Abstract
Innate lymphocytes are a diverse population of cells that carry out specialized functions in steady-state homeostasis and during immune challenge. While circulating cytotoxic natural killer (NK) cells have been studied for decades, tissue-resident innate lymphoid cells (ILCs) have only been characterized and studied over the past few years. As ILCs have been largely viewed in the context of helper T-cell biology, models of ILC lineage and function have been founded within this perspective. Notably, tissue-resident innate lymphocytes with cytotoxic potential have been described in an array of tissues, yet whether they are derived from the NK or ILC lineage is only beginning to be elucidated. In this review, we aim to shed light on the identities of innate lymphocytes through the lenses of cell lineage, localization, and timing of differentiation.
Similar content being viewed by others
Introduction
Lymphocytes are a fundamental component of the host immune response to challenge. Different classes of the lymphocyte response are best defined by the type of effector programs carried out by CD8+ or CD4+ T cells. CD8+ T cells are cytotoxic lymphocytes that mediate a type 1 immune response to intracellular threats such as viruses and tumor cells by directly killing infected or stressed cells in an antigen-dependent manner. CD4+ T cells can differentiate into three T-helper (Th) lineages based on the signals they receive during particular challenges. Th1 cells support a type 1 immune response, whereas Th2 and Th17 subsets support type 2 and type 3 immune responses, respectively, through cytokine production and immune cell recruitment to combat various intracellular and extracellular threats.
Recently, the field of lymphocyte biology has gained an appreciation for a more specific definition of lymphocytes based upon not only effector programs but also tissue localization. For example, T follicular helper (Tfh) cells substantiate their own lymphocyte subset.1 Although they exhibit Th1, Th2, and Th17 effector programs, Tfh cells play unique roles separate from their conventional counterparts. In a context-dependent manner, Tfh cells receive signals to induce Bcl-6, ICOS, and CXCR5 to induce homing to the follicles of secondary lymphoid organs.2 There, they have specialized roles in promoting the formation of germinal centers and generation of high-affinity antibodies and memory B cells.
Memory CD8+ T cells also exemplify how tissue localization and trafficking patterns define distinct subsets of lymphocytes. During infection, naive CD8+ T cells encounter antigens and proliferate to become effector T cells that circulate through the blood, secondary lymphoid organs and peripheral tissues. After infection clearance, most effector T cells are eliminated, but some persist long-term as memory T cells. Recently, four distinct subsets of memory T cells have been described.3 Central memory T (Tcm) cells are derived from CX3CR1− effector T cells and primarily circulate between the blood and secondary lymphoid organs. Resident memory T (Trm) cells, although also derived from CX3CR1− effector T cells, enter peripheral tissues where they are maintained locally by self-renewal and do not recirculate. Peripheral memory T cells are generated from CX3CR1 intermediate-expressing effector T cells and are the majority population that dynamically surveys peripheral tissues; yet unlike Trms, these cells can re-enter circulation. CX3CR1-high effector memory T cells are largely excluded from all tissues except the spleen; otherwise, they are found almost exclusively within the vascular compartment. These distinct trafficking patterns are a result of the differentiation from effector T cells with low, intermediate or high expression of CX3CR1 as well as expression of other homing molecules; these trafficking patterns further define subsets within the broad lineage of CD8+ T cells.
The third factor to be considered in lymphocytes characterization is time at which they differentiate and establish effector programs. For example, CD1d-restricted invariant NKT (iNKT) cells are tissue-resident innate-like T cells that express an invariant TCRα chain that pairs with a limited array of TCRβ chains.4,5 iNKT cells develop in the thymus from double positive thymocytes as a result of strong TCR stimulation with an endogenous agonist ligand and subsequently home to the periphery where they carry out T helper-like functions in detecting lipid antigens.6,7 Thus, the effector program of iNKT cells is specified during ontogeny under homeostatic conditions. Likewise, the unconventional intestinal intraepithelial lymphocytes (IELs) of the TCRαβ lineage are selected by agonistic antigens, constitutively home to the intestine and differentiate to effector lymphocytes with cytotoxic potential.8 The generation of iNKT cells and IELs are distinct from that of conventional CD4+ and CD8+ T cell subsets in which the effector and tissue-residency programs are induced downstream of the circulating naive stage during the course of infection or challenge.
Many parallels have been drawn between subsets of innate and adaptive lymphocytes; this has been helpful in understanding innate lymphocyte biology but has also potentially over simplified and even branded each subset into presumed subtypes. In this review, we aim to shed light on the heterogenous population of innate lymphocytes, in particular, cytotoxic innate lymphocytes in mice, through the three-way lens of lineage, placement and timing of differentiation. These three parameters dictate innate lymphocyte identity and functional outcome in tissue homeostasis and host defense.
Innate lymphocytes defined by lineage
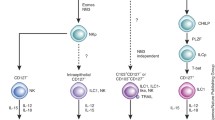
Over the last decade, the spectrum of innate lymphocytes has broadened with the recognition of distinct subsets of innate lymphoid cells (ILCs). Tentatively classified into five groups, innate lymphocytes include natural killer (NK) cells, ILC1s, ILC2s, ILC3s, and lymphoid tissue-inducer (LTi) cells.9,10,11 Many seminal studies have elucidated the development of innate lymphocytes, establishing that all innate lymphocytes arise from a CD127 (IL-7Rα)-expressing common innate lymphoid progenitor (CILp), which is downstream of the common lymphoid progenitor (CLP) from which T and B cells are also derived.12,13,14 CILps gives rise to the common helper innate lymphoid progenitor (CHILP) that gives rise to all helper subsets of ILCs as well as LTi cells.15 A more-restricted progenitor downstream of CHILPs, the innate lymphoid cell progenitor (ILCp), is defined by expression of PD-1 and the transcription factor PLZF.16,17 The ILCps can differentiate into all subsets of helper ILCs but has lost NK cell potential (Fig. 1a).
