Abstract
Background
Malnutrition is a common occurrence in critically ill patients, and has been related to poor prognosis in various diseases. Here, we assess the prognostic value of malnutrition using nutritional indices in intensive care units (ICU) patients.
Methods
We retrieved information on 2060 patients from the Medical Information Mart for Intensive Care III, and randomized the patients into training and validation cohorts, at a ratio of 7:3. We estimated their nutritional indices using prognostic nutritional index (PNI), geriatric nutritional risk index (GNRI), and controlling nutritional status (CONUT) score. Both multivariate regression analysis and the Kaplan–Meier (KM) survival curve were used to examine the prognostic role of nutritional indices in ICU mortality. Then we evaluated the additional predictive significance of each nutritional index beyond the baseline model including conventional risk factors.
Results
Multivariate regression analysis revealed that PNI, GNRI, and CONUT were independent predictors of in-hospital and 1-year mortality (all P < 0.001). KM curves showed higher 1-year mortality rates in having nutritional risk patients (PNI ≤ 38 or GNRI ≤ 98 or CONUT ≥ 2). Moreover, subgroup analyses revealed a significant association between each nutritional index and 1-year mortality in patients with different comorbidities. We also observed a pronounced additional impact on the predictive value of 1-year mortality when PNI, GNRI, and CONUT were separately added to the baseline model. The additional role of each nutritional index was further verified in the validation cohort.
Conclusions
Our results revealed that the nutritional indices at admission are significantly correlated with increased mortality rates in ICU adult patients.
Similar content being viewed by others
Introduction
Malnutrition in critically ill patients is a universal concern, owing to the fact that nutritional status of patients in intensive care units (ICU) deteriorated very early and rapidly especially during the first week of critical illness [1]. Previous studies have shown that malnourished patients are prone to higher complications, prolonged hospital stays, increased mortality, and healthcare costs [2, 3]. Although patients in ICU have a high risk of developing malnutrition, little is known regarding their initial nutrition status. Prognostic nutritional index (PNI), geriatric nutritional risk index (GNRI), and controlling nutritional status (CONUT) are different nutritional scoring systems that were generated by body mass index (BMI), total cholesterol (TC), serum albumin, and total lymphocyte count. Previous findings have reported that these three nutritional indices are correlated with unfavorable outcomes in patients with cancer [4, 5], heart failure (HF) [6, 7], acute myocardial infarction [8, 9], stable coronary artery disease (CAD) [10], and those on dialysis [11]. Assessment of PNI, GNRI, and CONUT is an inexpensive, and readily available approach, and is not biased by the operator. However, the association between nutritional indices and prognosis of ICU patients was not fully investigated. Therefore, we sought to assess the prognostic significance of nutritional indices on all-cause mortality in ICU patients.
Materials and methods
Study design
This was a retrospective and observational analysis, based on data obtained from the Medical Information Mart for Intensive Care III (MIMIC-III). Briefly, MIMIC-III contains rich deidentified clinical data for over 50,000 patients admitted to the ICU at the Beth Israel Deaconess Medical Center in Boston, Massachusetts, USA, between 2001 and 2012 [12]. To access the database, we completed the National Institutes of Health’s web-based course and passed the Protecting Human Research Participants exam. The study was exempted for ethical review by the Institutional Review Boards of Beth Israel Deaconess Medical Center and the Massachusetts Institute of Technology since the patients were anonymized to protect their personal information.
Inclusion and exclusion criteria
Patients aged above 18 years, at their first admission, with an ICU stay of ≥2 days were included in the study. Conversely, those who met the following criteria were excluded: (1) had missing data for serum albumin or lymphocyte count or TC or weight or height; and (2) were diagnosed with hematological diseases, such as leukemia or lymphoma. For patients with more than once ICU admission, we only used the data from the first admission.
