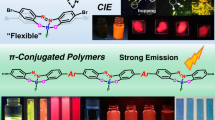
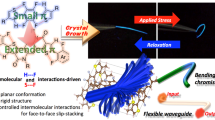
Abstract
Flexible molecules are unfavorable for designing luminescent dyes because their excitation states rapidly decay through molecular motions. We recently found that some flexible boron complexes, which potentially show a larger degree of structural relaxation in the excited state, and their polymers exhibit unique optical properties with high environmental sensitivity, such as aggregation-induced emission and luminochromism triggered by external stimuli, upon the addition of structural restrictions. Moreover, these optical properties were drastically changed by modulating the connecting points in the polymers. In this review, recent progress in the development of luminescent polymer films with stimuli responsiveness is illustrated. In particular, the influence of the alteration of connecting points on luminescent behaviors is explained. Polymerization is a versatile strategy not only for transforming a class of nonemissive molecules into luminescent dyes but also for precisely regulating the optical properties of film materials; the resulting materials are promising for application as scaffolds for advanced chemical sensors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
We recently proposed a new concept for material design based on an “element block,” which is a minimum functional unit containing heteroatoms [1, 2]. The creation of a wide variety of materials with unique functions originating from heteroatoms by the connection and assembly of even a single type of element block can be expected, and the resulting element-block polymers are likely to show not only superior properties to those of conventional organic materials but also diverse functions originating from the combination of element blocks. Because of the positive effects of boron complexation mentioned above, we also explored luminescent boron element blocks and found unique optical and electronic properties in conjugated polymers consisting of boron element blocks [3].
To obtain intense emission from organic materials, including polymers, the improvement of molecular rigidity at the chromophore unit is essential for suppressing energy-consuming molecular motions in the excited state (Scheme 1). Flexible molecules generally show poor luminescence in the absence of solid matrices. By boron complexation, molecular rigidity and planarity at local sites can be greatly improved [4]. These structural features are favorable for extending robust conjugation systems. Therefore, various types of luminescent dyes based on boron complexes have been developed [5]. In addition, useful electronic properties, such as carrier-transporting ability, are often observed in these rigid and planar structures. In particular, since these luminescent and electronic properties are feasible for fabricating highly efficient organic light-emitting diodes (OLEDs), boron complexes with π-conjugated ligands are known to be a promising platform for constructing advanced optoelectronic devices.
For example, boron dipyrromethene (BODIPY) is widely known to be a representative luminescent boron complex. By extending π-conjugation, deep red or near-infrared emission can be obtained [6, 7]. Owing to the intense emission properties derived from the rigid and planar dipyrromethene ligand reinforced by boron complexation, a series of light-absorbing and luminescent polymers can be obtained [8,9,10,11]. To avoid aggregation-caused quenching (ACQ), which is a critical annihilation process and is commonly observed in the condensed state of organic luminescent dyes, BODIPY-containing polymers were loaded into organic‒inorganic hybrid matrices [12]. Consequently, highly stable films possessing preserved emission properties were obtained [13]. As another example, by expanding the π-conjugated system of the dipyrromethene ligand through polymer main chains, longer-wavelength emission was observed (Fig. 1a) [14,15,16]. Moreover, it was shown that these polymers exhibited superior electron-carrier ability in the amorphous aluminum complexes conventionally utilized in light-emitting diodes (Fig. 1b) [17]. Thus, boron element blocks are potential units for constructing advanced functional polymers.
a Near-infrared emission and b superior electron-carrier ability of a BODIPY-containing polymer. Adapted with permission from [17]. Copyright 2014 American Chemical Society
To prepare the next generation of OLEDs, many studies have devoted their efforts to developing film-type optical sensors. If the optical properties can be changed by external stimuli, these films could be directly used to construct sensing materials. Owing to the various advantages of organic devices, such as lightness, flexibility, and thinness, these film sensors could be applied to develop advanced sensing tools, such as wearable devices and ultratiny sensors. To realize these future sensing technologies, the development of stimuli-responsive luminescent films is needed. For example, we manufactured elastic hybrid materials consisting of polyhedral oligomeric silsesquioxane (POSS)-capped polyurethane and polyfluorene [18]. The thermally stable hybrid films showed dual-emission properties involving intrinsic fluorescence from polyfluorene and excimer emission. Interestingly, emission color changes were detected upon stretching the film samples, which was attributable to the altered intensity ratio between fluorescence and excimer emission. When polyfluorene was replaced with doped poly(3-hexylthiophene), the conductivity could be varied by stretching [19]. Interchain interactions and polymer morphology, followed by the electronic properties of main-chain conjugation, were drastically changed by stretching the sample films. Hence, these responses were observed upon applying mechanical forces. These materials are promising paint-type sensors for detecting applied forces as well as distortions of product surfaces. We also found that dye-loaded POSS networks with water dispersibility can exhibit luminescent color changes in the presence of plastic particles [20]. Environmental changes around the dyes will be induced when the dyes are adsorbed onto particle surfaces. Furthermore, the size of the plastic particles could be discriminated by the degree of luminescent property changes. This system could be applied to evaluate water contamination by plastic particles through simple protocols. In these examples, luminescent materials were loaded onto physically flexible matrices. In contrast, we herein explain stimuli-responsive luminescent films based on “flexible” boron complexes [21,22,23,24,25,26], which potentially show a large degree of structural relaxation in the excited state (Scheme 1 and Fig. 2). As easily predicted, flexible boron complexes generally show extremely poor luminescence, while we recently found that their flexibility can be utilized to express unique stimuli responsiveness, especially in solid form, toward slight environmental changes and tiny external stimuli. Subsequently, a series of film-type sensors based on polymers containing flexible boron complexes were obtained. Therefore, we regard this class of flexible boron complexes as stimuli-responsive element blocks with luminescent properties for the fabrication of advanced sensors.
