Abstract
Background/Objectives
Radiotherapy (RT) is a component of therapy for head and neck cancer (HNC) with a negative nutritional impact. Our aim was to compare an early versus a conventional nutritional intervention.
Subjects and methods
Retrospective study of HNC patients undergoing RT. Evolution before and after the establishment of a fast-track circuit was evaluated. A conventional group (CG) made up of patients submitted to the nutrition unit during RT after nutritional deterioration, was compared to an early group (EG) represented by patients included in a fast-track circuit, starting nutritional follow-up before the beginning of RT. Only patients with preserved oral intake were involved. Demographic, nutritional and clinical variables were analyzed. Data of hospitalizations and deaths were collected up to three months after RT.
Results
135 subjects constituted the EG and 39 the CG. At baseline, the prevalence of malnutrition was lower in the EG (31.9% vs 69.5%, p = 0.0001), as was the need for nutritional supplements (40% vs 79.5%, p = 0.0001) or nasogastric tube (0% vs 12.8%, p = 0.0001) in comparison to the CG. Three months after RT, there were less patients with oral nutritional support in the EG (79.1% vs 96.9%, p = 0.018), and the number of emergency visits (0.75 vs 1.1 episodes per patient, p = 0.021) and hospitalizations was also lower in this group (29% vs 59%, p = 0.044).
Conclusions
The fast-track approach made early intervention possible. Therefore, patients maintained a better nutritional status, needed less nutritional support and their evolution improved, with a significant decrease in hospitalizations.
Similar content being viewed by others
Introduction
Head and neck cancer (HNC) is a collective term for cancers originating in the oral and nasal cavities, pharynx, larynx, hypopharynx and paranasal sinus [1].
In HNC patients, the incidence of malnutrition at diagnosis has been reported to be between 20 and 60% [2,3,4,5]. This percentage increases during treatment, affecting more than 70%, especially those patients with concomitant radiotherapy, due to the appearance of several symptoms such as mucositis, xerostomia, dysgeusia, among others, which may limit oral intake, resulting in unintended weight loss during and after treatment [6,7,8].
The causes of malnutrition amongst HNC patients are considered to be multifactorial and include the anatomical location of the tumor, lifestyle factors (excessive alcohol intake and/or smoking) and tumor factors [7]. Side effects of cancer therapies, patient-related concerns (physical deterioration, personal habits, psychological aspects, etc.), issues regarding personal health care (absence of nutritional assessment, lack of knowledge to detect malnutrition, delay of nutritional treatment, etc.) or aspects related to healthcare authorities (such as the lack of multidisciplinary care units) also play a significant role in the appearance of malnutrition in HNC patients [9].
The appearance of malnutrition in this scenario may lead to a range of complications, poor quality of life, a reduced response to chemotherapy and/or radiotherapy and an increase in its toxicity, treatment interruptions and hospital readmission rates, which have been associated with poor clinical outcomes such as an increased mortality rate [10,11,12].
An early, intensive and individualized nutritional intervention during RT may be beneficial in terms of decreasing the impact of side effects, decreasing unintended weight loss, and improving dietary intake and quality of life, minimizing acute toxicities and treatment interruptions and enhancing survival. Thus, it is recommended that nutritional intervention takes place before treatment is started and continued during and after treatment. According to published nutritional management guidelines, nutritional support should be part of HNC management [3, 5, 13,14,15,16,17,18].
The aim of this study was to evaluate the nutritional and prognostic impact of an early nutritional intervention before starting RT (fast-track circuit) in comparison with the conventional approach (nutritional intervention during RT) in HNC patients undergoing radiotherapy.
Methods
A retrospective and observational study was performed of HNC patients (≥18 years) undergoing RT (with or without concomitant chemotherapy) and evaluated in the nutrition unit of the University Hospital Complex of Santiago de Compostela, in the northwest of Spain, from 2013 to 2017.
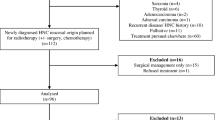
Prior to 2014, HNC patients were sent to the nutrition unit for evaluation when presenting deleterious effects of RT and a repercussion in nutritional status was evident. In 2014, a fast-track circuit was established to assess all HNC patients earlier, prior to RT, independently of their nutritional situation (Fig. 1).