Data extraction and calculations
The following parameters were selected from the MIMIC-III database: age, gender, weight, height, heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP), vasoactive drug use, and first Sequential Organ Failure Assessment (SOFA) score. These parameters, including weight, height, heart rate, BP, and SOFA score, were obtained from the first measured after admission. Vasoactive drugs comprised dopamine, dobutamine, epinephrine, and norepinephrine. Comorbidities included hypertension, diabetes, atrial fibrillation (AF), CAD, chronic kidney disease (CKD), acute kidney injury (AKI), chronic obstructive pulmonary disease (COPD), stroke, liver cirrhosis, HF, malignancy, and sepsis. Laboratory parameters included white blood cell (WBC) count, hemoglobin, platelet, neutrophil, and lymphocyte counts, as well as blood urea nitrogen (BUN), serum creatine (SCr), serum sodium, serum potassium, serum glucose, serum albumin, and TC levels. In cases where the subject received the same laboratory test more than once during hospitalization, we only selected results from the first laboratory test. BMI was calculated as weight in kilograms/(height in meters)2, neutrophil–lymphocyte ratio (NLR) as total neutrophil count/total lymphocyte count, whereas platelet–lymphocyte ratio (PLR) was calculated as total platelet count/total lymphocyte count. We then applied PNI, GNRI, and CONUT to evaluate the nutritional status of all patients at admission. Specifically, PNI was calculated using the following formula: 10 × serum albumin (g/dl) + 0.005 × total lymphocyte count (/ul) [6], while GNRI was calculated as follows: 14.89 × serum albumin (g/dl) + 41.7 × BMI/22 [13]. On the other hand, CONUT score was calculated based on serum albumin, and lymphocyte counts, as well as TC levels (Supplementary Table 1) [14]. We used previously reported cut-offs of 38, 2, 98 for PNI, CONUT score, and GNRI, respectively, to classify patients into having nutritional risk or no risk [6, 13, 14]. Follow-up time referred to the time from hospital admission to mortality, while primary and secondary outcomes were 1-year and in-hospital mortality, respectively.
Statistical analyses
Data were randomized into training and validation cohorts (at a ratio of 7:3). We used the training cohort to determine the association between nutritional indices at admission with mortality rates in ICU patients, whereas the validation cohort was carried out to validate the results. According to the Harrell guideline, the covariates in the prediction model should be less than 1/10 of the number of events. Data from continuous variables were reported as median (interquartile ranges), whereas categorical variables were reported as counts (percentage), after analysis using Mann–Whitney U and Pearson χ2 tests, respectively. Logistic regression models with odds ratios (OR) and Cox proportional hazards models with hazard ratios (HR) were used to determine in-hospital mortality and 1-year mortality rates, respectively, at 95% confidence interval (CI). The variables (P < 0.05) were included into a multivariate model to identify the independent predictors of mortality in ICU. We also included respective PNI, GNRI, and CONUT scores in the multivariable analyses, then generated Kaplan–Meier curves with the log-rank test to explore whether each nutritional index could predict the long-term mortality risk of ICU patients. Moreover, we applied interaction tests to determine whether the results persisted with different comorbidities. Stratification analyses included hypertension, CAD, and malignancy, since these were the only diseases that showed statistical significance across all multivariate Cox regression analyses. C-statistics, net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were constructed to assess the additional predictive significance of each nutritional index beyond the baseline model including conventional risk factors (variables were P < 0.05 in multivariate Cox proportional hazards analyses). Furthermore, we generated area under the curve (AUC) for different models then compared them using DeLong’s test. All statistical analyses were conducted using packages implemented in R software (version 3.6.3) and MedCalc version 19.1 (MedCalc Software, Belgium). Data followed by P < 0.05 were regarded as statistical significance.
Results
Baseline patient characteristics
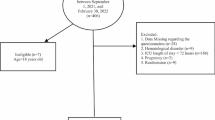
A total of 2060 subjects met our study criteria, and were enrolled in this study (Supplementary Fig. 1). These were randomized into training (n = 1444) and validation cohorts (n = 616). The total, training, and validation cohorts revealed in-hospital mortalities of 154 (7.5%), 109 (7.5%), and 45 (7.3%) patients, respectively, and there were no statistical significance among groups (P = 0.848). Moreover, the total, training, and validation cohorts recorded 1-year mortality rates of 19.7% (405/2060), 20.1% (290/1444), and 18.7% (115/616), respectively, although no significant difference were found among the groups (P = 0.460). Other variables were also not significantly different between the training cohort and validation cohort (Table 1). Patients in the training cohort were split into two groups, based on occurrence or lack of, in-hospital death. Their baseline characteristics are reported in Supplementary Table 2. Patients who died during hospitalization had significantly higher levels of CONUT, or lower levels of PNI or GNRI (all P < 0.001, Supplementary Table 2).
