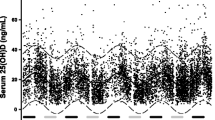
Abstract
Background/objectives
Our objective was to evaluate the degree of tracking for serum levels of 25-hydroxyvitamin D [25(OH)D] over time, by using data from three previously conducted surveys of the Tromsø study collected in the years 1994/1995 (Tromsø 4), 2007/2008 (Tromsø 6), and 2015/2016 (Tromsø 7).
Subjects/methods
Subjects with valid 25(OH)D measurements in all three surveys were included. 25(OH)D z-scores were used to adjust for seasonal variation. Z-scores and sextiles were used to illustrate tracking of 25(OH)D.
Results
1702 subjects (572 males, 1130 females) fulfilled the inclusion criteria. Median (5th, 95th percentiles) age for these subjects was 55 (33, 65) years in Tromsø 4, and mean (SD) 25(OH)D levels were 57 (18) nmol/L, 59 (19) nmol/L, and 72 (21) nmol/L for Tromsø 4, Tromsø 6, and Tromsø 7, respectively. There was significant tracking of serum 25(OH)D over the 21 years period between the surveys of the Tromsø study. The correlation coefficient r between 25(OH)D z-scores from Tromsø 4 and Tromsø 6 was 0.40, and declined to 0.29 for the correlation between Tromsø 4 and Tromsø 7. Twenty-six percent of the subjects in the lowest 25(OH)D z-score sextile in Tromsø 4 were in the three highest sextiles of 25(OH)D in Tromsø 7. Similarly, 35% of those in the highest sextile in Tromsø 4 were in the lowest three sextiles in Tromsø 7.
Conclusions
The degree of tracking for serum 25(OH)D declines over time, and the use of a single serum 25(OH)D measurement as an indicator of the vitamin-D status is questionable if used in long-lasting observational studies.
Similar content being viewed by others
Introduction
The importance of vitamin D for skeletomuscular health is well established [1, 2]. The discovery of vitamin-D receptors in almost all tissues of the body, also in those not related to calcium or bone metabolism [2], leads to discussions about its potential role in multiple heath-related issues. Following this discovery there have been many studies published on the association between vitamin-D status and an array of non-calcemic medical conditions, such as cardiovascular disease, cancer, obesity, depression, and dementia [3]. Currently, serum levels of 25-hydroxyvitamin D [25(OH)D] are viewed as the standard method to evaluate a subject’s vitamin-D status and most often this vitamin-D status assessment is based on a single measurement [4, 5].
In recent years there is new evidence that vitamin-D levels are not only dependent on sun exposure and diet [1], but are also to a significant degree determined genetically [6, 7]. This, and vitamin D’s possible association with numerous diseases, amplifies the value of being able to predict vitamin-D levels in an individual over time. Preferably, this prediction would be based on a single or a few serum values, and enable an appraisal of a subject’s vitamin-D status over time. This constancy of 25(OH)D level in a specific individual over a longer period of time is referred to as tracking.
In a previous article from 2010, our research group confirmed tracking of serum 25(OH)D within individuals based on samples taken 13 years apart in 2668 subjects. This was based on observations from the Tromsø study—an observational, longitudinal study, which is repeated on regular intervals in the form of surveys, examinations, and collection of biological data from the population in the Tromsø municipality [8]. Since then, several papers have been published regarding tracking of 25(OH)D [9,10,11,12,13,14,15,16,17,18,19,20,21]. However, these studies have been considerably smaller, and/or have had much shorter observation time, or have focused on population subgroups like pregnant women [11] or children/adolescents [17, 18, 20]. In general, they have confirmed our original observation of a high degree of tracking, as could be expected with a shorter observation time.
The seventh survey in the Tromsø study was performed in 2015/2016 and a considerable number of those included in our original tracking publication also participated in this survey. We therefore had the opportunity to evaluate the tracking of 25(OH)D over a 21 years time period.