Lineage, localization and timing of innate lymphocyte differentiation. a All lymphocytes develop from the common lymphoid progenitor (CLP). From the CLP, the common innate lymphoid progenitor (CILP) gives rise to the natural killer (NK) and innate lymphoid cell (ILC) lineages. Downstream of the CILP, the common helper innate lymphoid cell progenitor (CHILP) gives rise to all helper ILCs and lymphoid tissue induced (LTi) cells but has lost NK cell potential. Innate lymphoid progenitors (ILCp) give rise to ILC1, ILC2, and ILC3. In the liver, ILCk are derived from PLZF-expressing ILCp. However, it remains to be determined whether ILCk are differentiated via a distinct pathway downstream of ILCp or converted from helper ILC. The identity of an NK-committed progenitor downstream of the CILP remains unclear. Tissue-resident NK cells may be converted from conventional NK cells but may also be derived from an earlier progenitor. b The first wave of ILC hematopoiesis arises from the fetal liver starting at embryonic day E15.5. Bone marrow hematopoiesis begins just before birth and becomes the main site of hematopoiesis from weaning through adulthood. NK cell development is largely thought to occur in the bone marrow, with mature NK cells arising a few weeks after birth, whereas ILC development occurs during both waves
Historically, NK cell development has been described as linear stages downstream of the CLP; each intermediate is defined by stepwise acquisition of IL-2Rβ, loss of IL-7Rα, and gradual gain of NK cell markers such as NK1.1, CD49b (DX5), CD11b, and inhibitory/activating receptors of the NKG2 and Ly49 receptor families.18 More recent studies have identified NK precursors (NKP) as the earliest NK lineage committed cells, defined by Lin−CD244+CD27+CD127+Flt3− and further divided into CD122− (pre-NKP) and CD122+ (refined NKP, rNKP).19 However, a significant proportion of pre-NKPs and, to a lesser extent, rNKPs were fate-mapped by PLZF, indicating that both populations contain cells differentiated along the ILC pathway.20 In light of this and the separation of NK and ILC lines at the CHILP and ILCp stages, a dedicated NK progenitor downstream of CILp remains elusive.
The classical type 1 innate lymphocytes, NK cells and ILC1s, share features including their developmental requirement for the transcription factor Id2 and production of type 1 cytokines such as IFNγ.21 However, NK cells require the transcription factor Eomes and are largely circulating, whereas tissue-resident ILC1s are T-bet dependent and require Gata3 for their development.22,23,24,25 NK cells are the canonical killers of the innate immune system, functionally akin to cytotoxic CD8+ T cells via their expression of perforin and granzymes, whereas cytokine-producing ILC1s mirror CD4+ Th1 cells.26 NK cells and ILC1s protect against intracellular microbial threats and tumor cells. The relatively homogeneous population of ILC2s share many features with CD4+ Th2 cells in that they respond to IL-25, IL-33, and TSLP during helminth infections to produce IL-5 and IL-13 in a Gata3- and RORα-dependent fashion.27,28,29,30,31 ILC2s play important protective roles such as promoting tissue homeostasis and repair, but they also have pathogenic roles in allergy, asthma, and inflammation of tissues such as the skin and lung.32 ILC3s are more complex, consisting of natural cytotoxicity receptor-positive and -negative subsets and dependency on the RORγt transcription factor.33,34,35 In response to IL-23, ILC3s produce IL-17 and IL-22 to control bacterial infections and maintain gut homeostasis, analogous to CD4+ Th17 cells.36,37,38 Dysregulation of ILC3s can cause pathogenic inflammatory conditions such as colitis in the gut.36 LTi cells are RORγt-dependent and specialize in the instruction of secondary lymphoid organ generation during fetal development.39,40
Innate lymphocytes defined by localization
All ILCs are characterized by maintaining residency within peripheral tissues.23 ILC2s and ILC3s are enriched specifically at barrier tissues such as the mucosa and lung, whereas ILC1s have been reported in a wider array of tissues including the liver, intestine, adipose tissue, salivary gland, mammary gland and prostate.28 LTi cells are seeded embryonically into lymphoid tissues where they particularly instruct development of lymph nodes and Peyer’s patches.39 In the adult, LTi cells are found in secondary lymphoid organs where they are thought to aid in tissue repair, but their exact functions are not clear.40
Mature NK cells are phenotypically defined as circulating NK1.1+NKp46+CD49b+CD27−CD11b+ cells that equilibrate between hosts in parabiosis experiments, which have become the gold standard for determining tissue residency. Mature NK cells are abundant in the blood, bone marrow (BM), spleen, liver and lung and, to a lesser extent, in secondary lymphoid tissues.18
Over the years, there have been reports of “tissue-resident NK cells” in a variety of organs. Likewise, evidence for tissue-resident ILC-like cells with cytotoxic potential has emerged. The true identity of these tissue-resident cytotoxic innate lymphocytes across tissues is only starting to be uncovered. In this review, mature circulating CD11b+ NK cells will be termed “conventional NK” cells (cNK), whereas tissue-resident cells derived from the NK lineage will be termed “tissue-resident NK cells” (trNK). Similarly, the term helper ILC (ILCh) is reserved for purely cytokine-producing cells of the ILC lineage, whereas ILCp-derived cells with cytotoxic potential will be referred to as “killer ILCs” (ILCk) (Fig. 1a).
