Abstract
Since their initial identification more than 10 years ago, innate lymphoid cells (ILCs) have emerged as denizens of an immune realm parallel to that of T cells. Here I highlight basic similarities shared by all and underscore features related to development, tissue residency and regulation that distinguish ILCs from T cells. I further discuss the potential of ILCs as promising targets for therapeutic intervention in human diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 ILCs and T Cells: Identification of Two Parallel Universes
Until 2009, immunologists knew two types of innate lymphoid cells (ILCs): natural killer (NK) cells and lymphoid tissue inducer (LTi) cells. Extensive characterization of NK cells demarcated their innate capacity to kill tumor cells and virally infected cells without previous exposure [1,2,3,4]. Recruitment of LTi cells to embryonal anlagen was found to be essential for the development of lymph nodes and Peyer’s patches [5]. The limited diversity of ILCs was in stark contrast with that of T cells, which encompassed cytotoxic CD8 T cells and a variety of helper CD4 T cells, each with a distinct functional polarization, including TH1, TH2, TH17, TFH, and Treg. After 2009, a number of groups began to identify novel subsets of ILCs, each also defined by a discrete functional polarization, that are now called ILC1, ILC2, and ILC3. Remarkably, this diversity mirrors that of TH1, TH2, and TH17, respectively [6]. Assuming that cytotoxic NK cells are the innate counterparts of CD8 T cells, it became evident that T cells and ILCs represented two parallel universes of cell types with the same functional modules: cytotoxic (perforin and granzymes), type 1 (IFNγ), type 2 (IL-4, IL-5, IL-13), and type 3 (IL-17, IL-22). T cells differ from ILCs mainly in their capacity to recognize specific antigens through the T cell receptor. In retrospect this makes perfect sense: ILCs serve as tissue “first responders” by sensing the release of soluble inflammatory mediators during infections and tissue damage and rapidly communicating danger via cytokine secretion; in T cells these host defense modules are further equipped with the T cell receptor, which provides specificity and memory for a targeted pathogen.
1.2 The Importance of Tissue Residency
Why did immunologists miss ILCs for such a long time? At least two reasons contribute to this: first, ILCs comprise very small cell populations that were difficult to detect with the available tools years ago; second and perhaps more importantly, most of these cells reside in peripheral tissues rather than in blood and lymphoid organs. Despite encompassing crucial sites for immune responses, peripheral tissues fell below the radar of immunologists before 2009, especially in humans. Thus, another important outcome of the discovery of ILCs has been appreciating the relevance and diversity of immune cells in peripheral tissues.
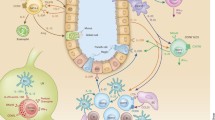
ILC diversity in tissues extends far beyond the strict definitions of NK cells, ILC1, ILC2, and ILC3. Single-cell RNA sequencing of ILCs in different tissues has unveiled substantial transcriptional heterogeneity, as each major ILC group presents distinct tissue-specific features that reflect the influence of the tissue microenvironment on phenotype and function [7,8,9,10,11,12]. Many tissue factors imprint ILCs: cytokines produced in steady state, such as TGFβ, or during tissue damage, particularly pro-inflammatory cytokines [13,14,15]; microbiota, bacterial products, and nutrients to which the tissue is exposed, such as short-chain fatty acids, ligands of the aryl hydrocarbon receptor, and vitamin A [16,17,18,19,20]; oxygen tension [21]; growth factors, like IGF-1 [22]; lipid mediators, such as prostaglandins [23, 24]; and neurotransmitters released by nerve fibers [25,26,27]. ILCs also express quorum-sensing molecules and chemokine receptors that further regulate their function based on cell density and spatial localization [28]. Overall, these observations emphasize the impact of tissue microenvironment on controlling ILC diversity and, conversely, the ability of ILCs to functionally adapt to local stimuli and tailor their responses to the tissue niche.