Materials and method
The Tromsø survey is a longitudinal, observational population study conducted by the University of Tromsø—The Arctic University of Norway, and the Norwegian National Heath Screening Service. Initiated in mid-1970 as a means of mapping cardiovascular disease in north Norwegian males, it has since evolved to include a large portion of Tromsø municipality inhabitants of both sexes and with a wide range of age groups [22, 23]. The focus has also shifted; from the narrow emphasis on cardiovascular disease, to a multi-focus study with detailed data gathered on lifestyle, and a wide range of health issues [23]. The study has been repeated with regular intervals and recently finished its seventh survey in 2015/2016. In the present study we have used data from the fourth, sixth, and seventh surveys:
-
– The fourth survey (“Tromsø 4”) was performed in 1994/1995 and invited all citizens aged 25 years or older living in Tromsø municipality (n = 37,558) of whom 27,158 subjects participated in the first phase of the survey. Furthermore, all men aged 55–74 years, all women aged 50–74 years, and 5–8% random samples of the other age groups <85 years were invited to a second visit with more extensive examinations. Among 10,542 eligible subjects 7965 attended this second phase, and 7156 (2269 smokers and 4887 non-smokers) had serum 25(OH)D measured.
-
– The sixth survey (“Tromsø 6”) was performed in 2007/2008. All who had participated in the second phase of Tromsø 4, a 10% random sample of those 20–39 years old, all subjects 40–42 or 60–87 years old, and a 40% random sample of those 43–59 years old were invited. Among the 19,762 subjects invited, 12,984 subjects attended, and 12,444 (2389 smokers and 10,055 non-smokers) had serum 25(OH)D measured.
-
– The seventh survey (“Tromsø 7”) was performed in 2015/2016 and all citizens aged 40 years or above living in Tromsø municipality (n = 32,591) were invited, among whom 21,084 participated and 20,720 (2878 smokers and 17,842 non-smokers) had serum 25(OH)D measured.
The Tromsø surveys are binary in design, with a questionnaire part followed by physical examination and blood sampling. The details regarding the questionnaires and examinations can be found on http://tromsoundersokelsen.uit.no/tromso/ (May to November 2019). The surveys contain information on age, sex, smoking habits, medication, use of cod-liver oil, and vitamin-D supplements.
The wording in the questionnaires regarding cod-liver oil and vitamin-D supplements differed between the surveys. In Tromsø 4 the cod-liver oil question was: “Have you used cod-liver oil or fish-oil capsules during the last 14 days (“yes”/”no”)?”. In Tromsø 6 and Tromsø 7 the corresponding question was “Do you use cod-liver oil or cod-liver-oil capsules?”. The answer options in Tromsø 6 were “yes, daily”/“sometimes”/“no,” and in Tromsø 7 “no”/“sometimes”/“daily during the winter season”/“daily.” In Tromsø 4 the vitamin-D supplement question was “Have you used vitamin-D supplements during the last 14 days?” and in Tromsø 7 “Do you use vitamin supplements with vitamin D?” with the same answer options as for cod-liver oil. Vitamin-D supplements were not specifically asked for in Tromsø 6.
The Tromsø 6 and 7 surveys also included questions regarding sunny vacations last 8 weeks (“yes”/”no”) and use of solarium or any form of light therapy during the last 7 days (“weekly”/“sometimes”/“never”). However, only 30% of the subjects answered these questions in Tromsø 6 and sunny vacation/use of solarium therefore not included in the tracking analyses.
Height and weight were measured with light clothing, and body mass index (BMI) calculated as kg/m2. Blood pressure was measured after a 2-min seated rest (in Tromsø 4 with Dinamap Vital Signs Monitor, Critikon Inc, Tampa, FL, USA; in Tromsø 6 and 7 with Dinamap ProCare 300 monitor, GE Healthcare, Oslo, Norway). The mean of the two last measurements was used in our analyses.