Previously described as tissue-resident NK cells, the type 1 innate lymphocytes in the liver have been extensively studied since the demarcation of ILC and NK lineages. PLZF-fate mapping and transfer experiments have demonstrated that most of them are derived from CHILP and ILCp, conclusively distinguishing them from the NK cell lineage.16 Furthermore, cytotoxic innate lymphocytes in the liver are severely reduced in T-bet-deficient mice but do not require Eomes for their development.22,41 These cells do not recirculate under steady-state conditions and have high expression of CD49a but lack CD49b expression. Notably, liver-resident type 1 innate lymphocytes have cytotoxic potential as exemplified by their relatively high expression of granzymes and TRAIL.42 This evidence collectively suggests that the liver-resident cytotoxic innate lymphocytes are better classified as ILCks instead of ILC1s.
A potential example of true tissue-resident NK cells is the uterine CD49a+ cytotoxic innate lymphocytes. They are present in the nonpregnant uterus but expand upon embryo implantation to contribute to arterial remodeling and tolerance at the maternal–fetal interface.43 They require IL-15, express cytotoxic molecules, produce IFNγ, and were shown to be tissue resident by parabiosis experiments.41 However, uterine tissue-resident NK cells do not require T-bet, as they are present in normal numbers in T-bet-deficient mice. CD49a+ uterine NK cells express a high level of Eomes and are generally CD127-negative; this evidence collectively suggests that they may be derived from the NK lineage.43 Fate-mapping and adoptive-transfer experiments are needed to establish the precursor(s) that give rise to these cells to conclusively determine the identity of uterine cytotoxic innate lymphocytes.
The repertoire of cytotoxic innate lymphocytes is less defined in other organs such as the salivary gland. All ILCs require the transcription factor Gata3 for their development, but cytotoxic innate lymphocytes in the salivary gland are largely unaffected upon its loss.44 Similarly, loss of T-bet has minimal effects on salivary gland type 1 innate lymphocytes, and a substantial fraction of them are not derived from the PLZF-expressing ILCps, distinguishing at least a portion from the liver ILCks.45 Conventional NK cells require Eomes for their development, but a proportion of cytotoxic lymphocytes in the salivary gland remain in Eomes-deficient mice.22 Collectively, these genetic data suggest that the population of cytotoxic lymphocytes in the salivary gland may represent a heterogenous mix of cells derived from the NK lineage and ILCp lineage, as well as a potentially yet-to-be identified lineage of cytotoxic innate lymphocytes.
Innate lymphocytes defined by timing of cell fate specification
Fetal liver versus BM hematopoiesis
ILCs initially arise during a wave of fetal liver hematopoiesis from embryonic day 13.5 through birth (Fig. 1b), at which point they become positioned in developing tissues, undergo marked tissue expansion, and acquire mature effector functions.15,46 The signals that dictate placement in peripheral tissues during ILC development in the fetal liver are still unclear, as is the developmental stage at which ILCs seed tissues. In the fetal intestine, a population of cells were found to represent a transitional state between fetal liver ILC progenitors and mature ILCs, as these precursor cells could give rise to ILC1, ILC2 and ILC3, indicating that ILC precursors can exit the site of hematopoiesis during fetal development.47
Just before birth, BM hematopoiesis is established, replaces fetal liver hematopoiesis during weaning and remains the site of hematopoiesis through adulthood48 (Fig. 1b). This second wave of BM hematopoiesis is different from fetal liver hematopoiesis in that hematopoietic stem cells (HSCs) of the fetal liver are highly proliferative and undergo symmetric division, which is likely to supply the hematopoietic demands during embryonic and early life development48. BM HSCs, on the other hand, are quiescent and undergo low self-renewal and asymmetric divisions; this is potentially due to lower demand in the adult. These marked differences may reflect inherent cellular differences but could be due to distinct niches that provide specific instructions to support fetal liver versus adult hematopoiesis. NK cell differentiation takes place during BM hematopoiesis; mature NK cells do not arise until 2 or 3 weeks after birth.15,49 The unique niches established during fetal liver versus BM hematopoiesis have intriguing implications for cell fate specification of early ILCs versus bone marrow-derived ILCs and NK cells.
It may be interesting to hypothesize that liver ILCks are derived from fetal liver hematopoiesis and become established early in life, whereas in adults, they maintain their pool locally and expand and activate when necessary. In fact, liver CD49a+CD49b− type 1 innate lymphocytes cannot be fully reconstituted upon BM transfer in irradiated hosts, suggesting that at least a portion of the pool of adult liver type 1 innate lymphocytes is established before BM hematopoiesis begins.50 Some organs, such as the uterus, develop and mature later in life, at which time the BM is the only site of hematopoiesis. Therefore, the major type 1 innate lymphocyte population that seeds these tissues may in fact be BM-derived cells of the NK lineage.