One of the latest developments in this regard is the recent observation that ILCs, particularly ILC3 in the gastrointestinal tract, adapt not only to their location, but also to circadian fluctuations of the tissue [29, 30]. The gastrointestinal tract, as many organs, is attuned to a circadian rhythm that primarily ensues in response to light and dark. ILC3 in the gastrointestinal tract express clock genes that undergo circadian oscillations and control the expression of genes encoding for cytokines. Moreover, ILC3 circadian oscillations are coordinated with the central clock in the suprachiasmatic nucleus of the central nervous system. As ILC3 expresses receptors for peptide neurotransmitters, the suprachiasmatic nucleus can connect long distance with ILC3 through enteric neurons secreting these peptides. Moreover, the daily cycle of nutrition can further contribute to entrain the ILC3 clock by food-induced circadian stimulation of enteric neurons [31]. It is likely that circadian rhythms control ILC functions in many other tissues to ensure timely coordination of ILC functions with those of the tissue.
1.3 ILC Versus T Cell Commitment: Shared and Unique Pathways
The parallel between ILC and T cell functional models extends to their development. Similar to T cells, all ILC subsets and NK cells originate from common lymphoid progenitors (CLPs). The commitment of CLPs towards ILCs rather than T cells occurs early on at the level of the common innate lymphoid progenitor CILP, the foremost progenitor with restricted potential to generate ILCs and NK cells [32, 33]. CILPs then differentiate into progenitors with more restricted potential, which together give rise to NK cells and all ILCs: the NK progenitor that gives rise to NK cells, the common helper lymphoid progenitor (CHILP), and the innate lymphoid cell progenitor (iLCP). The transcriptional repressor Id2 is a primary switch that propels the differentiation of CLPs towards NK cells and ILCs by blocking E2 family transcription factors that prompt T cell development [34,35,36]. However, the final delineation of NK cells, ILC1, ILC2, and ILC3 lineages from innate precursors largely depends on the same transcription factors that mediate polarization of T cells, i.e., Eomes, Tbet, GATA3, and Rorγt.
Epigenetic studies have confirmed the lineage and functional kinship of ILCs with their T cell counterparts. Analyses of gene regulatory circuitries of ILC-T pairs have revealed that each ILC type shares a circuitry devoted to lineage commitment and functional polarization with its Th counterpart [37]. However, epigenetic differences do exist between ILCs and their T cell counterparts, which are mainly related to their activating signals. T cell activation requires signals from TCR and co-stimulatory receptors, which drive expansion, differentiation, and cytokine expression. In contrast, ILC effector responses chiefly depend on tissue stimuli, endowing them with more rapid response profiles. Thus, ILCs and T cells employ both shared and divergent enhancers to express genes dependent on activating signals. The fundamental nature of these epigenetic differences in ILCs and T cells is still not well understood. Transcriptomic and chromatin accessibility studies on ILC subsets have been instrumental in comprehending the complexity of transcriptional modules in these cells. To build upon these initial findings, we should begin interrogating the epigenetic mechanisms that establish these modules, such as histone modifications, DNA methylation, and 3D chromatin conformation, as well as identifying ILC-specific regulatory elements.
Finally, it should be noted that ILCs have been shown to be rather plastic and can toggle their functional polarization in order to adapt their responses to disparate tissues and diverse pathogenic stimuli [13, 38]. Thus, it is possible that ILC lineage commitment is somewhat changeable and that gene regulatory circuitries may be flexible or reversible in certain contexts. It will be important to determine whether lineage commitment and plasticity are governed by distinct epigenetic modifications.
