Abstract
Cancer as a disease is a multifaceted foe which sometimes succumbs to the prescribed treatment and sometimes develops resistance against various therapies. Conventional cancer therapies suffer from many limitations, the least of which is their specificity and systemic side effects. In a majority of cases, acquired mutations render the cancer cells resistant to therapy and lower the prognostic outcome. In the constant effort to devise a therapeutic moiety which can comprehensively eliminate cancer cells, oncolytic viruses provide an attractive avenue as they selectively infect and lyse cancer cells sparing normal cells from their effects. Viruses can be engineered for their host specificity and toxicity as a promising anti-cancer tool. As it is essential to devise a strategy to address all targets involved in cancer development and progression, the idea of using oncolytic viruses with enhanced anti-cancer activity through arming with foreign genes gained merit and is showing promising advent in clinical studies. The use of oncolytic viruses as an agent of combination therapy for cancer treatment also gained much attention in the recent past. This review focuses on the emerging role of oncolytic viruses as vital components of anti-cancer regimen presenting a new dimension in an ever-changing cancer therapy scenario.
Similar content being viewed by others
Introduction
Current scenario in cancer therapy and lack of comprehensiveness
Cancer therapy is most commonly associated with a combination of surgery, chemotherapy and radiotherapy. The success of surgical removal of tumor relies heavily upon early diagnosis and ease of access but does not guarantee complete removal of primary tumor mass or of metastasized cells. Radiation therapy similarly is bound by inadequate specificity and systemic toxicity. In addition to precincts of efficacy, delivery and penetration of drugs to target site, chemotherapy is mired with various side effects as well as emergence of drug-resistant tumor cells. In addition, drug inactivation, target alteration, DNA mutation and damage repair, cell death inhibition, epigenetics and epithelial–mesenchymal transition [1] lead to cancer relapse unresponsive to established chemotherapeutic drugs.
Advances in molecular biology have led to the emergence of gene therapy as a viable tool for cancer treatment. Contrary to conventional cancer treatment, gene therapy projects a more sustainable therapeutic approach where genetic defects associated with cancer can be substituted or anti-tumor genes can be introduced. This transfer is facilitated by various viral, bacterial and chemical vectors among which viral vectors have garnered interest for their targeted approach. Viral vector-mediated gene therapy had immense success in the treatment of various monogenetic diseases but is unable to replicate it in the majority of cancers where genetic variations among the individuals or within different tumor sites in a patient is substantial [2]. All these approaches are quite effective on their own but their reliance on conjecture for disease progression and the dynamic nature of cancer cells has caused lacunae to develop a therapy which addresses the obstacles of targeted delivery such as efficient internalization at the effector site and efficient expression of anti-tumor genes. At present, there is a need to devise a therapeutic moiety which can be a template with provisions to make it effective towards any cancer type.
Recombinant viruses provide an attractive avenue towards the development of an all-encompassing cancer therapy
With the prior history of viruses causing spontaneous tumor regression [3, 4], the idea of viruses as an oncolytic agent has become a new reality of multimodal cancer therapy. Viruses as disease-causing pathogens exhibit traits such as host specificity, regulation of host cellular processes for efficient viral replication and the host cell lysis. These features of them can be exploited specifically against cancer cells for the generation of viruses as oncolytic agents.
Oncolytic viruses (OVs) are therapeutically useful anti-cancer agents that selectively infect and damage cancerous tissue without harming normal tissue [5, 6]. An ideal OV should exhibit a high replicative capacity in vivo, ability to infect both dividing and non-dividing cells, inability of chromosomal integration, lack of disease induction and absence of pre-existing antibodies to the virus in the host population. As of now, no single OV has all the desired features but various experimental approaches are being employed to develop a recombinant virus as a vital part of anti-cancer therapy.