When tissue-resident NK cells establish their effector program is unclear, but several possibilities exist. First, tissue-resident NK cells may be converted from conventional NK cells (Fig. 1a). Several studies have suggested that NK cells can adopt tissue-resident qualities under certain settings.51,52,53 One report showed that CD49b+ NK cells can express tissue-residency markers such as CD49a as well as induction of tissue-resident ILC-associated DNAM-1 and TRAIL expression in a transforming growth factor beta (TGF-β)-dependent manner both in vivo and in vitro.53 However, CD49b+ NK cells may consist of a mixture of CD11b+ cNK cells and CD27+ NK cells that are considered “immature” NK cells. Thus, it remains possible that trNKs may be converted from CD27+ progenitor cells rather than cNK cells (Fig. 1a). Conceptually, the differentiation of trNKs and cNKs may mirror the differentiation of Trms and Tems from naive T cells. Trm cells are derived from precursors that seed nonlymphoid tissues during the effector phase of an acute immune response and maintain residency within those tissues. In contrast, Tems continuously circulate through the bloodstream and are largely excluded from most tissues. These separate trafficking patterns suggest that inherent programs established during the progenitor phase dictate a mature cell’s ultimate placement and functional outcome. Fate-mapping studies and cell-transfer experiments with precisely defined NK cell populations will help clarify the origin of trNKs across different tissues.
Local expansion versus recruitment and continued differentiation
Despite the clarity of the ILC lineage downstream of CLP, when and where each stage of the differentiation pathway occurs remains incompletely understood. ILCs may exit sites of development as mature cells to seed peripheral tissues where they maintain their pools locally, or multipotent progenitors may seed the periphery and differentiate into mature ILCs upon receiving signals during immune challenge. Alternatively, circulating progenitors may contribute to and repopulate peripheral pools of ILCs as necessary. It may be that all three scenarios occur to maintain the proper balance of ILCs under homeostasis and during immune challenge, and this topic is under active investigation by several groups.
Under steady-state conditions, after the initial seeding and expansion of ILCs, there is limited evidence thus far for recruitment of progenitor populations in adult animals. Rather, tissue-specific signals appear to dictate local expansion and activation. Under inflammatory conditions, parabiosis experiments have revealed that ILCs undergo massive proliferation to expand their tissue pools locally rather than through recruitment of ILCs or progenitors from the blood.23,54 In addition, studies are emerging that demonstrate ILC2s expand and become activated in a tissue-dependent manner due to specific tissue-derived signals.55 For example, a recent study identified a circuit between intestinal tuft cells and ILC2s, whereby tuft cell sensing of succinate—a microbial-derived metabolite—through intestinal tuft cell-specific expression of the succinate receptor induces ILC2 expansion and activation.56
Still, the possibility for recruitment of mature ILCs from other sites or continued differentiation of progenitors outside of the BM exists. In humans, circulating ILCps have been identified from blood and shown to give rise to all subsets of ILCs in single-cell cloning assays.57 Their model suggests that progenitor seeding of peripheral tissues and subsequent tissue-derived signals induce differentiation of ILCps into mature ILCs in a process termed “ILC-poiesis”, rather than the model in which the BM instructs ILC subset generation from ILCps.58,59 However, circulating ILCps in mouse have yet-to-be identified. Interestingly, a recent report demonstrated that in mouse, “resting” ILC2s in the intestinal lamina propria can give rise to circulating inflammatory ILC2s during IL-25 activation or helminth infection.60 This is reminiscent of CD4+ helper subset induction from a resting or naive state and may support a model of continual differentiation rather than local expansion and activation.
Function and regulation of (tissue-resident cytotoxic) type 1 innate lymphocytes
Steady-state and challenge-associated type 1 innate lymphocyte response
The embryonic seeding of ILCs before the establishment of BM hematopoiesis may suggest an important function of ILCs in embryonic development and establishment of the early immunological niche. It is conceivable that a population of cells with cytotoxic potential is necessary to complete the immune cell repertoire necessary for protection early in life. M. Kotas and R. Locksley eloquently hypothesized that the initial seeding of ILC populations during gestation may provide instructions to tissues on how to support a tissue-resident immunological niche for organ maintenance later on and that the balance of ILCs established during embryonic development may have important outcomes during challenges in adulthood.61 These intriguing hypotheses remain untested.
Many studies are emerging that demonstrate a role for type 1 innate lymphocytes under inflammatory conditions; in particular, ILC1s are involved in the initial response to a variety of infections.62,63 For example, upon oral infection with the intracellular parasite Toxoplasma gondii, ILC1s produced significant amounts of IFNγ and TNFα, two cytokines known to be required for control of the parasite.15 In addition, T-bet-deficient mice that lack ILC1 had no IFNγ production and were unable to control parasite growth. In a model of Clostridium difficle infection, mice lacking T, B, and innate lymphocytes (Ragγc−/−) succumbed to infection much more rapidly than mice lacking only T and B cells (Rag−/−), and the transfer of ILC1 restored resistance to infection.64 In a model of mouse cytomegalovirus (MCMV) infection, Weizman et al.65 established that IFNγ derived from type 1 innate lymphocytes in the liver was essential for initial control of viral replication. Using Hobit-deficient mice—which were shown to be deficient in liver tissue-resident ILC1s (likely ILCks) but not circulating NK cells or ILC1s of other tissues—and Eomes-deficient mice (lacking conventional NK cells), they showed that liver-resident type 1 innate lymphocytes, but not cNK cells, were essential for limiting viral replication of MCMV in the liver.