1.4 Where Do ILCs Develop?
Recent studies on macrophage development have identified two types of progenitors [39, 40]. Tissue-resident macrophages derive mainly from embryonal progenitors, which colonize developing tissues and persist throughout life by self-renewing. Monocyte-derived macrophages are generated from bone marrow progenitors during definitive hematopoiesis and populate tissues during inflammation and remodeling. Although ILCs have been extensively shown to develop from bone marrow progenitors, some ILC subsets are predominantly present in fetal tissues and tend to decline with age. Thus, it is possible that ILCs, like macrophages, develop in part from embryonal or fetal progenitors and populate peripheral tissues, generating a subset of cells capable of self-maintenance [41]. ILC development has also been observed in the thymus when T cell progenitors deviate from their developmental trajectory and become ILCs rather than mature T cells [42]. Human studies have also suggested the possibility that ILCs and NK cells may develop in part from fetal liver and thymus. Thus, it is likely that ILC diversity depends not only on tissue localization, but also on developmental origin. Future studies should address the life span of ILCs originating from disparate origins in both steady state and disease. Moreover, it is important to see whether human ILCs generated in vitro from various hematopoietic and/or lymphopoietic sources can be eventually exploited in cell-based therapies.
1.5 Tregs and TFH: Why in T Cells Only?
It is of note that the similarity between ILCs and T cells seems limited to effector modules. In contrast to T cells, ILCs have not developed a separate lineage, such as Foxp3+ Tregs, dedicated to limiting the effector subsets. However, some ILCs can behave as regulatory cells. One subset of ILC2 can produce IL-10, acting as a regulatory cell in contexts in which ILC2 is exposed to IL-2 and IL-10 [43, 44]. ILC3 can induce tolerogenic T cells [45]. Through the expression of MHC class II, ILC3 can present antigens to T cells in a modality that induces tolerogenic rather than activated T cells, as ILC3 lacks costimulatory molecules. Additionally, ILC3 produces IL-2, which sustains Tregs [46]. These observations open interesting questions: Which mechanisms mediate antigen endocytosis and processing in ILC3? How does ILC3 antigen presentation differ from that of dendritic cells? What is the relative contribution of each to T cell responses? Do ILC3 and DC directly cooperate in T cell activation?
No direct counterpart of TFH has been identified in ILCs. However, ILCs can stimulate B cell responses through multiple mechanisms. Embryonal LTi cells promote the ontogenesis of secondary lymphoid organs, which are essential for B cell responses [5]. Similarly, LTi-like ILC3 promotes the postnatal generation of intestinal cryptopatches, the antecedents of B cell-rich lymphoid follicles that produce IgA [47] and enhance IgA production in lamina propria and Peyer’s patches by interacting with DCs [48, 49]. LTi cells and ILC3 also express lymphotoxins that promote B cell expansion and differentiation [50]. Thus, although ILCs have not developed a unique counterpart of TFH cells, they do provide help to B cell responses.
1.6 ILCs: A Target for Disease Treatment?
Since their discovery, ILCs have been implicated in the defense against intracellular bacteria, extracellular bacteria, fungi, parasites, and viruses [6]. On the other hand, their uncontrolled activation has been implicated in various autoimmune diseases and allergies [51, 52]. More recent studies have shown that ILCs control lipid absorption and metabolism in the gut and adipose tissue [31, 53,54,55] and can participate in immune responses to cancer, with either pro-tumorigenic or anti-tumorigenic effects [56,57,58]. Beyond mouse models, ILCs have also been implicated in human diseases, with beneficial effects in infections and detrimental effects in autoimmune and allergic diseases [59]. Given the broad impact of ILCs in diseases, can we consider ILCs as suitable targets for immunotherapy? What tools are available for modulating ILCs? There is a major barrier that prevents a satisfying answer to these questions. ILCs share many programs and molecules with T cells. Because of this original sin, it is difficult to dissect the role of ILCs from that of T cells as well as to target ILCs independently of T cells. There are only few animal models in which ILCs can be selectively investigated. Indeed, to date, the most frequently used mouse model is the Rag knockout mouse in which adaptive responses are missing. Better models should be developed to analyze the impact of ILCs in the context of intact adaptive responses. No antibodies have been developed that can selectively deplete ILCs without impacting T cells in mice or humans. Available drugs (antibodies and small molecules) targeting ILC cell surface molecules [60], cytokines (IFNγ, IL-5, IL-13, IL-17, IL-22), signaling mediators (such as JAKs), and transcription factors (such as Rorγt) equally target T cells. One essential direction for future studies is the development of more sophisticated approaches to specifically modify ILCs in the context of mouse models and human diseases. Additionally, given the development of NK cell adoptive therapies for treating cancer [61] it is important to determine whether ILCs can also be effectively generated in vitro and used for adoptive transfer therapies to enhance innate immune responses [62] or, conversely, to modulate adaptive responses, depending on the context. Nonetheless, ILCs do have the potential to become the focus of a new generation of immunotherapies.