We designed luminescent films with stimuli responsiveness based on main-chain-type conjugated polymers involving flexible boron complexes. In particular, we discovered that various environment-sensitive optical properties, such as aggregation-induced emission (AIE), can be induced by changing the connecting points in the polymer main chains (Scheme 1). By mainly referring to these studies, we will explain the results and basic chemistry of these materials.
First, we will explain the discovery of AIE-active boron ketoiminate, which is the first example of a flexible boron complex in our research (Fig. 2). Commercial boron complexes show intense emission only in solution, while some boron ketoiminates exhibit enhanced emission upon aggregation. Based on these molecules, we constructed polymers, and it was demonstrated that the optical properties changed between ACQ and AIE when the connecting points were changed. This result is a typical example demonstrating the tuning of material properties by simply altering the connecting motif with a single type of element block. To preserve the solid-state luminescent properties to improve the film emission efficiency, we designed a fused ketoiminate ligand, and the polymers showed relatively high efficiency. In particular, we also found that environmental dependency was enhanced by the introduction of the fused structure into boron ketoiminate. The resulting polymers with linear and zigzag structures showed thermochromic and mechanochromic luminescence, respectively. Furthermore, to improve flexibility, we designed boron diiminate in which weaker bonds were introduced into the boron complex moiety. The AIE properties of the polymers consisting of boron diiminate were clearly changed by altering the connecting points. Finally, we will explain the establishment from scratch of molecular designs that enable the switching of optical properties in polymer films. We theoretically explored flexible boron complexes and designed polymers with variable connecting points. By suppressing or releasing molecular motions that were predicted by quantum chemical calculations, the optical properties could be controlled to switch between ACQ and multistate emission. The further explanations on mechanisms in these materials are illustrated as follows.
Boron ketoiminate
Boron diketonate is a typical boron complex with luminescent properties [3]. By modulating π-conjugation, the luminescent properties can be tuned [27]. For instance, Fraser et al. synthesized a monoiodo complex and observed dual-emission properties involving intrinsic fluorescence and phosphorescence caused by the heavy-atom effect of iodine [28]. Only phosphorescence quenching followed by an alteration in the intensity ratio toward oxygen-resistant fluorescence can be induced under aerobic conditions. As a result, oxygen levels in tumor regions could be assessed by intensity ratio changes. Moreover, other types of boron ketoiminates show mechanochromic luminescent behaviors [29,30,31,32,33]. When mechanical forces are applied to crystal samples, the molecular distribution is drastically altered by morphological changes from crystal to amorphous. Correspondingly, luminescent chromism can be induced.
As introduced above, although some complexes show emission in solid form, most complexes commonly suffer from ACQ. We prepared a main-chain-type ligand polymer with a diketone structure and investigated the influence of boron complexation through polymer reactions on the optical properties (Fig. 3) [34]. Accordingly, it was clearly shown that boron complexation contributes to enhancing the emission intensity by forming robust main-chain conjugation. In the solution state, intense emission bands with higher quantum yields were obtained in the longer-wavelength region after boron complexation. However, critical ACQ was observed in the film samples.
To construct robust conjugation involving boron, ketoiminate polymers were designed and synthesized (Fig. 4) [35]. Intense green emission from the ligand polymer was observed only in the diluted solution, and ACQ was observed in the film. Moreover, red emission was induced by boron complexation, indicating that the conjugation system was elongated through the polymer main chain. Interestingly, the boron-containing polymer showed higher emission efficiency in film than in solution. This is typical AIE behavior.