In the context of cancer, the protective effects of group 1 innate lymphocytes have been demonstrated in models of chemically induced and transplantable tumors.66,67,68 Loss of NK1.1+ cells by antibody depletion or genetic model systems with NKp46+-specific diphtheria toxin expression demonstrated the importance of innate lymphocytes, as defined by NK1.1 and/or NKp46, in controlling tumor growth. We now know such studies cannot separate the relative contributions of conventional NK cells and other type 1 innate lymphocytes since trNKs, ILCks and ILChs express NK1.1, and NKp46 is a shared marker between NK cells, ILCks and some ILC3s. More recent studies aiming to elucidate their respective roles have utilized adoptive transfer of NKs or ILCs, as identified by cell type-specific markers, and probed each population’s effect on tumor growth. In these experiments, circulating CD49b+ NK cells provided tumor protection while tissue-resident innate lymphocytes not of the NK lineage did not display antitumor activity.53 However, in models of spontaneously induced breast and prostate cancers, which better recapitulate endogenous human disease, early protection against tumor growth was dependent on tissue-resident cytotoxic innate lymphocytes, whereas cNK cells were dispensable.69 As these cells are naturally found in nontransformed mammary tissue, they may serve as tissue sentinels of transformation and dominate antitumor responses early during tumorigenesis. Nonetheless, whether these tissue-resident cytotoxic innate lymphocytes are derived from ILC or NK cell lineages remains to be determined. Despite the discrepancies between tumor-specific models, harnessing tumor-infiltrating type 1 innate lymphocyte responses remains of therapeutic interest.
Regulation of cytotoxic innate lymphocytes by cytokines
IL-7 and IL-15, both of which signal through the common gamma chain (γc), have pleotropic roles in type 1 innate lymphocyte biology. In the BM, the heterogenous progenitor-containing population of Lin-CD127+CD122+ cells is maintained in the absence of IL-15, likely through compensation by IL-7 signaling.70 As NK cells mature, they lose expression of IL-7Rα and gain expression of IL-2Rβ at which point they become absolutely dependent on IL-15, as loss of this cytokine alone is sufficient to ablate the mature NK cell pool.49,71 Although a defining feature of innate lymphoid development is expression of IL-7Rα, development of ILCs is not solely dependent on IL-7 as IL-15 can support their differentiation in the absence of IL-7Rα, such as ILCks in the liver.72 Furthermore, absence of IL-15 significantly reduces tissue-resident cytotoxic innate lymphocytes in the liver, salivary gland, and small intestine lamina propria.15,73,74 In fact, IL-15 is a limiting factor for survival and/or maintenance of cytotoxic innate lymphocytes in the tumor.69
IL-15 is a unique cytokine in that the majority of signaling occurs through trans-presentation in the context of cell-membrane associated IL-15Rα, thereby rendering IL-15 signaling cell-to-cell contact dependent.75 IL-15 has a well-established role in supporting tissue-resident populations, including Trm cells in nonlymphoid tissues such as the skin.76 Interestingly, enterocyte-expressed IL-15 is sufficient to maintain the IEL population in the intestine in an otherwise IL-15-deficient animal.77 Evidence that IL-15 is dispensable for PD-1+ progenitors in the thymus further suggests that IL-15 is required for maintenance of mature IELs.78,79 It will be intriguing to determine whether IL-15 is sufficient to sustain cytotoxic innate lymphocytes under steady-state and challenge conditions; it will also be important to further assess whether tissue- and/or cell type-specific sources of IL-15 differentially regulate this heterogenous population.
TGF-β signaling also influences tissue-resident lymphocytes. It is required for maintenance of tissue-residency markers and cytotoxic potential in NKp46+ innate lymphocytes in the salivary gland as well as for formation of long-lived Trms in the skin.76,80 Several studies have demonstrated that regulation of the transcription factors T-bet and Eomes is intricately tied to signaling by TGF-β and IL-15 in the formation and maintenance of tissue-resident lymphocytes.79,81,82 It is interesting to hypothesize that as NK progenitors or ILC progenitors enter TGF-β- and IL-15-rich tissues, signaling by these cytokines may support the differentiation of progenitors towards NK or ILCk fates and the maintenance of the tissue-resident cells.
Regulation of type 1 innate lymphocytes by activating and inhibitory receptors
Conventional NK cells express an array of activating and inhibitory receptors on their surface; the balance and integration of these signals dictate their responsiveness to target cells and arm cNK cells with the ability to clear stressed or infected cells rapidly without prior priming.83,84 The inhibitory receptors interact with major histocompatibility complex (MHC) class I molecules that are constitutively expressed on healthy cells to prevent cNK cell lysis. The ability to discriminate healthy, self-cells versus unhealthy cells that have downregulated MHC class I, as is often the case in viral infections, is the result of “licensing”, a process that occurs during development in the BM.85 An interesting outstanding question in the field is to what extent Ly49 and NKG2 receptor-expressing ILChs and ILCks are regulated by interaction with MHC class I molecules. The ability to test this is limited by a current lack of genetic tools and models that can reliably discriminate the heterogenous populations of type 1 innate lymphocytes.