References
Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer. 2016;16(1):7–19.
Geiger TL, Sun JC. Development and maturation of natural killer cells. Curr Opin Immunol. 2016;39:82–9.
Lam VC, Lanier LL. NK cells in host responses to viral infections. Curr Opin Immunol. 2017;44:43–51.
Hammer Q, Ruckert T, Romagnani C. Natural killer cell specificity for viral infections. Nat Immunol. 2018;19(8):800–8.
Bar-Ephraim YE, Mebius RE. Innate lymphoid cells in secondary lymphoid organs. Immunol Rev. 2016;271(1):185–99.
Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells: 10 years on. Cell. 2018;174(5):1054–66.
Gury-Ben Ari M, Thaiss CA, Serafini N, Winter DR, Giladi A, Lara-Astiaso D, et al. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell. 2016;166(5):1231–46 e13.
Yu Y, Tsang JC, Wang C, Clare S, Wang J, Chen X, et al. Single-cell RNA-seq identifies a PD-1(hi) ILC progenitor and defines its development pathway. Nature. 2016;539(7627):102–6.
Xu H, Ding J, Porter CBM, Wallrapp A, Tabaka M, Ma S, et al. Transcriptional atlas of intestinal immune cells reveals that neuropeptide alpha-CGRP modulates group 2 innate lymphoid cell responses. Immunity. 2019;51(4):696–708 e9.
Bielecki P, Riesenfeld SJ, Hutter JC, Torlai Triglia E, Kowalczyk MS, Ricardo-Gonzalez RR, et al. Skin-resident innate lymphoid cells converge on a pathogenic effector state. Nature. 2021;592(7852):128–32.
McFarland AP, Yalin A, Wang SY, Cortez VS, Landsberger T, Sudan R, et al. Multi-tissue single-cell analysis deconstructs the complex programs of mouse natural killer and type 1 innate lymphoid cells in tissues and circulation. Immunity. 2021;54(6):1320–37 e4.
Hernandez DC, Juelke K, Muller NC, Durek P, Ugursu B, Mashreghi MF, et al. An in vitro platform supports generation of human innate lymphoid cells from CD34(+) hematopoietic progenitors that recapitulate ex vivo identity. Immunity. 2021;54(10):2417–32.
Colonna M. Innate lymphoid cells: diversity, plasticity, and unique functions in immunity. Immunity. 2018;48(6):1104–17.
Domingues RG, Hepworth MR. Immunoregulatory sensory circuits in group 3 innate lymphoid cell (ILC3) function and tissue homeostasis. Front Immunol. 2020;11:116.
Meininger I, Carrasco A, Rao A, Soini T, Kokkinou E, Mjosberg J. Tissue-specific features of innate lymphoid cells. Trends Immunol. 2020;41(10):902–17.
Eberl G. Development and evolution of RORgammat+ cells in a microbe's world. Immunol Rev. 2012;245(1):177–88.
Belkaid Y, Harrison OJ. Homeostatic immunity and the microbiota. Immunity. 2017;46(4):562–76.
Britanova L, Diefenbach A. Interplay of innate lymphoid cells and the microbiota. Immunol Rev. 2017;279(1):36–51.
Chun E, Lavoie S, Fonseca-Pereira D, Bae S, Michaud M, Hoveyda HR, et al. Metabolite-sensing receptor Ffar 2 regulates colonic group 3 innate lymphoid cells and gut immunity. Immunity. 2019;51(5):871–84 e6.