Relationship between nutritional indices and mortality
Logistic regression and Cox proportional hazards models were utilized to examine significant predictors of in-hospital and 1-year mortality rates. Univariate logistic regression analysis, in the training cohort, revealed that age, SBP, hypertension, diabetes, CKD, AKI, sepsis, vasoactive, SOFA score, BUN, PLR, NLR, PNI, GNRI, and CONUT were correlated with increased risk of in-hospital mortality (details are shown in Supplementary Table 3). Even after accounting for these confounders, PNI, GNRI, and CONUT were still significant predictors of in-hospital mortality [OR (95% CI): 0.94 (0.92–0.97), P < 0.001, for PNI; OR (95% CI): 0.98 (0.96–0.99), P < 0.001, for GNRI; OR (95% CI): 1.24 (1.14–1.34), P < 0.001, for CONUT, respectively] (Table 2). Multivariate COX regression analysis showed similar results. PNI, GNRI, and CONUT were significantly independently related to 1-year mortality (all P < 0.001, Table 2), independent of variables (including age, SBP, hypertension, CAD, HF, AF, AKI, malignancy, sepsis, vasoactive, SOFA score, WBC count, hemoglobin, BUN, Scr, PLR, and NLR) that were significant in univariate COX regression analysis in training set (details are shown in Supplementary Table 3).
Kaplan–Meier curves reported the 1-year cumulative survival rates, according to different nutritional status (Fig. 1). In the training cohort, patients in the low PNI (PNI ≤ 38) group had significantly higher risks of 1-year mortality than those in high PNI (PNI > 38) group (Log-rank P < 0.001, Fig. 1A). Compared with high GNRI (GNRI > 98) group, 1‐year cumulative mortality rates were remarkably higher in the low GNRI (GNRI ≤ 98) group (Log-rank P < 0.001, Fig. 1B). Patients with high CONUT (CONUT ≥ 2) scores showed higher incidence of 1-year mortality than those with low CONUT (CONUT < 2) scores (Log-rank P < 0.001, Fig. 1C). Similar results were observed in the validation cohort (all Log-rank P < 0.001, Fig. 1D–F).
Subgroup analyses
Subgroup analysis indicated the relationship between each nutritional index with 1-year mortalities of ICU patients with different comorbidities (Fig. 2). Specifically, both PNI and GNRI were consistently correlated with 1-year mortality across different subgroups, including those with or without hypertension, CAD, and malignancy. Notably, the predictive value of PNI and GNRI seemed to be more prominent in patients with CAD (Pinteraction = 0.017 and Pinteraction = 0.004 for PNI and GNRI, respectively) and patients with malignancy (Pinteraction = 0.03 and Pinteraction = 0.011 for PNI and GNRI, respectively). We found no significant interaction between CONUT scores with any of the subgroups (all Pinteraction > 0.05).
Incremental prognostic value of each nutritional indices
The addition of PNI improved the predictive power of 1-year mortality, as evidenced by a significant increase in the AUC (P < 0.001, Table 3). Moreover, inclusion of GNIR yielded a remarkable increase in the AUC (P = 0.034), while addition of CONUT to the baseline model caused a remarkable increase in the AUC (P < 0.001, Table 3). NRI and IDI significantly improved following addition of PNI, GNRI, and CONUT to the baseline model (all P < 0.001, Table 3).
The validation cohort corroborated these results (Table 4). Briefly, C-statistics analysis revealed an increase in the predictive value of 1-year mortality when PNI, GNRI, and CONUT were separately added to the baseline model (all P < 0.05). Furthermore, addition of PNI, GNRI, and CONUT to the baseline model mediated a significant increase in NRI and IDI (all P < 0.05).
Discussion
This paper was the first to demonstrate that the nutritional indices at admission are significant predictors for mortality in ICU adult patients; and adding PNI, GNRI, and CONUT, respectively, to the baseline model significantly improved prognostic ability in ICU mortality.