The idea of AIE was first reported by Tang et al. with respect to modified silole compounds, and tetraphenylethene was also found to be an AIE-active molecule [36, 37]. Owing to their versatile properties, such as environment-switchable luminescent properties, AIE-active materials have been applied in material science as well as biotechnology [38,39,40,41,42,43]. It has been suggested that in these AIE-active dyes, molecular motions in the excited state are responsible for emission annihilation in the solution state. To understand the mechanism of the AIE properties of boron ketoiminate polymers, we prepared boron complexes and examined their optical properties (Fig. 5) [44]. ACQ and AIE were observed in boron diketonate and ketoiminates, respectively. Moreover, under high-viscosity and frozen conditions where molecular motions are critically restricted, significant emission enhancements were observed in both states, indicating that molecular motions in the excited state play an important role in emission annihilation, similar to previous AIE dyes. From a series of mechanistic studies including quantum calculations, a plausible mechanism was proposed. By replacing one of the oxygen atoms with nitrogen, the molecular rigidity is lowered because of the weaker bond strength of B‒N than that of B‒O [45]. Therefore, emission annihilation can be induced in solution due to molecular motions. In the solid-state, nonradiation paths caused by these molecular motions will be suppressed owing to structural restriction. Furthermore, according to quantum calculations, localization of the highest occupied molecular orbitals at the oxygen side in boron ketoiminate was proposed, implying that intermolecular interactions can be disturbed even in the condensed state. As a result, luminescence can be preserved in the solid. In conclusion, AIE properties are obtained by combining emission annihilation in solution and solid-state luminescence.
Different types of polymers containing boron ketoiminate were prepared by changing the connecting points (Fig. 6) [46]. According to optical measurements, solid-state luminescence was observed, although ACQ was also detected. These data suggest that molecular motions in phenyl groups at the base of ketones play a critical role in the AIE properties of boron ketoiminates. Switching between AIE and ACQ was demonstrated upon changing the connecting points in polymer main chains.
To avoid ACQ, one of the conventional strategies is modification with bulky substituents [47,48,49,50,51,52,53,54,55,56,57,58]. Since intermolecular interactions are disturbed in the condensed state, intrinsic emission can be preserved. Another strategy is to employ transparent matrices. When luminescent dyes are loaded onto polymers or organic‒inorganic hybrids, chromophores will be located in an environment similar to the solution state [12]. As a result, solid-state emission can be maintained. However, these strategies are unfavorable for obtaining stimuli-responsive materials. As is often the case with the above materials, environmental sensitivity is reduced. Basically, in these strategies, chromophores tend to be isolated from any interactions, resulting in insensitivity to environmental changes and external stimuli. Hence, it is still challenging to simultaneously realize solid-state emission and environmental responsiveness. In contrast, it was found that the solid-state luminescent properties of flexible boron complexes had environmental sensitivity [21]. Therefore, luminochromic behaviors were observed upon changing the molecular morphology in aggregation as well as in the solid state.
For instance, boron ketoiminate was introduced into a hydrogel matrix, and the optical properties were monitored by the swelling and shrinking (Fig. 7) [59]. As we expected, AIE was observed in the modified gel. Interestingly, we found that the AIE color varied upon soaking the hydrogel in organic solvents to shrink the hydrogel. It was proposed that aggregation-induced blueshift emission is induced in relatively polar organic solvents by the formation of tight aggregates. Based on these color changes upon shrinking, protein sensing was demonstrated. In the presence of proteins, blueshifted emission was obtained through adsorption followed by aggregation. Unique sensing materials can be obtained owing to the environmental sensitivity of boron ketoiminate.
Schematic illustration of the aggregation-induced blueshifted emission of a boron ketoiminate-modified hydrogel. Reprinted with permission from [57]. Copyright 2016 Wiley-VCH Verlag GmbH & Co. KGaA
Fused boron ketoiminate
Flexible boron complexes with luminochromic properties show very low emission efficiency. For example, most of the emission quantum yields of mechanochromic luminescent molecules involving the boron ketoiminate structure remained within several percentage points before and after mechanical treatment [60,61,62]. To enhance the environmental responsiveness and emission efficiency of boron ketoiminate, a complex with a fused ligand was designed (Fig. 8) [63]. By expanding the π-conjugated system and anchoring the boron complex moiety, it was expected that solid-state emission in the AIE behavior could be preserved in any state without critical loss of environmental responsiveness. A series of fused boron ketoiminates were synthesized, and their optical properties were monitored in many phases. Accordingly, constant emission efficiencies were observed in the solution, crystal and amorphous states from each complex. In addition, peak shifts in emission bands were observed after applying mechanical forces to the crystal powders, indicating that mechanochromic luminescent properties can be realized with good emission efficiencies.
By utilizing the fused complexes as monomers, polymers were obtained (Fig. 9) [64]. The obtained polymers exhibited intense emission in both the solution and film states. Interestingly, we found that one of the linear polymers presented thermochromic luminescence in solution. The emission spectrum of the polymer solution in chloroform showed two emission bands at ~530 and 580 nm below 30 °C, while upon heating the polymer solution, the emission band at ~580 nm drastically decreased and almost disappeared at 50 °C. Only the emission band at ~530 nm was detected after heating. Because of these optical changes, thermochromic luminescence was obtained from the solution sample containing the linear-shaped polymer. From a series of mechanistic analyses, it was shown that chain assembly was responsible for the luminescent color changes.