Oncolytic virotherapy relies on cancer-specific replication of virus triggering tumor cell death by a number of mechanisms including direct lysis, expression of toxic proteins, autophagy and induction of apoptosis. In addition, OVs can mediate the killing of uninfected cancer cells by indirect mechanisms such as the induction of anti-angiogenic response [7], anti-cancer immune response [8] or through the specific activities of transgene encoded proteins expressed from engineered viruses [5, 6]. There are a number of viruses with natural preference to infect cancer cells such as parvovirus, reovirus, Newcastle Disease Virus (NDV), Mumps virus and Moloney leukemia virus, whereas viruses such as measles, adenovirus, vesicular stomatitis virus (VSV), vaccinia and herpes simplex virus (HSV) can be adapted to infect cancer cells by repeated laboratory cultures [9] or engineered for cancer specificity. However, for a virus to be an oncolytic agent, stringent criteria related to safety of the population from pathogenic reversion or evolution of a novel strain or of person-to-person transmission of the OV have to be followed.
Development of a virus into an oncolytic agent
Virus that may or may not be naturally inclined for oncolysis can be developed into an oncolytic agent by manipulating its genome to enhance its specificity and toxic profile against cancer cells. Viruses can also be engineered to encode additional transcriptional units to modulate virus biology against cancer cells [10]. Following the advent of molecular biology techniques, the early era of OVs was dominated by attenuation of viruses for better safety profile and retargeting viral entry to non-natural hosts. The first-generation OVs were attenuated viruses. Their tumor specificity was natural or was acquired through laboratory passages, but the anti-tumor effect was limited to a small percentage of tumor types. Second-generation OVs were retargeted through genetic modifications for selective internalization or selective replication. Selective internalization was achieved by modifying the viral proteins engaged with specific receptors or their mutants overexpressed in tumor cells. Specificity was also attained for viral gene expression by introducing tumor-specific promoters or generating deletion mutants of viruses where the deletion is substituted in cancer cells. Targeting was also done based on tumor microenvironment and the expression profile of cancer cells towards cell death. Extensive work was done to generate recombinant viruses for oncolytic activity with desirable safety and selectivity. Numerous studies have reported the construction; modification and retargeting of OVs and some examples where tumor specificity and regression was achieved are listed in the Table 1.
Third-generation OVs are not only attenuated and retargeted, but also armed with additional genetic element(s) of viral or non-viral origin to enhance their anti-tumor activity. Generally, OVs are designed to exploit the pathways responsible for induction of apoptosis and to multiply by exploiting the abrogated cell cycle machinery which may induce cell death. The general targets are cancer cells with defective or downregulated p53 tumor-suppressor protein, RAS/PKR, IFN/PKR, p16/Rb pathways or other pro-apoptotic signals [11]. Here we discuss about the armed OVs and their application in cancer therapy in combination with pre-existing cancer regimens.
Arming of OVs to address cancer-specific adaptations
As stated earlier, robust anti-tumor activity can be achieved by inserting cytotoxic elements into OV genome. This ‘arming’ of OV potentiates the therapeutic index of virus-mediated anti-cancer gene therapy by efficient delivery and expression of transgene. Although the use of viral vectors for anti-cancer gene therapy has been quite popular in cases such as Gendicine, an Ad5 vector approved by China’s State Food and Drug Administration for treatment of head and neck cancer in 2004 [12], these were mainly replication-incompetent viruses which served as one-time effector molecule. Thus, the development of replication-competent and conditionally replicating OVs has provided a platform where the viral genome can be equipped with transgenes to generate a stable and sustainable production of both transgene proteins and viral progeny. Transgenes encoding functions of tumor suppression, apoptosis, anti-angiogenesis and immunomodulation [13] are largely chosen for insertion into the OV genome.