MHC class I-like molecules are indicators of the evolutionarily ancient intracellular stress response.86 These molecules are expressed on the surface of cells that have undergone cell stress, damage, infection or transformation. Detection of these molecules by activating receptors informs lymphocytes of their need for elimination. Presumably, the presence of activating receptors on various cytotoxic innate lymphocytes indicates that these cells can detect stressed, transformed, or otherwise disrupted tissues. One of the better characterized activating receptors is NKG2D, which is expressed on some conventional T cells, NK cells and ILCks.87,88 NKG2D has been implicated in mechanisms of tumor immunosurveillance as well as in sensing tissue injury and infection by pathogens.89,90,91 It will be interesting to explore whether such an evolutionarily conserved mechanism for detecting cellular stress is applicable for tissue-resident cytotoxic innate lymphocytes as well.
Concluding remarks
The presence of such a diverse array of innate lymphocytes that mirrors the adaptive immune system in many ways begs the questions of why such heterogeneity is necessary and whether innate lymphocytes simply provide redundancy. Innate lymphocyte lineages parallel those of conventional CD8+ and CD4+ T cell as well as unconventional innate-like T cell subsets in terms of their transcription factor dependency and effector programs employed, which clearly reflects a certain degree of redundancy. In fact, despite their activation and instruction of inflammatory responses after establishment, there have been few reports in which ILCs proved essential for survival.92 Nevertheless, the innate properties of these cells dictate that they respond quickly to insults in a generally nonspecific manner, whereas the adaptive immune response requires days to weeks to fully potentiate a response. Furthermore, their distinctive localizations within peripheral tissues perfectly poises innate lymphocytes to both monitor the overall state of a tissue and manage tissue-specific affronts they are intended to combat.
In addition to lineage and localization, the repertoire of innate lymphocytes and their ultimate function can be viewed in terms of their timing of differentiation and cell fate specification. During embryonic development, ILC differentiation in the fetal liver establishes pools of ILC-derived cells, some with cytotoxic potential, in peripheral tissues early in life. Interestingly, this is reminiscent of two populations of tissue-resident innate-like T cells, IELs and iNKT cells. These cell types are both selected by agonist ligands during development in the thymus at which point they gain effector functions and are instructed to home to peripheral tissues. Conceivably, establishment of a range of cytokine-producing helper subsets and cytotoxic cells completes the immunological niches necessary for host protection during development until BM hematopoiesis takes over. Therefore, waves of differentiation may establish particular niches in the growing and adult organism, where fetal liver- and/or BM-derived innate lymphocytes carry out their functions supporting overall host protection.
References
Nurieva, R. I. et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149 (2008).
Vinuesa, C. G., Linterman, M. A., Yu, D. & MacLennan, I. C. Follicular helper T Cells. Annu. Rev. Immunol. 34, 335–368 (2016).
Gerlach, C. et al. The chemokine receptor CX3CR1 defines three antigen-experienced CD8 T cell subsets with distinct roles in immune surveillance and homeostasis. Immunity 45, 1270–1284 (2016).
Godfrey, D. I., Uldrich, A. P., McCluskey, J., Rossjohn, J. & Moody, D. B. The burgeoning family of unconventional T cells. Nat. Immunol. 16, 1114–1123 (2015).
Godfrey, D. I., Stankovic, S. & Baxter, A. G. Raising the NKT cell family. Nat. Immunol. 11, 197–206 (2010).
Zhou, D. et al. Lysosomal glycosphingolipid recognition by NKT cells. Science 306, 1786–1789 (2004).
Kronenberg, M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 23, 877–900 (2005).
Cheroutre, H., Lambolez, F. & Mucida, D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 11, 445–456 (2011).
Vivier, E. et al. Innate lymphoid cells: 10 years on. Cell 174, 1054–1066 (2018).
Gury-BenAri, M. et al. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell 166, 1231–46 e13 (2016).
Robinette, M. L. et al. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat. Immunol. 16, 306–317 (2015).
Yu X., et al. The basic leucine zipper transcription factor NFIL3 directs the development of a common innate lymphoid cell precursor. Elife 3 (2014).
Yang, Q. et al. TCF-1 upregulation identifies early innate lymphoid progenitors in the bone marrow. Nat. Immunol. 16, 1044–1050 (2015).
Serafini, N., Vosshenrich, C. A. & Di Santo, J. P. Transcriptional regulation of innate lymphoid cell fate. Nat. Rev. Immunol. 15, 415–428 (2015).
Klose, C. S. N. et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 157, 340–356 (2014).
Constantinides, M. G., McDonald, B. D., Verhoef, P. A. & Bendelac, A. A committed precursor to innate lymphoid cells. Nature 508, 397–401 (2014).
Seillet, C. et al. Deciphering the innate lymphoid cell transcriptional program. Cell Rep. 17, 436–447 (2016).
Yu, J., Freud, A. G. & Caligiuri, M. A. Location and cellular stages of natural killer cell development. Trends Immunol. 34, 573–582 (2013).
Fathman, J. W. et al. Identification of the earliest natural killer cell-committed progenitor in murine bone marrow. Blood 118, 5439–5447 (2011).
Constantinides, M. G. et al. PLZF expression maps the early stages of ILC1 lineage development. Proc. Natl. Acad. Sci. USA 112, 5123–5128 (2015).
Spits, H. et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149 (2013).
Daussy, C. et al. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J. Exp. Med. 211, 563–577 (2014).
Gasteiger, G., Fan, X., Dikiy, S., Lee, S. Y. & Rudensky, A. Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350, 981–985 (2015).
Gordon, S. M. et al. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity 36, 55–67 (2012).