Previous studies have reported use of multiple tools in screening for nutritional parameters in patients. For example, Mini-Nutritional Assessment [15] and Subjective Global Assessment [16], which are based on subjective assessments by trained professionals, have been described. In contrast, PNI, GNRI, and CONUT, which can only be obtained from objective data, are widely applied for malnutrition assessment. Moreover, nutritional assessment using multiple indices may better reflect the actual status of a patient. Numerous studies have reported that PNI, GNRI, and CONUT scores are associated with adverse outcomes across various cardiovascular diseases, including HF [17,18,19], stable CAD [10, 20, 21], and acute myocardial infarction [9, 22, 23]. Other research works have shown that these three nutritional indices are vital markers for predicting mortality rates in differ cancers [4, 5, 24]. PNI was correlated with short-term and long-term adverse prognosis in patients receiving coronary artery bypass grafting [25]. On the other hand, GNRI was not only correlated with unfavorable outcomes in dialysis patients [26], but also with adverse outcomes in patients with non-dialysis stage 3–5 CKD [27]. Additional evidence have demonstrated the relationship between GNRI and CONUT with poor prognosis in patients with stroke [28, 29]. Taken together, the aforementioned evidences may explain the potential relationship between the three nutritional indicators with ICU mortality rates observed in the present study. Patients in ICU often have various complications, and their clinical situation is often complex. Our analysis further extends the population to critically ill patients. Multivariate analysis revealed that the three nutritional indices were significant predictors of mortality in the ICU patients, even after adjusting for potential confounders.
Diseases have been shown to cause or aggravate malnutrition in a variety of ways [30]. Our subgroup analysis revealed that the association between PNI, GNRI, and CONUT with 1-year mortality rates persisted in this group of patients, and this was further stratified by hypertension, CAD, and malignancy. This shows that the prognostic value of nutritional indices in the ICU may be not affected by the disease types. Interestingly, the predictive significance of PNI and GNRI seemed to be more pronounced in patients with CAD and malignancy, suggesting that both nutritional indices may be more suitable for nutritional assessment of patients with CAD and cancer.
The predictive value of nutritional indices under complex clinical conditions shows that there are common pathways, although the specific mechanisms may be different. Several risk factors have been implicated in malnutrition, with diseases representing one of the most significant players. Most chronic and acute diseases, especially critical illness, negatively affect appetite and absorption, leading to insufficient intake [30]. In addition, disease-related inflammatory responses upregulate catabolic cytokines and accelerate muscle catabolism, thereby increasing losses [31, 32]. On the other hand, proinflammatory cytokines, such as interleukin-6 or tumor necrosis factor-alpha, affect albumin synthesis in hepatocytes, causing malnutrition [33, 34]. Since patients in ICU are basically confined to bed, prolonged immobility and stress-related hypercortisolemia cause severe catabolism that reduce muscle protein synthesis [35]. Critically ill patients often require a large number of oral or intravenous drugs, such as radiotherapy, chemotherapy, digoxin, sedative, and antipsychotic drugs, which may cause side effects thereby suppressing appetite. Apart from these reasons, negligence by clinicians may also contribute to the development of undernutrition. Only a small part of patients with malnutrition received nutritional treatment. It has been reported that the rate of nutritional support for patients with nutritional risk was between 14 and 25% [36, 37]. Malnutrition mainly suppresses immunity and delays recovery from illnesses, thereby predisposing patients to increased morbidity and mortality. A previous retrospective study, comprising 1171 critically ill patients, showed that increasing caloric intake to 70% of resting energy expenditure was enough to lower mortalities [38], whereas another randomized double-blind study in HF patients with cachexia identified that appropriate oral nutritional supplementation could improve the quality of life of patients [39].
Some limitations of our analysis should be considered. First, although we performed internal validation and successfully verified our results, it is a pity that we did not conduct an external validation. Second, three nutritional indicators were evaluated only once at admission. Dynamic changes in nutritional indicators, which may be better predictors of unfavorable outcomes, were not evaluated in our paper. Finally, although we attempted to minimize the confounding covariates, our analysis was prone to selection bias owing to its retrospective nature. In the future, a multicenter prospective study is needed to validate the observed association between nutritional status at admission with ICU mortality.
Conclusion
In summary, our findings indicate that undernutrition at admission is strongly correlated with the mortality rates of patients in ICU. We recommend use of these simple nutritional scores during identification of patients at nutritional risk, as they can guide development of effective and timely intervention measures.
References
Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–600.
Correia MI, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22:235–39.