Introduction of OVs has opened up new avenues of establishing host immune response against tumor cells. Generally, tumor cells produce immunosuppressive cytokines (e.g., transforming growth factor-β) and recruit cells to inhibit immune response (e.g. regulatory T cells) to halt the host defense mechanism [14]. With OVs, it is now possible to combine debulking of tumor and attack on tumor vasculature due to virus-induced cell lysis with effective activation of adaptive and innate immune response [6, 15]. In fact, the 2015 FDA (The Food and Drug Administration) approved T-Vec, an HSV-based therapy for the treatment of surgically unresectable melanoma, supports this possibility, as in addition to double deletion of γ34.5 and α47genes, it has granulocyte-macrophage colony-stimulating factor (GM-CSF) at the deleted γ34.5 loci [16].
Viruses are also being armed with secretory factors inducing apoptosis, functional p53 gene, prodrug activation gene as well as immune checkpoints which are abrogated in tumor cells. For example, in case of gene directed enzyme prodrug therapy, viral vectors are armed with suicide genes which can convert low cytotoxicity prodrugs into potent cytotoxic agents against cancer cells. The case in point is GLV-1h68, a strain of vaccinia virus carrying β-galactosidase (lacZ), that, supplied with prodrug derived from a seco-analog of the natural antibiotic duocarmycin SA, caused tumor regression and activation of intrinsic apoptotic pathway in human G1-101A breast cancer xenografts [17] by circumventing the presence of anti-apoptotic viral genes in favor of toxicity of converted prodrug.
Viruses have also been armed to exploit antibody-based cancer therapy, where complement activation and cytotoxic effects of antibody towards tumor vasculature enhanced the therapeutic efficacy. One such example can be seen in case of orthotopic hepatoma-bearing mice treated with velogenic NDV Italien strain armed with chimeric mouse–human antibody targeting CD147 (cHAb18) overexpressed in hepatocellular carcinoma (HCC). The recombinant virus (rNDV-18HL) showed tumor specificity and inhibition of intra-hepatic metastasis of HCC causing prolonged survival [18]. Another study explored the sequential administration of NDV and adenovirus armed with a cytokine, oncostatin M (human), which promotes antigen presentation and co-stimulatory signals triggering anti-tumor immunity. It showed significant anti-tumor activity and immune response leading to increased survival of orthotopic model of pancreatic ductal adenocarcinoma in Syrian hamster. This study elucidated the optimal expression of transgene and low serum concentration of oncostatin M is desirable for maximum effect and low systemic toxicity as well as principle of prime boost by using two antigenically unrelated OVs to overcome the neutralizing antibody interference [19].
Oncolytic virus in combination therapies
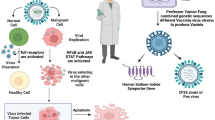
Armed OVs can either be developed as a standalone anti-cancer regimen or as a synergistic component of an established anti-cancer approach. As a standalone therapy, OVs are sometimes restricted by the tumor microenvironment, host-mounted anti-viral response as well as pre-existing neutralizing antibodies decreasing the overall effectivity. In addition, the repertoire of approved candidates constitutes only T-Vec with other OVs still at various stages of clinical trials, resulting in a narrow window of selection and efficiency for treatment of various cancers. Thus, it may be beneficial to combine OVs with conventional anti-cancer therapies to improve the treatment outcome as the multi component regimen can address the shortcomings of each component as a standalone thereby making it more effective (Fig. 1). However, in doing so there is a possibility of either chemotherapy or radiotherapy negatively affecting the viral replication [20, 21]. Thus, it is imperative to analyze the significance of anti-cancer potential of OVs amidst all the established therapies and their role in instituting a comprehensive treatment module.