Pikovskaya, O. et al. Cutting edge: eomesodermin is sufficient to direct type 1 innate lymphocyte development into the conventional NK lineage. J. Immunol. 196, 1449–1454 (2016).
Sun, J. C. & Lanier, L. L. NK cell development, homeostasis and function: parallels with CD8(+) T cells. Nat. Rev. Immunol. 11, 645–657 (2011).
Wong, S. H. et al. Transcription factor RORalpha is critical for nuocyte development. Nat. Immunol. 13, 229–236 (2012).
Artis, D. & Spits, H. The biology of innate lymphoid cells. Nature 517, 293–301 (2015).
Moro, K. et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463, 540–544 (2010).
Neill, D. R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010).
Mjosberg, J. et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity 37, 649–659 (2012).
Zhu, J. Mysterious ILC2 tissue adaptation. Nat. Immunol. 19, 1042–1044 (2018).
Satoh-Takayama, N. et al. Microbial flora drives interleukin 22 production in intestinal NKp46+cells that provide innate mucosal immune defense. Immunity 29, 958–970 (2008).
Sanos, S. L. et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+cells. Nat. Immunol. 10, 83–91 (2009).
Luci, C. et al. Influence of the transcription factor RORgammat on the development of NKp46+cell populations in gut and skin. Nat. Immunol. 10, 75–82 (2009).
Buonocore, S. et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464, 1371–1375 (2010).
Klose, C. S. & Artis, D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 17, 765–774 (2016).
Song, C. et al. Unique and redundant functions of NKp46+ILC3s in models of intestinal inflammation. J. Exp. Med. 212, 1869–1882 (2015).
Eberl, G. et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5, 64–73 (2004).
Lane, P. J. et al. Lymphoid tissue inducer cells: bridges between the ancient innate and the modern adaptive immune systems. Mucosal Immunol. 2, 472–477 (2009).
Sojka, D. K. et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife 3, e01659 (2014).
Takeda, K. et al. TRAIL identifies immature natural killer cells in newborn mice and adult mouse liver. Blood 105, 2082–2089 (2005).
Peng, H. & Tian, Z. Diversity of tissue-resident NK cells. Semin. Immunol. 31, 3–10 (2017).
Yagi, R. et al. The transcription factor GATA3 is critical for the development of all IL-7Ralpha-expressing innate lymphoid cells. Immunity 40, 378–388 (2014).
Erick, T. K., Anderson, C. K., Reilly, E. C., Wands, J. R. & Brossay, L. NFIL3 expression distinguishes tissue-resident NK cells and conventional NK-like cells in the mouse submandibular glands. J. Immunol. 197, 2485–2491 (2016).
Juelke, K. & Romagnani, C. Differentiation of human innate lymphoid cells (ILCs). Curr. Opin. Immunol. 38, 75–85 (2016).
Bando, J. K., Liang, H. E. & Locksley, R. M. Identification and distribution of developing innate lymphoid cells in the fetal mouse intestine. Nat. Immunol. 16, 153–160 (2015).
Mikkola, H. K. & Orkin, S. H. The journey of developing hematopoietic stem cells. Development 133, 3733–3744 (2006).
Kim, S. et al. In vivo developmental stages in murine natural killer cell maturation. Nat. Immunol. 3, 523–528 (2002).
Peng, H. et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J. Clin. Invest. 123, 1444–1456 (2013).
Hydes, T. et al. IL-12 and IL-15 induce the expression of CXCR6 and CD49a on peripheral natural killer cells. Immun. Inflamm. Dis. 6, 34–46 (2018).
Cortez, V. S. et al. SMAD4 impedes the conversion of NK cells into ILC1-like cells by curtailing non-canonical TGF-beta signaling. Nat. Immunol. 18, 995–1003 (2017).
Gao, Y. et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat. Immunol. 18, 1004–1015 (2017).
Moro, K. et al. Interferon and IL-27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat. Immunol. 17, 76–86 (2016).
Ricardo-Gonzalez, R. R. et al. Tissue signals imprint ILC2 identity with anticipatory function. Nat. Immunol. 19, 1093–1099 (2018).
Nadjsombati, M. S. et al. Detection of succinate by intestinal tuft cells triggers a type 2 innate immune circuit. Immunity 49, 33–41 e7 (2018).
Lim, A. I. et al. Systemic human ILC precursors provide a substrate for tissue ILC differentiation. Cell 168, 1086–100 e10 (2017).
Lim, A. I. & Di Santo, J. P. ILC-poiesis: ensuring tissue ILC differentiation at the right place and time. Eur. J. Immunol. 49, 11–18 (2018).
Cherrier, D. E., Serafini, N. & Di Santo, J. P. Innate lymphoid cell development: a T cell perspective. Immunity 48, 1091–1103 (2018).
Huang, Y. et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 359, 114–119 (2018).
Kotas, M. E. & Locksley, R. M. Why innate lymphoid cells? Immunity 48, 1081–1090 (2018).
Diefenbach, A. Innate lymphoid cells in the defense against infections. Eur. J. Microbiol Immunol. (Bp) 3, 143–151 (2013).
Fuchs, A. ILC1s in tissue inflammation and infection. Front. Immunol. 7, 104 (2016).
Abt, M. C. et al. Innate immune defenses mediated by two ILC subsets are critical for protection against acute clostridium difficile infection. Cell Host. Microbe 18, 27–37 (2015).