Lim SL, Ong KC, Chan YH, Loke WC, Ferguson M, Daniels L. Malnutrition and its impact on cost of hospitalization, length of stay, readmission and 3-year mortality. Clin Nutr. 2012;31:345–50.
Xie H, Tang S, Wei L, Gan J. Geriatric nutritional risk index as a predictor of complications and long-term outcomes in patients with gastrointestinal malignancy: a systematic review and meta-analysis. Cancer Cell Int. 2020;20:530.
Okadome K, Baba Y, Yagi T, Kiyozumi Y, Ishimoto T, Iwatsuki M, et al. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. 2020;271:693–700.
Sze S, Pellicori P, Kazmi S, Rigby A, Cleland JGF, Wongand K, et al. Prevalence and prognostic significance of malnutrition using 3 scoring systems among outpatients with heart failure: a comparison with body mass index. JACC Heart Fail. 2018;6:476–86.
Chien SC, Lo CI, Lin CF, Sung KT, Tsai JP, Huang WH, et al. Malnutrition in acute heart failure with preserved ejection fraction: clinical correlates and prognostic implications. ESC Heart Fail. 2019;6:953–64.
Chen QJ, Qu HJ, Li DZ, Li XM, Zhu JJ, Xiang Y, et al. Prognostic nutritional index predicts clinical outcome in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Sci Rep. 2017;7:3285.
Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Impacts of geriatric nutritional risk index on prognosis of patients with non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Nutr Metab Cardiovasc Dis. 2020;30:1685–96.
Wada H, Dohi T, Miyauchi K, Doi S, Konishi H, Naito R, et al. Prognostic impact of nutritional status assessed by the controlling nutritional status score in patients with stable coronary artery disease undergoing percutaneous coronary intervention. Clin Res Cardiol. 2017;106:875–83.
Kobayashi I, Ishimura E, Kato Y, Okuno S, Yamamoto T, Yamakawa T, et al. Geriatric nutritional risk index, a simplified nutritional screening index, is a significant predictor of mortality in chronic dialysis patients. Nephrol Dial Transpl. 2010;25:3361–5.
Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035.
Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82:777–83.
Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, Gonzalez P, Gonzalez B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20:38–45.
Guigoz Y, Vellas B, Garry PJ. Assessing the nutritional status of the elderly: the mini nutritional assessment as part of the geriatric evaluation. Nutr Rev. 1996;54:S59–65.
Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, et al. What is subjective global assessment of nutritional status? J Parenter Enter Nutr. 1987;11:8–13.
Candeloro M, Di Nisio M, Balducci M, Genova S, Valeriani E, Pierdomenicoand SD, et al. Prognostic nutritional index in elderly patients hospitalized for acute heart failure. ESC Heart Fail. 2020;7:2479–84.
Kato T, Yaku H, Morimoto T, Inuzuka Y, Tamaki Y, Yamamoto E, et al. Association with controlling nutritional status (CONUT) score and in-hospital mortality and infection in acute heart failure. Sci Rep. 2020;10:3320.
Nishi I, Seo Y, Hamada-Harimura Y, Yamamoto M, Ishizu T, Sugano A, et al. Geriatric nutritional risk index predicts all-cause deaths in heart failure with preserved ejection fraction. ESC Heart Fail. 2019;6:396–405.
Wada H, Dohi T, Miyauchi K, Jun S, Endo H, Doi S, et al. Relationship between the prognostic nutritional index and long-term clinical outcomes in patients with stable coronary artery disease. J Cardiol. 2018;72:155–61.
Wada H, Dohi T, Miyauchi K, Doi S, Naito R, Konishi H, et al. Prognostic impact of the geriatric nutritional risk index on long-term outcomes in patients who underwent percutaneous coronary intervention. Am J Cardiol. 2017;119:1740–5.
Keskin M, Hayiroglu MI, Keskin T, Kaya A, Tatlisu MA, Altay S, et al. A novel and useful predictive indicator of prognosis in ST-segment elevation myocardial infarction, the prognostic nutritional index. Nutr Metab Cardiovasc Dis. 2017;27:438–46.
Deng X, Zhang S, Shen S, Deng L, Shen L, Qian J, et al. Association of controlling nutritional status score with 2-year clinical outcomes in patients with ST elevation myocardial infarction undergoing primary percutaneous coronary intervention. Heart Lung Circ. 2020;29:1758–65.