Effects induced by oncolytic virotherapy combined with other anti-cancer therapeutics. Use of different treatment modalities such as chemotherapy, radiotherapy, immunotherapy and HDACi for antitumor activity in combination with viruses engineered for oncolysis facilitates the replication of recombinant viruses, thus inducing enhanced lysis of cancer cells. Likewise, oncolytic viruses sensitize the tumors to other therapeutics and enable them to exert anti-cancer effects efficiently even at lower doses. By and large, the effects induced by virotherapy and another therapeutic component together lead to tumor reduction and improved treatment outcome
Radiation therapy
Oncolytic virotherapy and radiotherapy are two different treatment modalities, but pre-clinical studies have indicated their synergistic anti-tumor role. This combination has exhibited a significant enhancement of anti-cancer activity with various OVs. Variants of recombinant HSV have shown increased viral load [22] and appreciable toxicity against various carcinomas when combined with radiation therapy. For example, mutated HSV (G207) with ICP6/γ34.5 deletions combined with radiation exhibited multi-fold increased toxicity and reduction of carcinoma in cervical cancer mouse models [23]. Similar effects were reported in the case of colorectal cancer mouse xenograft where the combination of G207 and low-dose radiation resulted in upregulation of ribonucleotide reductase causing increased anti-cancer toxicity [24]. Another HSV variant NV1066, with ICP0/ICP4/γ34.5 deletions administered in combination with radiation, resulted in the reduction of tumor mass in xenogenic mice tumor flank model of non-small-cell lung carcinoma [25] and mesothelioma [26]. This synergism can be attributed to upregulation of GADD34 in response to radiation-induced DNA damage, as carboxy terminus of mammalian GADD34 shares structural homology with deleted viral neurovirulence gene ICP34.5 and substitutes its action in cells to favor viral replication leading to enhanced oncolysis [25]. Temporal sequestration of radiation with respect to viral gene expression has been reported to cause regression in high-grade glioma mouse models as irradiation is known to enhance the late promoter genes of HSV-1 [27]. Similarly, measles virus encoding for human carcinoembryonic antigen (MV-CEA) in combination with radiation therapy has shown a significant regression of tumors in subcutaneous model of human gliomas [28].
Combination of oncolytic adenovirus and radiation has also shown significantly greater toxicity as compared to single-agent treatment modalities [29, 30]. ONYX-015, a mutant adenovirus with E1B-55k gene deletion, has been reported to enhance radiation-induced cytotoxicity in vitro and in vivo in mice xenograft model of anaplastic thyroid cancer [31] as well as in mice xenograft model derived from primary human malignant glioma [32]. Two prostate-specific adenoviral vectors CV706 [33] and CV787 [34] in combination with radiation resulted in reduction of tumor mass and serum prostate-specific antigens in xenograft mouse models for prostate cancer. Gendicine (E1/E3 deletions expressing p53 under RSV promoter) in combination with radiation [35] and chemotherapy [12] has been approved as intra-tumoral therapy against head and neck squamous cell carcinoma in China. AdΔ24 (24-bp deletion in C2 domain of E1A region) and AdΔ24-p53 (p53 gene in deleted E3 region) have shown increased anti-tumor efficacy in combination with radiation in mice xenograft model of therapy-resistant glioma [29].
VSV expressing tumor-associated antigens (TAAs) has shown significant reduction in locally established and metastasized mouse model of oligometastatic melanoma in combination with stereotactic ablative radiation therapy. The tumor regression was associated with priming of substantial tumor-infiltrative CD8+T-cell response [36].
Reovirus in combination with radiation in murine-human colorectal carcinoma model has shown synergistic oncolytic effect as compared to individual therapy even at low input of virus. This combination resulted in a statistically significant death of cell lines relatively resistant to reovirus-mediated oncolysis, suggesting that this synergism is not simply additive but is causative due to increased apoptosis and bystander effect [37]. The combination of T3D, a non-pathogenic reovirus with radiation, showed increased viral replication due to CUG2 upregulation causing downregulation of PKR and eIF2-α. It activates mitochondrial apoptotic signaling in wild type (WT) and in both mutant BRAF-Ras cell line and BRAF mutant xenograft mouse model of malignant melanoma which is generally chemotherapy and radiation resistant [38]. GLV-1h68, a construct of oncolytic vaccinia virus, showed induction of intrinsic apoptotic pathway by downregulation of anti-apoptotic Bcl-2 proteins when combined with external beam radiation leading to decreased tumor mass and increased survival in a rat–human orthotopic model of advanced extremity sarcoma [39].