Fachi JL, Secca C, Rodrigues PB, Mato FCP, Di Luccia B, Felipe JS, et al. Acetate coordinates neutrophil and ILC3 responses against C. difficile through FFAR2. J Exp Med. 2020;217(3):20190489.
Pral LP, Fachi JL, Correa RO, Colonna M, Vinolo MAR. Hypoxia and HIF-1 as key regulators of gut microbiota and host interactions. Trends Immunol. 2021;42(7):604–21.
Zhou L, Sonnenberg GF. In situ support of ILC precursors. Immunity. 2020;52(2):207–9.
Kabata H, Moro K, Koyasu S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol Rev. 2018;286(1):37–52.
Konya V, Mjosberg J. Lipid mediators as regulators of human ILC2 function in allergic diseases. Immunol Lett. 2016;179:36–42.
Veiga-Fernandes H, Mucida D. Neuro-immune interactions at barrier surfaces. Cell. 2016;165(4):801–11.
Klose CS, Artis D. Neuronal regulation of innate lymphoid cells. Curr Opin Immunol. 2019;56:94–9.
Godinho-Silva C, Cardoso F, Veiga-Fernandes H. Neuro-immune cell units: a new paradigm in physiology. Annu Rev Immunol. 2019;37:19–46.
Secca C, Bando JK, Fachi JL, Gilfillan S, Peng V, Di Luccia B, et al. Spatial distribution of LTi-like cells in intestinal mucosa regulates type 3 innate immunity. Proc Natl Acad Sci. 2021;118:23.
Burrows K, Mortha A. Going green with solar-powered ILC3 homeostasis. Sci Immunol. 2019;4(40):0433.
Jacquelot N, Belz GT, Seillet C. Neuroimmune interactions and rhythmic regulation of innate lymphoid cells. Front Neurosci. 2021;15:657081.
Talbot J, Hahn P, Kroehling L, Nguyen H, Li D, Littman DR. Feeding-dependent VIP neuron-ILC3 circuit regulates the intestinal barrier. Nature. 2020;579(7800):575–80.
Yu X, Wang Y, Deng M, Li Y, Ruhn KA, Zhang CC, et al. The basic leucine zipper transcription factor NFIL3 directs the development of a common innate lymphoid cell precursor. Elife. 2014;3:e04406.
Ishizuka IE, Constantinides MG, Gudjonson H, Bendelac A. The innate lymphoid cell precursor. Annu Rev Immunol. 2016;34:299–316.
Bedoui S, Gebhardt T, Gasteiger G, Kastenmuller W. Parallels and differences between innate and adaptive lymphocytes. Nat Immunol. 2016;17(5):490–4.
Qian L, Bajana S, Georgescu C, Peng V, Wang HC, Adrianto I, et al. Suppression of ILC2 differentiation from committed T cell precursors by E protein transcription factors. J Exp Med. 2019;216(4):884–99.
Spinner CA, Lazarevic V. Transcriptional regulation of adaptive and innate lymphoid lineage specification. Immunol Rev. 2021;300(1):65–81.
Fernando N, Sciume G, O'Shea JJ, Shih HY. Multi-dimensional gene regulation in innate and adaptive lymphocytes: a view from regulomes. Front Immunol. 2021;12:655590.
Bal SM, Golebski K, Spits H. Plasticity of innate lymphoid cell subsets. Nat Rev Immunol. 2020;20(9):552–65.
Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol. 2015;15(12):731–44.
Bleriot C, Chakarov S, Ginhoux F. Determinants of resident tissue macrophage identity and function. Immunity. 2020;52(6):957–70.
Turner JE, Gasteiger G. Innate lymphoid cells: key players in tissue-specific immunity. Semin Immunopathol. 2018;40(4):315–7.
Wang HC, Qian L, Zhao Y, Mengarelli J, Adrianto I, Montgomery CG, et al. Downregulation of E protein activity augments an ILC2 differentiation program in the thymus. J Immunol. 2017;198(8):3149–56.