Blood samples were non-fasting. Serum cholesterol and serum calcium were analyzed as previously described [24, 25]. These methods have a total analytic coefficient of variation (CV) of <2 % and 2.5%, respectively.
The samples from Tromsø 4 were stored at −70 °C and together with the samples from Tromsø 6 analyzed for 25(OH)D in batch with ECLIA (Roche) using an automated clinical chemistry analyzer (Modular E170, Roche Diagnostics). This method, which overestimates serum 25(OH)D in smokers has been described in detail previously and has a CV of 7.3% (26). The samples from Tromsø 7 were analyzed consecutively with an in-house LC–MS/MS method, which has a CV of <9% [26, 27].
Statistical analyses
Only subjects with valid serum 25(OH)D measurements in all three surveys were included in the analyses. The effect of season was adjusted by calculating month-specific z-scores for serum 25(OH)D for each survey. Since the assay used in Tromsø 4 and 6 overestimates serum 25(OH)D in smokers, the z-scores were calculated separately for smokers and non-smokers in all three surveys. Z-scores for smokers and non-smokers were then combined in the tracking analyses. We excluded subjects who changed smoking status between the surveys.
Normal distribution was evaluated with visual inspection of histograms and plots, and by assessing kurtosis and skewness. Distribution was normal for the dependent variables BMI, systolic blood pressure, serum calcium, serum cholesterol, and serum 25(OH)D. The dataset was assessed using the Pitman–Morgan test for related samples, displaying homogeneity of variances.
Tracking was evaluated by Pearson’s correlation coefficient r. Blood pressure and cholesterol analyses only included subjects not using blood pressure or lipid medication, respectively. A linear regression model was used to evaluate predictors of serum 25(OH)D and of change in serum 25(OH)D z-score (delta z-score: z-score in Tromsø 7 minus z-score in Tromsø 4) with covariates as appears in the tables. In addition, the 25(OH)D z-scores from Tromsø 4 and 7 were divided into sextiles and cross-tabled to illustrate degree of tracking.
P < 0.05 (two-tailed) is considered statically significant. Data are presented as mean (SD) for normally distributed values, and as median (5th, 95th percentiles) for non-normally distributed values. All statistical analyses are performed using IBM SPSS version 26 software.
Results
A total of 1702 subjects (572 males, 1130 females) with valid 25(OH)D measurements in the Tromsø 4, 6, and 7 surveys, and without change in smoking status were included in the present study. Their mean (SD) serum 25(OH)D levels were 57 (18) nmol/L, 59 (19) nmol/L, and 72 (21) nmol/L for Tromsø 4, 6, and 7, respectively. As expected, the serum 25(OH)D levels were higher during the summer months, as shown for Tromsø 6 in Fig. 1. Other characteristics from the separate surveys are displayed in Table 1.
In a linear regression model, sex, age, BMI, recent sunny vacation, intakes of cod-liver oil, and vitamin-D supplements were significant predictors of serum 25(OH)D (Table 2).
The correlation coefficient r between serum 25(OH)D z-scores from Tromsø 4 and 6 was 0.40, and declined to 0.29 for the correlation between Tromsø 4 and 7 (Table 3). In comparison, correlations between Tromsø 4 and 7 for BMI, systolic blood pressure (in subjects not using blood pressure medication), serum calcium, and serum total cholesterol (in subjects not using lipid lowering medication) were slightly higher, at 0.78, 0.45, 0.31, and 0.53, respectively (Table 3). The degree of tracking for serum 25(OH)D was higher in males, non-smokers, age > 55 years in Tromsø 4, change in BMI < 1.1 kg/m2, and with continuous use of cod-liver oil or vitamin-D supplements (Table 4).
The importance of BMI, use of cold liver oil, and vitamin-D supplements for tracking of serum 25(OH)D was confirmed in a linear regression model where change in BMI and change in intakes of vitamin D were significant predictors of change in serum 25(OH)D z-scores (Table 5).