Weizman, O. E. et al. ILC1 confer early host protection at initial sites of viral infection. Cell 171, 795–808 (2017).
Glasner, A. et al. Recognition and prevention of tumor metastasis by the NK receptor NKp46/NCR1. J. Immunol. 188, 2509–2515 (2012).
Smyth, M. J., Crowe, N. Y. & Godfrey, D. I. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int. Immunol. 13, 459–463 (2001).
Halfteck, G. G. et al. Enhanced in vivo growth of lymphoma tumors in the absence of the NK-activating receptor NKp46/NCR1. J. Immunol. 182, 2221–2230 (2009).
Dadi, S. et al. Cancer immunosurveillance by tissue-resident innate lymphoid cells and innate-like T cells. Cell 164, 365–377 (2016).
Vosshenrich, C. A. et al. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J. Immunol. 174, 1213–1221 (2005).
Ranson, T. et al. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood 101, 4887–4893 (2003).
Robinette, M. L. et al. IL-15 sustains IL-7R-independent ILC2 and ILC3 development. Nat. Commun. 8, 14601 (2017).
Cortez, V. S., Fuchs, A., Cella, M., Gilfillan, S. & Colonna, M. Cutting edge: salivary gland NK cells develop independently of Nfil3 in steady-state. J. Immunol. 192, 4487–4491 (2014).
Satoh-Takayama, N. et al. IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+cell subsets from Id2-dependent precursors. J. Exp. Med. 207, 273–280 (2010).
Burkett, P. R. et al. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+T cell homeostasis. J. Exp. Med. 200, 825–834 (2004).
Mackay, L. K. et al. The developmental pathway for CD103(+)CD8+tissue-resident memory T cells of skin. Nat. Immunol. 14, 1294–1301 (2013).
Ma, L. J., Acero, L. F., Zal, T. & Schluns, K. S. Trans-presentation of IL-15 by intestinal epithelial cells drives development of CD8alphaalpha IELs. J. Immunol. 183, 1044–1054 (2009).
Lai, Y. G. et al. IL-15 does not affect IEL development in the thymus but regulates homeostasis of putative precursors and mature CD8 alpha alpha+IELs in the intestine. J. Immunol. 180, 3757–3765 (2008).
Klose, C. S. et al. The transcription factor T-bet is induced by IL-15 and thymic agonist selection and controls CD8alphaalpha(+) intraepithelial lymphocyte development. Immunity 41, 230–243 (2014).
Cortez, V. S. et al. Transforming growth factor-beta signaling guides the differentiation of innate lymphoid cells in salivary glands. Immunity 44, 1127–1139 (2016).
Mackay, L. K. et al. T-box transcription factors combine with the cytokines TGF-beta and IL-15 to control tissue-resident memory T cell fate. Immunity 43, 1101–1111 (2015).
Intlekofer, A. M. et al. Effector and memory CD8+T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6, 1236–1244 (2005).
Pegram, H. J., Andrews, D. M., Smyth, M. J., Darcy, P. K. & Kershaw, M. H. Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol. 89, 216–224 (2011).
Lanier, L. L. Up on the tightrope: natural killer cell activation and inhibition. Nat. Immunol. 9, 495–502 (2008).
Yokoyama, W. M. & Kim, S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol. Rev. 214, 143–154 (2006).
Gleimer, M. & Parham, P. Stress management: MHC class I and class I-like molecules as reporters of cellular stress. Immunity 19, 469–477 (2003).
Bauer, S. et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285, 727–729 (1999).
Jamieson, A. M. et al. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity 17, 19–29 (2002).
Savage, P. A. et al. Recognition of a ubiquitous self antigen by prostate cancer-infiltrating CD8 + T lymphocytes. Science 319, 215–220 (2008).
Raulet, D. H. & Guerra, N. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat. Rev. Immunol. 9, 568–580 (2009).
Smyth, M. J. et al. NKG2D function protects the host from tumor initiation. J. Exp. Med. 202, 583–588 (2005).
Bando, J. K. & Colonna, M. Innate lymphoid cell function in the context of adaptive immunity. Nat. Immunol. 17, 783–789 (2016).
Acknowledgements
We thank Chun Chou, Briana Nixon, and Efstathios Stamatiades of the Li lab for helpful discussions and critical reading of the manuscript. Work in the Li laboratory was supported by NIAID (R01 CA198280–01 to M.O.L), HHMI (Faculty Scholar Award to M.O.L.), the Ludwig Center for Cancer Immunology (M.O.L.), and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kansler, E.R., Li, M.O. Innate lymphocytes—lineage, localization and timing of differentiation. Cell Mol Immunol 16, 627–633 (2019). https://doi.org/10.1038/s41423-019-0211-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-019-0211-7
- Springer Nature Limited
This article is cited by
-
Comprehensive immune cell spectral library for large-scale human primary T, B, and NK cell proteomics
Scientific Data (2024)
-
Reversing immunosuppression in the tumor microenvironment of fibrolamellar carcinoma via PD-1 and IL-10 blockade
Scientific Reports (2024)
-
The secretome of Staphylococcus aureus strains with opposite within-herd epidemiological behavior affects bovine mononuclear cell response
Veterinary Research (2023)
-
Chimeric antigen receptor- and natural killer cell receptor-engineered innate killer cells in cancer immunotherapy
Cellular & Molecular Immunology (2021)