Chemical structures, (a) thermochromic luminescence in solution and (b) mechanochromic luminescence of fused boron ketoiminate polymers. Reproduced from [62] with permission from The Royal Society of Chemistry
Mechanochromic luminescent properties were observed in a zigzag-type polymer in the solid state (Fig. 9) [64]. The pristine powder sample exhibited an orange luminescent color, while yellow emission was induced by grinding. From differential scanning calorimetry, the melting temperature of the pristine sample disappeared after grinding. This result implied that the polymer chain assembly could be collapsed by applying mechanical forces. This morphological change will provide environmental alteration, followed by different luminescent colors. These data involving thermochromic luminescent behaviors indicate that the expansion of π-conjugated systems should contribute to enhancing not only emission efficiency but also environmental sensitivity. As a result, luminochromism originating from morphological changes can be induced.
Boron diiminate
By introducing a B‒N bond, flexibility was improved, and subsequently, AIE and stimuli-responsive luminochromism could be induced. Therefore, it can be expected that higher environmental sensitivity, followed by stimuli responsiveness, will be obtained if molecular flexibility is enhanced. Based on this idea, boron diiminates in which another oxygen atom in boron ketoiminate was also replaced with nitrogen were designed and synthesized (Fig. 10) [65, 66]. It was found that boron diiminates with various substituent groups exhibit CIE as well as AIE with variable luminescent color in the crystal state. It is likely that more structural restriction is necessary to suppress emission annihilation by molecular motions. Similar to boron ketoiminates, main-chain-type polymers were synthesized, and it was observed that the emission intensity and color could be tuned by the substituent effects (Fig. 11a) [67]. By modifying the side-chain substituents to tune the properties of the materials, film-type sensors were developed. For instance, when the film of a dimethylamine-substituted polymer was fumed to acid vapor, red emission turned yellow. Subsequently, by fuming basic vapor, the emission color was recovered to red. This result occurred because electronic conjugation will be drastically changed by acidification at the side chains of dimethylamine. Thus, such vapochromic luminescent behaviors can be detected. As another example, a methyl sulfide-substituted polymer was synthesized, and cast films were prepared (Fig. 11b) [68]. The pristine sample showed slight yellow emission, whereas the emission intensity increased upon soaking the film in an aqueous solution containing hydrogen peroxide, which is a reactive oxygen species and plays a crucial role in oxidative damage in cells. By oxidation at the methyl sulfide group, methyl sulfoxide was generated, according to the results from 1H NMR analyses. Oxidation changed the electronic properties of the substituent from electron donating to electron accepting. Finally, the emission intensity was enhanced. A film-type sensor for biosignificant molecules can be readily constructed based on the substituent effects.
Further enhancement of molecular flexibility was also accomplished by replacing boron with a heavier element. For example, we prepared diamine complexes with aluminum and gallium, which belong to the same group as boron in the periodic table [69, 70]. Both complexes show CIE, and gallium diiminate exhibits vapochromic luminescent properties. It should be mentioned that the degree of luminescent chromism was critically dependent not on the chemical components of the captured volatile organic compounds but on their radius of gyration. It was proposed that crystal‒crystal transitions could readily occur owing to the lower packing density of gallium diiminate than that of boron diiminate. Gallium diiminate-containing polymers were also prepared, and AIE was observed [71]. These results also suggest that the enhancement of molecular flexibility obtained by introducing a heavier element will also improve environmental sensitivity.
To examine the influence of the alteration of connecting points in detail, a series of alternating copolymers composed of boron diiminate were prepared (Fig. 12) [72]. All polymers had AIE properties, and various colors from green to orange were observed depending on the type of comonomer, such as fluorene and bithiophene, and the connecting points were observed in the film. To clarify the mechanism of such clear color changes, several optical measurements were performed. Accordingly, it was shown that the charge transfer characteristics between boron diiminate and comonomer units were critically changed by changing the connecting points. Theoretical investigation suggested that boron diiminate will be a strong electron acceptor when the comonomer is connected to one or both of the phenyl groups on the nitrogen atoms. On the other hand, weak electron-donating properties were expressed in polymers where the comonomers were connected at the phenyl groups on the carbon atoms in the boron-containing six-membered ring. It was demonstrated that the luminescent properties can be tuned simply by changing the connection motif with the flexible boron complex.
Theoretical predictions
The final topic concerns the establishment from scratch of material designs for stimuli-responsive luminescent polymers composed of flexible boron complexes. As mentioned above, flexibility in this manuscript means the degree of structural transformation caused by relaxation in the excited molecule [45]. We presumed that the degree of structural relaxation can be estimated by comparing the optimized structures in the ground and excited states. Thus, flexible boron complexes can be designed via a theoretical approach. Based on this idea, the pyridinoiminate complex BPI was obtained (Fig. 13) [73, 74]. A large difference between the optimized structures in the ground and excited states, obtained by calculations with density functional theory (DFT) and time-dependent DFT methods, respectively, was proposed [73]. Therefore, it was expected that boron pyridinoiminate will exhibit AIE behavior. The fused molecule FBPI, which shows minimal structural transformation because of the anchoring effect of the fused structure, was also prepared as a comparison. In summary, as we expected, BPI showed AIE, while FBPI exhibited ACQ [73]. Moreover, in the optical spectra, a larger degree of Stokes shift was observed only in BPI, suggesting that structural relaxation occurred. These data indicate that flexible boron complexes can be predicted from scratch through the quantum chemical approach. This result raises another significant issue. According to the comparison data, the movable points in the excited state can be estimated. Therefore, we assumed that sensitivity toward environmental factors could be controlled by connecting at movable or immovable points. Similar to the results for polymers with a fused boron ketoiminate structure, it was presumed that polymers composed of BPI units might show multistate emission properties.