Dietary counseling was carried out by a dietitian with frequent individualized visits in an attempt to maintain and/or improve patients’ energy and protein intake, adjusting diet during RT treatment and taking into account its side effects.
In patients who did not meet their calculated needs, nutritional support was prescribed according to usual clinical practice. The type and amount of this support were adjusted depending on the situation of the patient, their food intake and the presence of RT-related symptoms.
Ethical considerations
The present study was reviewed and approved by the Clinical Research Ethics Committee of Santiago, Spain (CEIC 2019/365).
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Inclusion and exclusion criteria
Two different groups of HNC patients constituted our study population. The conventional group (CG) included patients referred to the nutrition unit during RT after some degree of nutritional deterioration was detected, following the usual clinical practice prior to the establishment of the fast-track. The early group (EG) represents those patients included in the fast-track circuit that came to our unit before beginning RT by protocol. In both groups, only patients with preserved oral intake were involved. Patients with enteral nutritional support via nasogastric tubes or ostomies before RT were excluded.
Study variables
Demographic, nutritional and clinical variables were collected for both groups.
At baseline, age, gender, tumor site and stage, toxic habits, treatment and number of RT sessions before evaluation in the nutrition unit were recorded. The patients were asked about the presence of symptoms associated with HNC which could interfere with oral intake, such as anorexia or dysphagia.
Nutritional assessment included anthropometry (weight, height, body mass index, percentage of weight loss) and laboratory tests. Nutritional classification of patients was established following definitions from SENPE (Spanish Society of Clinical Nutrition and Metabolism) and SEDOM (Spanish Society of Medical Documentation) [19]. In every visit with the dietitian, height and weight were measured using standard protocols on a calibrated scale. The nutritional intervention carried out was also recorded.
Blood tests included levels of albumin, prealbumin, transferrin and retinol binding protein (PBR). These were measured following the usual practice of our laboratory.
We compared the patients’ evolution before and after the establishment of the fast-track circuit. Anthropometric variables, nutritional intervention, data on emergency department visits, unplanned hospitalizations and deaths were collected up to 3 months after the end of RT.
Statistical analysis
Statistical analysis was performed using Statistical Package for Social Sciences version 22.0 (SPSS Inc., Chicago, IL, USA). The normal distribution of quantitative variables was examined by the Kolmogorov-Smirnov test. Variables matching normal distribution were presented in terms of mean and standard deviation (SD). Categorical variables were expressed as percentages. Quantitative variables with normal distribution were compared using the Student’s t-test. Categorical variables were compared using the Chi-squared test. A p value lower than 0.05 was considered statistically significant.
Results
A total of 174 patients were included. 135 subjects constituted the EG and 39 the CG.
Patients in the CG had received an average of 10.9 ± 9.1 radiotherapy sessions before being referred to the nutrition unit. On the contrary, patients in the EG had not started radiotherapy (following the protocol of the fast-track circuit).
In both groups, almost all subjects were treated with chemoradiotherapy (74.8% in the EG and 74.4% in the CG). In relation to toxic habits, 124 (71%) patients had a smoking habit and/or abused alcohol at diagnosis (104 in the EG and 20 in the CG).
The baseline characteristics separated by groups are summarized in Table 1.
Significant differences were found between both groups in the mean BMI at baseline, being higher in the EG. The prevalence of malnutrition at the onset was significantly lower in the EG. Furthermore, statistically significant differences were also observed in the percentage of patients who needed oral nutritional support and a nasogastric tube from the first visit, being lower in the EG.
3-month follow-up after ending radiotherapy
The summary of the results obtained 3 months after the end of radiotherapy treatment is shown in Table 2.
During radiotherapy treatment, practically all of the subjects in both groups needed some type of nutritional support. However, 3 months after finishing the oncological treatment, a significantly lower percentage of patients who still needed ONS and NGT in the EG compared to the CG was observed (Table 2).
Weight and BMI decreased in a similar way in the two groups of patients over the course of the treatment and 3 months later. No significant differences were found between both groups in the final mean BMI.
The number of emergency department visits and unplanned hospitalizations was statistically lower in the EG. There were no significant differences between both groups in the death data. However, there was a trend toward lower mortality in the EG.