To explore reasons for the higher tracking for serum 25(OH)D for males and for subjects >55 years, the serum 25(OH)D, BMI, intakes of cod-liver oil, and vitamin-D supplement in relation to sex and age are shown for the three surveys in Table 6. In the females there was an increase in use of vitamin-D supplementation from 8% in Tromsø 4 to 29% in Tromsø 7, whereas in the males the corresponding increase was only from 3 to 14%. This could possibly explain the higher tracking in the males. However, there was in our data no obvious explanation for the higher tracking of serum 25(OH)D in those with age >55 years.
To further illustrate the degree of tracking, the change in distribution of sextiles of 25(OH)D z-scores from Tromsø 4 to 7 is shown in Table 7. Among those in the lowest sextile in Tromsø 4, 74% were still in the three lowest sextiles of 25(OH)D in Tromsø 7, but accordingly, 26% had shifted to the three highest percentiles. Similarly, among those in the highest sextile in Tromsø 4, 65% were still in the three highest sextiles in Tromsø 7, and 35% were now in the three lowest.
Discussion
In the present study, based on data from three surveys in the Tromsø study, we have found significant tracking of serum 25(OH)D over a 21 years period. Tracking was observed in both sexes, and in all age groups, with a correlation coefficient r ranging from 0.25 to 0.40 in the various subgroups between the first and last serum 25(OH)D measurement.
As expected, the degree of tracking declined over time. The correlation coefficient r was for all subjects 0.40 between the fourth and the sixth surveys (13 years apart), and dropped to 0.29 between the forth and the seventh surveys (21 years apart). In comparison, 1-year tracking data for participants receiving placebo in a vitamin-D intervention study was as high as 0.80 [8].
When dividing the cohort into serum 25(OH)D z-score sextiles in the fourth and seventh surveys, 26% of those in the lowest sextile in Tromsø 4 were in the upper half of the cohort in Tromsø 7. Conversely, 35% of those in the highest sextile in Tromsø 4 were in the lower half in Tromsø 7. Consequently, the use of a single serum 25(OH)D measurement as an indicator of the vitamin-D status, as has been frequently done in case-control studies [28, 29], appears to be highly questionable, at least if the observation time is long. Likewise, in prospective studies lasting for more than a few years, repeated measurements of serum 25(OH)D should be used for estimation of vitamin-D status before occurrence of the outcome in question. Furthermore, the high degree of tracking for serum 25(OH)D over shorter periods of time, like a few years, is an argument against repeated serum 25(OH)D measurements in clinical practice.
The serum 25(OH)D level is partly genetically determined, and several single nucleotide polymorphisms in enzymes necessary for production, transport, and degradation of the active vitamin-D metabolite 1,25-dihydroxyvitamin D have been described [6, 30]. These genetic differences may account for a large part of the variation in serum 25(OH)D levels [7]. However, the main determinants of serum 25(OH)D levels are amendable factors related to lifestyle, like time spent in the sun, intake of vitamin-D-rich food like fatty fish, and the use of vitamin-D supplement [1, 30]. Furthermore, it appears as body size, in particular adipose tissue, is of importance by increasing volume of distribution [31]. It was therefore no surprise that tracking was more pronounced in subjects who continuously used cod-liver oil and/or vitamin-D supplements, and that changes in intake of these substances, as well as change in BMI, were associated with greater change in delta serum 25(OH)D z-scores. In Norway, which does not receive as much UV light during the summer as countries further south, the importance of these factors are perhaps more important for 25(OH)D tracking than seasonal changes, which are more pronounced in countries like England [32]. We also found a lower degree of tracking for serum 25(OH)D in the females, which could possibly be explained by an increase in their use of vitamin-D supplements.
