angiogenesis, and lymphangiogenesis by directly downregulating VEGF-A. Gene Ther 29, 28–40 (2022). https://doi.org/10.1038/s41434-020-0167-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-020-0167-3
- Springer Nature Limited
This article is cited by
-
MiR-503-5p alleviates peripheral neuropathy-induced neuropathic pain in T2DM mice by regulating SEPT9 to inhibit astrocyte activation
Scientific Reports (2024)
-
miR-575/RIPK4 axis modulates cell cycle progression and proliferation by inactivating the Wnt/β-catenin signaling pathway through inhibiting RUNX1 in colon cancer
Molecular and Cellular Biochemistry (2024)
-
Regulation of VEGF-A expression and VEGF-A-targeted therapy in malignant tumors
Journal of Cancer Research and Clinical Oncology (2024)
-
Redefining the significance of quinoline containing compounds as potent VEGFR-2 inhibitors for cancer therapy
Medicinal Chemistry Research (2024)
-
Carcinogenic roles of MAFG-AS1 in human cancers
Clinical and Translational Oncology (2023)