Discussion
This study has shown that early nutritional intervention via a protocolized fast-track circuit in HNC patients before undergoing radiotherapy/chemoradiotherapy treatment was associated with a reduced need for oral and enteral nutritional support, a lower number of emergency assistances and hospitalizations and a tendency towards a lower death rate.
At baseline, our CG patients presented a higher prevalence of malnutrition compared to earlier studies [3, 4, 6, 8, 11, 12, 16, 20, 21] and, in consequence, the percentage of patients requiring oral supplements from the beginning of the follow-up was higher than those previously reported [4, 6, 12, 13, 22]. Regarding the EG, the percentage of patients who were underweight before starting RT was lower than in the CG (3.6% vs 7.9%) and in comparison to patients in other studies [3, 12, 20, 23]. Likewise, the prevalence of malnutrition at baseline in this group compared to the CG was also lower (31.9% vs 69.5%). Therefore, the establishment of the fast-track circuit made it possible to begin nutritional monitoring of the patients before there was a marked nutritional deterioration.
During treatment, deterioration in nutritional status was evident in our sample, independently of the moment of the intervention. Most patients experienced weight loss during the follow-up period, with no differences between both groups. In this sense, adherence to dietary recommendations and nutritional treatment is usually complicated due to the acute toxicity of chemoradiotherapy [4, 6, 7, 12, 24]. Previous studies with populations of similar characteristics have presented similar results, with weight loss ranging widely between 5 and 33% during radiotherapy [6, 12, 25]. A sharp decline in body weight was also found, fundamentally in patients at an advanced stage/age, with a high-risk tumor site, and with the use of concomitant chemotherapy [12, 20]. Thus, weight loss and the nutritional impact of RT seem inevitable, but it is clear that beginning the treatment with a better-conserved nutritional state is of the utmost importance. In this way, it is important to note that the EG subjects were referred to the nutrition unit before having started the treatment and the deleterious effects of RT were not yet evident, which is probably why the final outcomes in this group were better.
Regarding the number of unplanned admissions, the establishment of a fast-track circuit was related with a significant decrease in unplanned hospitalizations, as previously reported [13]. Consequently, the CG showed a higher percentage of hospitalizations (59%) compared to the data reported by other authors [26, 27]. Other studies not focused on the nutritional treatment of these patients, showed a higher incidence of admissions in subjects with HNC treated with intensity-modulated radiation therapy or chemoradiotherapy compared to our EG [28,29,30,31], which supports the importance of an early nutritional assessment as an integral component of HNC patient care.
Although no significant differences were found in the number of deaths between the two analyzed groups, a trend towards a lower percentage of deaths was observed in the group with early nutritional intervention. Despite the presence of conflicting evidence [32], several studies have reported an association between malnutrition and morbimortality in HNC patients, emphasizing the importance of the identification and optimal treatment of malnutrition before, during and after cancer treatment [27, 33, 34].
The approach proposed in this work is in line with the suggestions of international guidelines for avoiding treatment-related weight loss and unplanned interruptions in RT [35]. In order to achieve this goal, the best strategy is multidisciplinary supportive care, which enables proper assessment of nutritional status and requirements, dietary counseling and monitoring of its compliance, as well as the timely management of symptoms [8].
This work has the limitation of being a retrospective study, which has led to a greater degree of difficulty in collecting data from the patients in the CG, resulting in a relatively small sample size in this group. In addition, it may seem that the CG is highly selected. However, the two groups belong to the same cohort of patients, and the percentage of subjects received in our clinic per year is almost the same for each group. Only the moment of referral varies: before starting RT and independently of their nutritional status in the case of the EG, or after starting RT when repercussion in nutritional status was evident in the case of the CG. On the other hand, there are some strengths, such as the fact of having two comparison groups with follow-up not only during the treatment but also up to 3 months after its finalization. Moreover, the population sample analyzed had very similar onset characteristics to that of previous studies. This leads us to think that our results not only have internal validity in our environment but that they could also be applicable to other populations with similar characteristics.
In conclusion, the establishment of a fast-track circuit allowed for early intervention, with which the patient maintained a better nutritional status. Thus, this early approach reduced the need for nutritional support, improving patients’ outcomes, with a significant decrease in hospitalizations and a tendency towards a lower death rate.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Van Bokhorst-de van der Schueren MA, van Leeuwen PA, Sauerwein HP, Kuik DJ, Snow GB, Quak JJ. Assessment of malnutrition parameters in head and neck cancer and their relation to postoperative complications. Head Neck. 1997;19:419–25.