a Chemical structures of BPI and FBPI and plausible models of the difference in the degree of structural relaxation in the excited states. b Changes in intensity ratios induced by aggregation formation in THF solutions with variable water concentrations and (c) the appearance of the solutions under UV irradiation (365 nm). Reproduced from [71] with permission from The Royal Society of Chemistry
To evaluate the validity of this idea, polymers were prepared with BPI or FBPI (Fig. 14) [75]. From optical measurements, it was observed that the FBPI polymer had intense luminescent properties only in the solution state and showed ACQ. In contrast, the BPI polymer exhibited multistate emission. Similar emission intensities were obtained in both the solution and film states. By connecting at a potential movable point in the excited state predicted by quantum calculations, the optical properties could be switched between ACQ and multistate luminescence. This strategy is a valid strategy for designing ACQ-resistant luminescent polymers.
Conclusion
The emission intensity and color of small-molecule dyes with solid-state luminescent properties occasionally depend on the molecular distribution in the solid. In these cases, luminochromism can be correspondingly induced by morphological changes. On the other hand, when these stimuli-responsive luminescent molecules are polymerized, the environmental responsiveness is often reduced, especially in films. Due to ACQ, the emission intensity in the film state tends to be critically lowered. Furthermore, since polymer films generally form amorphous structures, only monotonous optical properties can be obtained. Even though crystallization can be partially induced, only a minimal influence on the optical properties will be obtained. Furthermore, the mobility of flexible boron complexes in the excited state is restricted by incorporation into polymer main chains. This kind of “frustrated state” is the origin of environmental sensitivity, followed by stimuli responsiveness in the form of luminochromism. From a conventional standpoint, this class of flexible complexes is not a potential target for obtaining luminescent dyes. In contrast, it is demonstrated in this manuscript that material designs based on flexible element blocks are very likely to yield highly sensitive stimuli-responsive polymer films. Hence, it can be said that not only the exploration of new flexible element blocks but also clarification of structural relaxation in the excited state could be key areas of study for making a breakthrough in the development of a new series of advanced luminescent devices and sensors.
References
Chujo Y, Tanaka K. New polymeric materials based on element-blocks. Bull Chem Soc Jpn. 2015;88:633–43.
Gon M, Sato K, Tanaka K, Chujo Y. Controllable intramolecular interaction of 3D arranged π-conjugated luminophores based on a POSS scaffold, leading to highly thermostable and emissive materials. RSC Adv. 2016;6:78652–60.
Tanaka K, Chujo Y. Recent progress of optical functional nanomaterials based on organoboron complexes with β-diketonate, ketoiminate and diiminate. NPG Asia Mater. 2015;7:e223.
Buyuktemiz M, Duman S, Dede Y. Luminescence of BODIPY and dipyrrin: an MCSCF comparison of excited states. J Phys Chem A. 2013;117:1665–9.
Tanaka K, Chujo Y. Advanced luminescent materials based on organoboron polymers. Macromol Rapid Commun. 2012;33:1235–55.
Tanaka K, Yamane H, Yoshii R, Chujo Y. Efficient light absorbers based on thiophene-fused boron dipyrromethene (BODIPY) dyes. Bioorg Med Chem. 2013;21:2715–9.
Yamane H, Ohtani S, Tanaka K, Chujo Y. Synthesis of furan-substituted aza-BODIPYs having strong near-infrared emission. Tetrahedron Lett. 2017;58:2989–92.
Yoshii R, Yamane H, Tanaka K, Chujo Y. Synthetic strategy for low-band gap oligomers and homopolymers using characteristics of thiophene-fused boron dipyrromethene. Macromolecules. 2014;47:3755–60.
Yeo H, Tanaka K, Chujo Y. Effective light-harvesting antennae based on BODIPY-tethered cardo polyfluorenes via rapid energy transferring and low concentration quenching. Macromolecules. 2013;46:2599–605.
Yeo H, Tanaka K, Chujo Y. Tunable optical property between pure red luminescence and dual-emission depended on the length of light-harvesting antennae in the dyads containing the cardo structure of BODIPY and oligofluorene. Macromolecules. 2016;49:8899–904.
Yamane H, Tanaka K, Chujo Y. Pure-color and dual-color emission from BODIPY homopolymers containing the cardo boron structure. Polym Chem. 2018;9:3917–21.
Gon M, Tanaka K, Chujo Y. Creative synthesis of organic–inorganic molecular hybrid materials. Bull Chem Soc Jpn. 2017;90:463–74.
Kajiwara Y, Nagai A, Tanaka K, Chujo Y. Efficient simultaneous emission from RGB-emitting organoboron dyes incorporated into organic-inorganic hybrids and preparation of white light-emitting. Mater J Mater Chem C. 2013;1:4437–44.