Seehus CR, Kadavallore A, Torre B, Yeckes AR, Wang Y, Tang J, et al. Alternative activation generates IL-10 producing type 2 innate lymphoid cells. Nat Commun. 2017;8(1):1900.
Bando JK, Gilfillan S, Di Luccia B, Fachi JL, Secca C, Cella M, et al. ILC2s are the predominant source of intestinal ILC-derived IL-10. J Exp Med. 2020;217(2):e20191520.
Sonnenberg GF, Hepworth MR. Functional interactions between innate lymphoid cells and adaptive immunity. Nat Rev Immunol. 2019;19(10):599–613.
Zhou L, Chu C, Teng F, Bessman NJ, Goc J, Santosa EK, et al. Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature. 2019;568(7752):405–9.
Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov II, et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29(2):261–71.
Kruglov AA, Grivennikov SI, Kuprash DV, Winsauer C, Prepens S, Seleznik GM, et al. Nonredundant function of soluble LTalpha3 produced by innate lymphoid cells in intestinal homeostasis. Science. 2013;342(6163):1243–6.
Reboldi A, Arnon TI, Rodda LB, Atakilit A, Sheppard D, Cyster JG. IgA production requires B cell interaction with subepithelial dendritic cells in Peyer’s patches. Science. 2016;352(6287):aaf 4822.
Magri G, Miyajima M, Bascones S, Mortha A, Puga I, Cassis L, et al. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat Immunol. 2014;15(4):354–64.
Ebbo M, Crinier A, Vely F, Vivier E. Innate lymphoid cells: major players in inflammatory diseases. Nat Rev Immunol. 2017;17(11):665–78.
Friedrich M, Pohin M, Powrie F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity. 2019;50(4):992–1006.
O'Sullivan TE, Rapp M, Fan X, Weizman OE, Bhardwaj P, Adams NM, et al. Adipose-resident group 1 innate lymphoid cells promote obesity-associated insulin resistance. Immunity. 2016;45(2):428–41.
Guendel F, Kofoed-Branzk M, Gronke K, Tizian C, Witkowski M, Cheng HW, et al. Group 3 innate lymphoid cells program a distinct subset of IL-22BP-producing dendritic cells demarcating solitary intestinal lymphoid tissues. Immunity. 2020;53(5):1015–32.
Cardoso F, Klein Wolterink RGJ, Godinho-Silva C, Domingues RG, Ribeiro H, da Silva JA, et al. Neuro-mesenchymal units control ILC2 and obesity via a brain–adipose circuit. Nature. 2021;597(7876):410–4.
Panda SK, Colonna M. Innate lymphoid cells: a potential link between microbiota and immune responses against cancer. Semin Immunol. 2019;41:101271.
Bald T, Wagner M, Gao Y, Koyasu S, Smyth MJ. Hide and seek: plasticity of innate lymphoid cells in cancer. Semin Immunol. 2019;41:101273.
Stamatiades EG, Li MO. Tissue-resident cytotoxic innate lymphoid cells in tumor immunosurveillance. Semin Immunol. 2019;41:101269.
Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17(7):765–74.
Guia S, Fenis A, Vivier E, Narni-Mancinelli E. Activating and inhibitory receptors expressed on innate lymphoid cells. Semin Immunopathol. 2018;40(4):331–41.
Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18(2):85–100.
Cobb LM, Verneris MR. Therapeutic manipulation of innate lymphoid cells. JCI Insight. 2021;6(6):e146006.
Acknowledgements
I would like to thank Susan Gilfillan, Vincent Peng, and Marina Cella for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Colonna, M. (2022). Overview: Themes in Innate Lymphoid Cell Biology. In: Sun, XH. (eds) Innate Lymphoid Cells. Advances in Experimental Medicine and Biology, vol 1365. Springer, Singapore. https://doi.org/10.1007/978-981-16-8387-9_1
Download citation
DOI: https://doi.org/10.1007/978-981-16-8387-9_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-8386-2
Online ISBN: 978-981-16-8387-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)