Van den Berg MG, Rasmussen-Conrad EL, Wei KH, Lintz-Luidens H, Kaanders JH, Merkx MAW. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. Br J Nutr. 2010;104:872–7.
Arribas L, Hurtós L, Taberna M, Peiró I, Vilajosana E, Lozano A, et al. Nutritional changes in patients with locally advanced head and neck cancer during treatment. Oral Oncol. 2017;71:67–74.
Isenring EA, Capra S, Bauer JD. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. Br J Cancer. 2004;91:447–52.
Citak E, Tulek Z, Uzel O. Nutritional status in patients with head and neck cancer undergoing radiotherapy: a longitudinal study. Support Care Cancer. 2019;27:239–47.
Lees J. Incidence of weight loss in head and neck cancer patients on commencing radiotherapy treatment at a regional oncology centre. Eur J Cancer Care. 1999;8:133–6.
Ravasco P, Monteiro-Grillo I, Marques Vidal P, Camilo ME. Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck. 2005;27:659–68.
Ocón Bretón MJ, Luengo Pérez LM, Virizuela JA, Álvarez Hernández J, Jiménez, Fonseca P, et al. Nutritional support and parenteral nutrition in cancer patients: an expert consensus report. Endocrinol Diabetes Nutr. 2018;65:17–23.
Talwar BP. Head and neck cancer. In: Shaw C, editor. Nutrition and Cancer. Oxford: Wiley Blackwell Science Ltd, 2010; 188–220.
Valentini V, Marazzi F, Bossola M, Miccichè F, Nardone L, Balducci M, et al. Nutritional counselling and oral nutritional supplements in head and neck cancer patients undergoing chemoradiotherapy. J Hum Nutr Diet. 2012;25:201–8.
Jeffery E, Sherriff J, Langdon C. A clinical audit of the nutritional status and need for nutrition support amongst head and neck cancer patients treated with radiotherapy. Australas Med J. 2012;5:8–13.
Alhambra Expósito MR, Herrera-Martínez AD, Manzano García G, Espinosa Calvo M, Bueno Serrano CM, Gálvez Moreno MA. Early nutrition support therapy in patients with head-neck cancer. Nutr Hosp. 2018;35:505–10.
Licitra L, Keilholz U, Tahara M, Lin JC, Chomette P, Ceruse P, et al. Evaluation of the benefit and use of multidisciplinary teams in the treatment of head and neck cancer. Oral Oncol. 2016;59:73–79.
Gorenc M, Kozjek NR, Strojan P. Malnutrition and cachexia in patients with head and neck cancer treated with (chemo)radiotherapy. Rep Pr Oncol Radiother. 2015;20:249–58.
Langius JA, Bakker S, Rietveld DH, Kruizenga HM, Langendijk JA, Weijs PJM, et al. Critical weight loss is a major prognostic indicator for disease-specific survival in patients with head and neck cancer receiving radiotherapy. Br J Cancer. 2013;109:1093–9.
Talwar B, Donnelly R, Skelly R, Donaldson M. Nutritional management in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130:S32–S40.
Findlay M, Bauer J, Brown T, Committee HaNGS. Evidence-based practice guidelines for the nutritional management of adult patients with head and neck cancer. Sydney: Cancer Council Australia, 2014.
Álvarez J, Del Río J, Planas M, García Peris P, García de Lorenzo A, Calvo V, et al. SENPE-SEDOM documento on coding of hospital hyponutrition. Nutr Hosp. 2008;23:536–40.
Van den Berg MG, Rasmussen-Conrad EL, Gwasara GM, Krabbe PF, Naber AH, Merkx MA. A prospective study on weight loss and energy intake in patients with head and neck cancer, during diagnosis, treatment and revalidation. Clin Nutr. 2006;25:765–72.
Kang WX, Li W, Huang SG, Dang Y, Gao H. Effects of nutritional intervention in head and neck cancer patients undergoing radiotherapy: a prospective randomized clinical trial. Mol Clin Oncol. 2016;5:279–82.