Yoshii R, Nagai A, Tanaka K, Chujo Y. Highly NIR emissive boron di(iso)indomethene (BODIN)-based polymer: drastic change from deep-red to NIR emission via quantitative polymer reaction. J Polym Sci Part A. 2013;51:1726–33.
Yamane H, Tanaka K, Chujo Y. Synthesis of a near-infrared light-absorbing polymer based on thiophene-substituted aza-BODIPY. Polym J 2018;50:271–5.
Tanaka K, Yanagida T, Yamane H, Hirose A, Yoshii R, Chujo Y. Liquid scintillators with near infrared emission based on organoboron conjugated polymers. Bioorg Med Chem Lett 2015;25:5331–4.
Yoshii R, Yamane H, Nagai A, Tanaka K, Taka H, Kita H, et al. π-Conjugated polymers composed of BODIPY or aza-BODIPY derivatives exhibiting high electron mobility and low threshold voltage in electron-only devices. Macromolecules. 2014;47:2316–23.
Gon M, Kato K, Tanaka K, Chujo Y. Elastic and mechanofluorochromic hybrid films with POSS-capped polyurethane and polyfluorene. Mater Chem Front. 2019;3:1174–1180.
Kato K, Gon M, Tanaka K, Chujo Y. Stretchable conductive hybrid films consisting of cubic silsesquioxane-capped polyurethane and poly(3-hexylthiophene). Polymers. 2019;11:1195.
Nakamura R, Narikiyo H, Gon M, Tanaka K, Chujo Y. An optical sensor for discriminating the chemical compositions and sizes of plastic particles in water based on water-soluble networks consisting of polyhedral oligomeric silsesquioxane presenting dual-color luminescence. Mater Chem Front. 2019;3:2690–5.
Gon M, Tanaka K, Chujo Y. Concept of excitation-driven boron complexes and their applications for functional luminescent materials. Bull Chem Soc Jpn. 2019;92:7–18.
Gon M, Wakabayashi J, Tanaka K, Chujo Y. Unique substitution effect at 5,5’-positions of fused azobenzene–boron complexes with a N=N π‐conjugated system. Chem Asian J. 2019;14:1837–43.
Ohtani S, Gon M, Tanaka K, Chujo Y. Construction of the luminescent donor–acceptor conjugated systems based on boron-fused azomethine acceptor. Macromolecules. 2019;52:3387–93.
Gon M, Tanaka K, Chujo Y. A highly efficient near-infrared-emissive copolymer with a N=N double-bond π-conjugated system based on a fused azobenzene-boron complex. Angew Chem Int Ed. 2018;57:6546–51.
Matsumoto T, Takamine H, Tanaka K, Chujo Y. Design of bond-cleavage-induced intramolecular charge transfer emission with dibenzoboroles and their application to ratiometric sensors for discriminating chain lengths of alkanes. Mater Chem Front. 2017;1:2368–75.
Ohtani S, Gon M, Tanaka K, Chujo Y. Flexible fused azomethine–boron complex: thermally-induced switching of crystalline-state luminescent property and thermosalient behaviors based on phase transition between polymorphs. Chem Eur J. 2017;23:11827–33.
Hirose A, Tanaka K, Tamashima K, Chujo Y. Synthesis for dual-emissive organometallic complexes containing heterogeneous metal elements. Tetrahedron Lett. 2014;55:6477–81.
Zhang G, Palmer GM, Dewhirst MW, Fraser CL. A dual-emissive-materials design concept enables tumour hypoxia imaging. Nat Mater. 2009;8:747–51.
Wang F, DeRosa CA, Daly ML, Song D, Sabat M, Fraser CL. Multi-stimuli responsive luminescent azepane-substituted β-diketones and difluoroboron complexes. Mater Chem Front. 2017;1:1866–74.
Morris WA, Butler T, Kolpaczynska M, Fraser CL. Stimuli responsive furan and thiophene substituted difluoroboron β-diketonate materials. Mater Chem Front. 2017;1:158–66.
Zhang G, Lu J, Sabat M, Fraser CL. Polymorphism and reversible mechanochromic luminescence for solid-state difluoroboron avobenzone. J Am Chem Soc. 2010;132:2160–2.
Wang L, Wang K, Zou B, Ye K, Zhang H, Wang Y. Luminescent chromism of boron diketonate crystals: distinct responses to different stresses. Adv Mater. 2015;27:2918–22.
Galer P, Korošec RC, Vidmar M, Šket B. Crystal structures and emission properties of the BF2 complex 1-phenyl-3-(3,5-dimethoxyphenyl)-propane-1,3-dione: multiple chromisms, aggregation- or crystallization-induced emission, and the self-assembly effect. J Am Chem Soc. 2014;136:7383–94.
Tanaka K, Tamashima K, Nagai A, Okawa T, Chujo Y. Facile modulation of optical properties of diketonate-containing polymers by regulating complexation ratios with boron. Macromolecules. 2013;46:2969–75.