In addition to lifestyle factors, the degree of tracking is also influenced by the precision of the laboratory analyses, and for serum 25(OH)D the CV was higher than for serum calcium and total cholesterol. It should be noted that the tracking of serum 25(OH)D was lower than that found for serum calcium and serum total cholesterol, as well as for BMI and systolic blood pressure. For observational studies that rely on a single measurement these variables are therefore probably better suited than serum 25(OH)D.
In general, other studies have found a similar degree of tracking for serum 25(OH)D as we have [11, 17, 18, 20]. However, most of these studies have been of short duration, and to our knowledge, ours is the longest running by far. Some of these studies have included subjects in certain age groups or in particular periods of life. Thus, Thordisdottir et al. found in a group of 139 children a correlation coefficient between serum 25(OH)D at age 1 and 6 years of 0.34 [17]. Similarly, Zhu et al. found in a group of 821 children with serum 25(OH)D measured at ages 6, 14, 17, and 20 years, correlation coefficients ranging from 0.35 to 0.56 depending on time interval [20]. On the other hand, Poopedi et al. found no significant correlation for 25(OH)D between age 11 and 20 years in a group of 76 adolescents (r = 0.15), whereas between ages 15 and 20 the correlation was highly significant (r = 0.65) [18]. And finally, in 1753 pregnant women, Moon et al. found a correlation coefficient of 0.53 between season-corrected serum 25(OH)D measurements at 11 and 34 weeks of gestation [11].
The mean serum 25(OH)D levels were similar in Tromsø 4 and 6, which were 13 years apart, but in Tromsø 7 8 years later the serum 25(OH)D was ~20% higher. There was a change in 25(OH)D assay from Tromsø 6 to 7, and in view of the time line, this is the most likely explanation for the apparent increase in serum 25(OH)D. Additional factors could be the increase in use of daily vitamin-D supplements from 7 to 24% from Tromsø 4 to 7, as well as nutritional changes that were not recorded in our study. Sunny vacation the last 2 months was a strong predictor of the serum 25(OH)D level in Tromsø 7, but was not asked for in the Tromsø 4 survey. Most likely, such vacations were more frequent in 2015/2016 than in 1994/1995 and could thus have contributed to the apparent increase in serum 25(OH)D in this cohort.
Our study has several weaknesses. In Tromsø 7, we used an LC–MS/MS assay for determination of serum 25(OH)D levels, whereas in Tromsø 4 and 6 an immunological assay was used. We could therefore not relate the degree of tracking to changes in nmol/L of serum 25(OH)D, but had to make a z-score transformation. The assay used in Tromsø 4 and 6 overestimated serum 25(OH)D in smokers. We therefore calculated z-scores separately for smokers and non-smokers, before combining them into one group. Since we made month-specific z-scores to adjust for seasonal variation, some of the smoker groups became small, which can have made these z-scores less accurate. We had limited information regarding intakes of vitamin D, as well as sun exposure, which could have improved the analysis of factors affecting the degree of tracking. The questionnaires regarding cod-liver oil and vitamin-D supplements differed slightly between the surveys and made evaluation of changes in these intakes difficult. We had to exclude subjects with change in smoking habits, which might have resulted in an overestimation of the serum 25(OH)D tracking. Only subjects who lived in Tromsø at all three time points could be included, and in societies with a high degree of mobility the tracking of serum 25(OH)D is likely to be lower. On the other hand, our study also has strengths as it was performed on the general population, included a large number of subjects of both sexes and of different ages, and the follow-up period was 21 years.
In conclusion, we have found a significant degree of tracking for serum 25(OH)D over a period of 21 years. However, the degree of tracking declined over time, and using a single serum 25(OH)D measurement as an indicator of vitamin-D status in long-term observational studies might be questionable.
References
DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80 6 Suppl:1689S–96S.
Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80 6 Suppl:1678S–88S.
Pludowski P, Holick MF, Pilz S, Wagner CL, Hollis BW, Grant WB, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality—a review of recent evidence. Autoimmun Rev. 2013;12:976–89.