Silander E, Jacobsson I, Bertéus-Forslund H, Hammerlid E. Energy intake and sources of nutritional support in patients with head and neck cancer-a randomised longitudinal study. Eur J Clin Nutr. 2013;67:47–52.
Kubrak C, Olson K, Jha N, Jensen L, McCargar L, Seikaly H, et al. Nutrition impact symptoms: key determinants of reduced dietary intake, weight loss, and reduced functional capacity of patients with head and neck cancer before treatment. Head Neck. 2010;32:290–300.
Paccagnella A, Morello M, Da Mosto MC, Baruffi C, Marcon ML, Gava A, et al. Early nutritional intervention improves treatment tolerance and outcomes in head and neck cancer patients undergoing concurrent chemoradiotherapy. Support Care Cancer. 2010;18:837–45.
Beaver ME, Matheny KE, Roberts DB, Myers JN. Predictors of weight loss during radiation therapy. Otolaryngol Head Neck Surg. 2001;125:645–8.
Leistra E, Eerenstein SE, van Aken LH, Jansen F, de van der Schueren MA, Twisk JW, et al. Effect of early individualized dietary counseling on weight loss, complications, and length of hospital stay in patients with head and neck cancer: a comparative study. Nutr Cancer. 2015;67:1093–103.
Capuano G, Grosso A, Gentile PC, Battista M, Bianciardi F, Di Palma A, et al. Influence of weight loss on outcomes in patients with head and neck cancer undergoingconcomitant chemoradiotherapy. Head Neck. 2008;30:503–8.
Eskander A, Krzyzanowska MK, Fischer HD, Liu N, Austin PC, Irish JC, et al. Emergency department visits and unplanned hospitalizations in the treatment period for head and neck cancer patients treated with curative intent: a population-based analysis. Oral Oncol. 2018;83:107–14.
Ling DC, Kabolizadeh P, Heron DE, Ohr JP, Wang H, Johnson J, et al. Incidence of hospitalization in patients with head and neck cancer treated with intensity-modulated radiation therapy. Head Neck. 2015;37:1750–5.
Gupta S, Kong W, Booth CM, Mackillop WJ. Impact of concomitant chemotherapy on outcomes of radiation therapy for head-and-neck cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2014;88:115–21.
Moore ZR, Pham NL, Shah JL, Nedzi L, Sumer BD, Andrew T. et al. Risk of unplanned hospital encounters in patients treated with radiotherapy for head and neck squamous cell carcinoma. J Pain Symptom Manag. 2019;57:738–45.
Ehrsson YT, Langius-Eklöf A, Laurell G. Nutritional surveillance and weight loss in head and neck cancer patients. Support Care Cancer. 2012;20:757–65.
Datema FR, Ferrier MB, Baatenburg, de Jong RJ. Impact of severe malnutrition on short-term mortality and overall survival in head and neck cancer. Oral Oncol. 2011;47:910–4.
Van Bokhorst-de van der Schuer, van Leeuwen PA, Kuik DJ, Klop WM, Sauerwein HP, Snow GB, et al. The impact of nutritional status on the prognoses of patients with advanced head and neck cancer. Cancer. 1999;86:519–27.
Arends J, Bodoky G, Bozzetti F, Fearon K, Muscaritoli M, Selga G. ESPEN (European Society for Parenteral and Enteral Nutrition) et al. ESPEN guidelines on enteral nutrition: non-surgical oncology. Clin Nutr. 2006;25:245–59.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
The authors’ contributions were as follows: MGR and RVT were responsible for designing the research; MGR, RVT, MASD and SFF were responsible for conducting the research; MGR, RVT, AFP and MPC were responsible for extracting and analyzing the data and performing the statistical analysis; MGR, RVT and AFP were responsible for interpreting the results. MGR, RVT and AFP were responsible for writing the manuscript; ACB and MAMO provided feedback on the research. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
González-Rodríguez, M., Villar-Taibo, R., Fernández-Pombo, A. et al. Early versus conventional nutritional intervention in head and neck cancer patients before radiotherapy: benefits of a fast-track circuit. Eur J Clin Nutr 75, 748–753 (2021). https://doi.org/10.1038/s41430-020-00786-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-020-00786-1
- Springer Nature Limited