Yoshii R, Tanaka K, Chujo Y. Conjugated polymers based on tautomeric units: regulation of main-chain conjugation and expression of aggregation induced emission property via boron-complexation. Macromolecules. 2014;47:2268–78.
Luo J, Xie Z, Lam JW, Cheng L, Chen H, Qiu C, et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem Commun. 2001:1740–1.
Tong H, Hong Y, Dong Y, Häußler M, Lam JWY, Li Z, et al. Fluorescent “light-up” bioprobes based on tetraphenylethylene derivatives with aggregation-induced emission characteristics. Chem Commun. 2006:3705‒7.
Hu YB, Lam JWY, Tang BZ. Recent progress in AIE-active polymers. Chin J Polym Sci. 2019;37:289–301.
Qiu Z, Liu X, Lam JWY, Tang BZ. The marriage of aggregation-induced emission with polymer science. Macromol Rapid Commun. 2019;40:1800568.
Qin A, Lam JWY, Tang BZ. Luminogenic polymers with aggregation-induced emission characteristics. Prog Polym Sci. 2012;37:182–209.
Hu R, Kang Y, Tang BZ. Recent advances in AIE polymers. Polym J. 2016;48:359–70.
Tang BZ. Luminogenic polymers. Macromol Chem Phys. 2009;210:900–2.
Mei J, Leung NL, Kwok RT, Lam JW, Tang BZ. Aggregation-induced emission: Together We Shine, United We Soar! Chem Rev. 2015;115:11718–940.
Yoshii R, Nagai A, Tanaka K, Chujo Y. Highly emissive boron ketoiminate derivatives as new class of aggregation-induced emission fluorophores. Chem Eur J. 2013;19:4506–12.
Tanaka K, Nishino K, Ito S, Yamane H, Suenaga K, Hashimoto K, et al. Development of solid-state emissive o-carborane and theoretical investigation of the mechanism of the aggregation-induced emission behaviors of organoboron “element-blocks”. Faraday Discuss. 2017;196:31–42.
Yoshii R, Nagai A, Tanaka K, Chujo Y. Boron ketoiminate-based polymers: fine-tuning of the emission color and expression of strong emission both in the solution and film state. Macromol Rapid Commun. 2014;35:1315–9.
Zhao C-H, Wakamiya A, Yamaguchi S. Highly emissive poly(aryleneethynylene)s containing 2,5-diboryl-1,4-phenylene as a building unit. Macromolecules. 2007;40:3898–3900.
Zhao C-H, Sakuda E, Wakamiya A, Yamaguchi S. Highly emissive diborylphemlene-containing bis(phenylethynyl)benzenes: Structure-photophysical property correlations and fluoride ion sensing. Chem Eur J. 2009;15:10603–12.
Yin X, Guo F, Lalancette RA, Jäkle F. Luminescent main-chain organoborane polymers: highly robust, electron-deficient poly(oligothiophene borane)s via stille coupling polymerization. Macromolecules. 2016;49:537–46.
Meng B, Ren Y, Liu J, Jäkle F, Wang L. p-π conjugated polymers based on stable triarylborane with n-type behavior in optoelectronic devices. Angew Chem Int Ed. 2018;57:2183–7.
Yamane H, Tanaka K, Chujo Y. Simple and valid strategy for the enhancement of the solid-emissive property based on boron dipyrromethene. Tetrahedron Lett. 2015;56:6786–90.
Yamane H, Ito S, Tanaka K, Chujo Y. Preservation of main-chain conjugation through BODIPY-containing alternating polymers from electronic interactions with side-chain substituents by cardo boron structures. Polym Chem. 2016;7:2799–807.
Yeo H, Tanaka K, Chujo Y. Synthesis and energy transfer through heterogeneous dyes-substituted fluorene-containing alternating copolymers and their dual-emission properties. J Polym Sci A. 2015;53:2026–35.
Yeo H, Tanaka K, Chujo Y. Synthesis of dual-emissive polymers based on ineffective energy transfer through cardo fluorene-containing conjugated polymers. Polymer. 2015;60:228–33.
Nishino K, Yamamoto H, Tanaka K, Chujo Y. Development of solid-state emissive materials based on multi-functional o-carborane-pyrene dyads. Org Lett. 2016;18:4064–7.
Naito H, Nishino K, Morisaki Y, Tanaka K, Chujo Y. Luminescence color tuning of stable luminescent solid materials from blue to NIR based on bis-o-carborane-substituted oligoacenes. Chem Asian J. 2017;12:2134–8.
Naito H, Nishino K, Morisaki Y, Tanaka K, Chujo Y. Highly-efficient solid-state emissions of the anthracene–o-carborane dyads with various substituents and their thermochromic luminescent properties. J Mater Chem C. 2017;4:10047–54.
Mori H, Nishino K, Wada K, Morisaki Y, Tanaka K, Chujo Y. Modulation of luminescent chromic behaviors and environment-responsive intensity changes by substituents in bis-o-carborane-substituted conjugated molecules. Mater Chem Front. 2018;2:573–9.