Jorde R, Grimnes G. Serum cholecalciferol may be a better marker of vitamin D status than 25-hydroxyvitamin D. Med Hypotheses. 2018;111:61–5.
Sachs MC, Shoben A, Levin GP, Robinson-Cohen C, Hoofnagle AN, Swords-Jenny N, et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the multi-ethnic study of atherosclerosis. Am J Clin Nutr. 2013;97:1243–51.
Jolliffe DA, Walton RT, Griffiths CJ, Martineau AR. Single nucleotide polymorphisms in the vitamin D pathway associating with circulating concentrations of vitamin D metabolites and non-skeletal health outcomes: review of genetic association studies. J Steroid Biochem Mol Biol. 2016;164:18–29.
Bahrami A, Sadeghnia HR, Tabatabaeizadeh SA, Bahrami-Taghanaki H, Behboodi N, Esmaeili H, et al. Genetic and epigenetic factors influencing vitamin D status. J Cell Physiol. 2018;233:4033–43.
Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol. 2010;171:903–8.
Mirfakhraee S, Ayers CR, McGuire DK, Maalouf NM. Longitudinal changes in serum 25-hydroxyvitamin D in the Dallas Heart Study. Clin Endocrinol. 2017;87:242–8.
van Schoor NM, Knol DL, Deeg DJ, Peters FP, Heijboer AC, Lips P. Longitudinal changes and seasonal variations in serum 25-hydroxyvitamin D levels in different age groups: results of the Longitudinal Aging Study Amsterdam. Osteoporos Int. 2014;25:1483–91.
Moon RJ, Crozier SR, Dennison EM, Davies JH, Robinson SM, Inskip HM, et al. Tracking of 25-hydroxyvitamin D status during pregnancy: the importance of vitamin D supplementation. Am J Clin Nutr. 2015;102:1081–7.
McKibben RA, Zhao D, Lutsey PL, Schneider AL, Guallar E, Mosley TH, et al. Factors associated with change in 25-hydroxyvitamin D levels over longitudinal follow-up in the ARIC Study. J Clin Endocrinol Metab. 2016;101:33–43.
Major JM, Graubard BI, Dodd KW, Iwan A, Alexander BH, Linet MS, et al. Variability and reproducibility of circulating vitamin D in a nationwide U.S. population. J Clin Endocrinol Metab. 2013;98:97–104.
Meng JE, Hovey KM, Wactawski-Wende J, Andrews CA, Lamonte MJ, Horst RL, et al. Intraindividual variation in plasma 25-hydroxyvitamin D measures 5 years apart among postmenopausal women. Cancer Epidemiol Biomark Prev. 2012;21:916–24.
Kluczynski MA, Wactawski-Wende J, Platek ME, DeNysschen CA, Hovey KM, Millen AE. Changes in vitamin D supplement use and baseline plasma 25-hydroxyvitamin D concentration predict 5-y change in concentration in postmenopausal women. J Nutr. 2012;142:1705–12.
Sonderman JS, Munro HM, Blot WJ, Signorello LB. Reproducibility of serum 25-hydroxyvitamin d and vitamin D-binding protein levels over time in a prospective cohort study of black and white adults. Am J Epidemiol. 2012;176:615–21.
Thorisdottir B, Gunnarsdottir I, Steingrimsdottir L, Palsson GI, Birgisdottir BE, Thorsdottir I. Vitamin D Intake and status in 6-year-old Icelandic children followed up from infancy. Nutrients. 2016;8:75.
Poopedi MA, Norris SA, Micklesfield LK, Pettifor JM. Does vitamin D status track through adolescence? Am J Clin Nutr. 2015;102:1025–9.
Berger C, Greene-Finestone LS, Langsetmo L, Kreiger N, Joseph L, Kovacs CS, et al. Temporal trends and determinants of longitudinal change in 25-hydroxyvitamin D and parathyroid hormone levels. J Bone Min Res. 2012;27:1381–9.