Kuroda D, Sawayama H, Kurashige J, Iwatsuki M, Eto T, Tokunaga R, et al. Controlling nutritional status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. 2018;21:204–12.
Keskin M, Ipek G, Aldag M, Altay S, Hayiroglu MI, Borklu EB, et al. Effect of nutritional status on mortality in patients undergoing coronary artery bypass grafting. Nutrition. 2018;48:82–6.
Panichi V, Cupisti A, Rosati A, Di Giorgio A, Scatena A, Menconi O, et al. Geriatric nutritional risk index is a strong predictor of mortality in hemodialysis patients: data from the Riscavid cohort. J Nephrol. 2014;27:193–201.
Lin TY, Hung SC. Geriatric nutritional risk index is associated with unique health conditions and clinical outcomes in chronic kidney disease patients. Nutrients. 2019;11:2769.
Kang MK, Kim TJ, Kim Y, Nam KW, Jeong HY, Kim SK, et al. Geriatric nutritional risk index predicts poor outcomes in patients with acute ischemic stroke – automated undernutrition screen tool. PLoS One. 2020;15:e228738.
Cai ZM, Wu YZ, Chen HM, Feng RQ, Liao CW, Ye SL, et al. Being at risk of malnutrition predicts poor outcomes at 3 months in acute ischemic stroke patients. Eur J Clin Nutr. 2020;74:796–805.
Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27:5–15.
Guigoz Y, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition.The Mini Nutritional Assessment. Clin Geriatr Med. 2002;18:737–57.
Roubenoff R. Sarcopenia and its implication for the elderly. Eur J Clin Nutr. 2000;54 Suppl 3:S40–7.
Peters SJ, Vanhaecke T, Papeleu P, Rogiers V, Haagsman HP, van Norren K. Co-culture of primary rat hepatocytes with rat liver epithelial cells enhances interleukin-6-induced acute-phase protein response. Cell Tissue Res. 2010;340:451–7.
Vavrova L, Rychlikova J, Mrackova M, Novakova O, Zakand A, Novak F. Increased inflammatory markers with altered antioxidant status persist after clinical recovery from severe sepsis: a correlation with low HDL cholesterol and albumin. Clin Exp Med. 2016;16:557–69.
Paddon-Jones D, Sheffield-Moore M, Cree MG, Hewlings SJ, Aarsland A, Wolfeand RR, et al. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J Clin Endocrinol Metab. 2006;91:4836–41.
Kondrup J, Johansen N, Plum LM, Bak L, Larsen IH, Martinsen A, et al. Incidence of nutritional risk and causes of inadequate nutritional care in hospitals. Clin Nutr. 2002;21:461–8.
Liang X, Jiang ZM, Nolan MT, Efron DT, Kondrup J. Comparative survey on nutritional risk and nutritional support between Beijing and Baltimore teaching hospitals. Nutrition 2008;24:969–76.
Zusman O, Theilla M, Cohen J, Kagan I, Bendavid I, Singer P. Resting energy expenditure, calorie and protein consumption in critically ill patients: a retrospective cohort study. Crit Care. 2016;20:367.
Rozentryt P, von Haehling S, Lainscak M, Nowak JU, Kalantar-Zadeh K, Polonski L, et al. The effects of a high-caloric protein-rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition, and inflammation markers: a randomized, double-blind pilot study. J Cachexia Sarcopenia Muscle. 2010;1:35–42.
Acknowledgements
We thank Jeremiah Machuki for language editing.
Funding
Chengdu Science and Technology Agency (NO. 2020-YF05-00184-SN).
Author information
Authors and Affiliations
Contributions
YS was responsible for designing protocol, conducting the search, extracting and analyzing data from MIMIC-III, interpreting results, and creating “Summary of findings” tables. Q-cL was responsible for designing the review protocol and extracting data. QD and PG contributed to updating reference lists and provided feedback on the report. LY contributed to analyzing data, interpreting results, as well as creating tables and figures, and writing paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Shao, Y., Lai, Qc., Duan, Q. et al. Nutritional indices at admission are associated with mortality rates of patients in the intensive care unit. Eur J Clin Nutr 76, 557–563 (2022). https://doi.org/10.1038/s41430-021-00994-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-021-00994-3
- Springer Nature Limited