Suenaga K, Yoshii R, Tanaka K, Chujo Y. Sponge-type emissive chemosensors for the protein detection based on boron ketoiminate-modifying hydrogels with aggregation-induced blueshift emission property. Macromol Chem Phys. 2016;217:414–7.
Yoshii R, Suenaga K, Tanaka K, Chujo Y. Mechanofluorochromic materials based on aggregation-induced emission-active boron ketoiminates: regulation of the direction of the emission color changes. Chem Eur J. 2015;21:7231–7.
Yamaguchi M, Ito S, Hirose A, Tanaka K, Chujo Y. Modulation of sensitivity to mechanical stimulus in mechanofluorochromic properties by altering substituent positions in solid-state emissive diiodo boron diiminates. J Mater Chem C. 2016;3:5314–9.
Suenaga K, Tanaka K, Chujo Y. Heat-resistant mechanoluminescent chromism of the hybrid molecule based on boron ketoiminate-modified octa-substituted polyhedral oligomeric silsesquioxane. Chem Eur J. 2017;23:1409–14.
Suenaga K, Tanaka K, Chujo Y. Design and luminescent chromism of fused boron complexes having constant emission efficiencies in solution and in the amorphous and crystalline states. Eur J Org Chem. 2017;2017:5191–6.
Suenaga K, Uemura K, Tanaka K, Chujo Y. Stimuli-responsive luminochromic polymers consisting of multi-states emissive fused boron ketoiminate. Polym Chem. https://doi.org/10.1039/C9PY01733J.
Yoshii R, Hirose A, Tanaka K, Chujo Y. Boron diiminate with aggregation-induced emission and crystallization-induced emission enhancement characteristics. Chem Eur J. 2014;20:8320–4.
Tanaka K, Yanagida T, Hirose A, Yamane H, Yoshii R, Chujo Y. Synthesis and color tuning of boron diiminate conjugated polymers with aggregation-induced scintillation properties. RSC Adv. 2015;5:96653–9.
Yoshii R, Hirose A, Tanaka K, Chujo Y. Functionalization of boron diiminates with unique optical properties: multicolor tuning of crystallization-induced emission and introduction into the main chain of conjugated polymers. J Am Chem Soc. 2014;136:18131–9.
Hirose A, Tanaka K, Yoshii R, Chujo Y. Film-type chemosensors based on boron diminate polymers having oxidation-induced emission properties. Polym Chem. 2015;6:5590–5.
Ito S, Tanaka K, Chujo Y. Characterization and photophysical properties of a luminescent aluminum hydride complex supported by a β-diketiminate ligand. Inorganics. 2019;7:100.
Ito S, Hirose A, Yamaguchi M, Tanaka K, Chujo Y. Size-discrimination for volatile organic compounds utilizing gallium diiminate by luminescent chromism of crystallization-induced emission via encapsulation-triggered crystal-crystal transition. J Mater Chem C. 2016;3:5564–71.
Ito S, Hirose A, Yamaguchi M, Tanaka K, Chujo Y. Synthesis of aggregation-induced emission-active conjugated polymers composed of group 13 diiminate complexes with tunable energy levels via alteration of central element. Polymers. 2017;9:68–78.
Yamaguchi M, Ito S, Hirose A, Tanaka K, Chujo Y. Luminescent color tuning with polymer films composed of boron diiminate conjugated copolymers by changing connection points to comonomers. Polym Chem. 2018;9:1942–6.
Yamaguchi M, Ito S, Hirose A, Tanaka K, Chujo Y. Control of aggregation-induced emission versus fluorescence aggregation-caused quenching by bond existence at a single site in boron pyridinoiminate complexes. Mater Chem Front. 2017;1:1573–9.
Yamaguchi M, Tanaka K, Chujo Y. Design of conjugated molecules presenting short-wavelength luminescence by utilizing heavier atoms of the same element group. Chem Asian J. 2018;13:1342–7.
Yamaguchi M, Tanaka K, Chujo Y. Control of solution and solid-state emission with conjugated polymers based on the boron pyridinoiminate structure by ring fusion. Polymer. 2018;142:127–31.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tanaka, K., Chujo, Y. Modulation of the solid-state luminescent properties of conjugated polymers by changing the connecting points of flexible boron element blocks. Polym J 52, 555–566 (2020). https://doi.org/10.1038/s41428-020-0316-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-0316-y
- Springer Nature Limited
This article is cited by
-
New strategy for lowering the energy levels of one frontier molecular orbital in conjugated molecules and polymers based on Aza-substitution at the isolated HOMO or LUMO
Polymer Journal (2024)
-
π-Conjugated polymers based on flexible heteroatom-containing complexes for precise control of optical functions
Polymer Journal (2023)
-
Frustrated element-blocks: A new platform for constructing unique stimuli-responsive luminescent materials
Polymer Journal (2023)
-
PPV-type π-conjugated polymers based on hypervalent tin(IV)-fused azobenzene complexes showing near-infrared absorption and emission
Polymer Journal (2021)
-
Evaluation-oriented exploration of photo energy conversion systems: from fundamental optoelectronics and material screening to the combination with data science
Polymer Journal (2020)