Zhu K, Oddy WH, Holt P, Ping-Delfos WCS, Mountain J, Lye S, et al. Tracking of vitamin D status from childhood to early adulthood and its association with peak bone mass. Am J Clin Nutr. 2017;106:276–83.
Mitchell DM, Ruppert K, Udupa N, Bassir F, Darakananda K, Solomon DH, et al. Temporal increases in 25-hydroxyvitamin D in midlife women: longitudinal results from the Study of Women’s Health Across the Nation. Clin Endocrinol. 2019;91:48–57.
Forde OH, Thelle DS. The Tromso heart study: risk factors for coronary heart disease related to the occurrence of myocardial infarction in first degree relatives. Am J Epidemiol. 1977;105:192–9.
Jacobsen BK, Eggen AE, Mathiesen EB, Wilsgaard T, Njolstad I. Cohort profile: the Tromso Study. Int J Epidemiol. 2012;41:961–7.
Jorde R, Sundsfjord J, Fitzgerald P, Bonaa KH. Serum calcium and cardiovascular risk factors and diseases: the Tromso study. Hypertension. 1999;34:484–90.
Jorde R, Grimnes G. Exploring the association between serum 25-hydroxyvitamin D and serum lipids-more than confounding? Eur J Clin Nutr. 2018;72:526–33.
Grimnes G, Almaas B, Eggen AE, Emaus N, Figenschau Y, Hopstock LA, et al. Effect of smoking on the serum levels of 25-hydroxyvitamin D depends on the assay employed. Eur J Endocrinol. 2010;163:339–48.
Sollid ST, Hutchinson MY, Fuskevåg OM, Figenschau Y, Joakimsen RM, Schirmer H, et al. No effect of high-dose vitamin D supplementation on glycemic status or cardiovascular risk factors in subjects with prediabetes. Diabetes Care. 2014;37:2123–31.
Pereira-Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obesity and vitamin D deficiency: a ≤systematic review and meta-analysis. Obes Rev. 2015;16:341–9.
Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035.
Engelman CD, Fingerlin TE, Langefeld CD, Hicks PJ, Rich SS, Wagenknecht LE, et al. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab. 2008;93:3381–8.
Drincic AT, Armas LAG, van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity. 2012;20:1444–8.
Hyppönen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85:860–8.
Acknowledgements
This article would not be possible without the work of the people engaged in the Tromsø survey and the Tromsø population taking part in the surveys year after year. We would also like to thank Yngve Figenschau at the Division of Diagnostic Services and Department of Clinical Medicine—UiT/UNN for assistance with the CV of the analyses included in the “Materials and method” section.
Funding
The study was supported by grants from the North Norway Regional Health Authorities (Grant number SFP1277-16) and UiT The Arctic University of Norway.
Author information
Authors and Affiliations
Contributions
RJ and EK were responsible for designing the protocol. JK was responsible for doing the analyses and drafting the manuscript. JK, EK, and RJ all participated in finalizing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The Tromsø Study is approved by the Regional Committee for Medical Research Ethics (REK) and this investigation is covered by this approval.
Informed consent
All included subjects signed a written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kubiak, J., Kamycheva, E. & Jorde, R. Tracking of serum 25-hydroxyvitamin D during 21 years. Eur J Clin Nutr 75, 1069–1076 (2021). https://doi.org/10.1038/s41430-020-00814-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-020-00814-0
- Springer Nature Limited
This article is cited by
-
The association between circulating 25-hydroxyvitamin D and pancreatic cancer: a systematic review and meta-analysis of observational studies
European Journal of Nutrition (2024)
-
25-Hydroxyvitamin D reference percentiles and the role of their determinants among European children and adolescents
European Journal of Clinical Nutrition (2022)
-
Longitudinal changes in vitamin D concentrations and the association with type 2 diabetes mellitus: the Tromsø Study
Acta Diabetologica (2022)