Chemotherapy
The road to develop OVs as an efficient standalone therapy is still not completely paved, and thus all major studies and trials focus towards using viruses with chemotherapeutic modalities. Chemotherapeutic drugs generally inhibit DNA replication or disrupt the microtubule structures. As the mechanism of action of OV varies from that of cytotoxic drugs used, the effects exerted by combination therapy depend on the nature of the virus used and the synergism created between two therapeutic components. It is thought that the expression of viral genes and their interaction with cellular factors determine the sensitivity of the tumor to chemotherapy [40]. For example, Gendicine, approved for the treatment of head and neck squamous cell carcinoma, in combination with chemotherapy is also being used in clinical studies involving both the agents against other cancers such as HCC. In many cases the use of Gendicine in combination with doxorubicin, camptothecin or 5-flurouracil resulted in better quality of life and increased patient survival [41]. Oncorine (H101), a derivative of ONYX-015, showed promising anti-cancer effects in various pre-clinical studies involving tumor cells having either mutated or normal p53 gene. It also showed enhanced anti-tumor effects in nasopharyngeal and squamous cell carcinoma patients in phase III clinical trials especially in combination with cisplatin and 5-fluorouracil [35, 42] and was approved as therapy for head and neck squamous cell carcinoma in China. Similarly, Advexin (adenovirus with E1/E3 deletions expressing p53 under CMV promoter) in combination with methotrexate showed enhanced toxicity as compared to both the therapies given independent of each other in phase III clinical trials against advanced recurrent head and neck squamous cell carcinoma [43]. Silica implants bearing Ad5-Δ24-RGD and Ad-Δ24-RGD-GM-CSF in combination with gemcitabine have shown marked increase in survival of mouse and hamster xenograft model of peritoneal disseminated pancreatic cancer [44]. In addition, both the viral constructs showed decrease in tumor marker expression and conversion of progressive state of different cancers to stable disease in almost 50% of patient population tested [45].
Oncolytic WT reovirus has shown significant synergistic anti-tumor toxicity with low dose of docetaxel in murine flank model of hormone refractory metastatic prostate cancer as compared to modest or negligible affects respectively as a single therapeutic agent. This effect was partially due to microtubular stabilization of cells by docetaxel promoting mitotic arrest resulting in apoptotic induction [46]. The combination of chemotherapy and virotherapy has also been exploited to overcome the constraints imposed by neutralizing antibodies in the patients by using chemotherapeutic agent as an immuno-modulator. For example, administration of cyclophosphamide prior to the use of reovirus for the treatment of refractory or metastatic solid tumors in phase I clinical trials resulted in no rise of neutralizing antibody baseline level [47]. Similar effects were seen during co-administration of gemcitabine and Reolysin [48]. Combination of cisplatin, paclitaxel and Reolysin also showed significant rise in overall survival of refractory or metastatic head and neck cancer patients in phase II clinical trials [49]. Cisplatin in combination with NV1066, an oncolytic HSV-1 with γ34.5 deletion, showed increase in viral replication and cytotoxicity due to upregulation of DNA damage-inducible protein GADD34 in human malignant mesothelioma cell lines [50]. A study involving paclitaxel in combination with oncolytic rhabdovirus, Maraba-MG1, showed prolonged survival in various murine breast cancer models [51]. Pre-clinical studies with doxorubicin and rituximab in combination with NDV have shown enhanced toxicity against hematological malignancies such as plasmacytoma and non-Hodgkin lymphoma in vitro [52].
Recombinant vaccinia virus, GLV-1h68 with cyclophosphamide in mice model of human lung adenocarcinoma has shown complete loss of characteristic hemorrhagic phenotype of the disease in addition to reduction in tumor growth, angiogenesis further leading to epidermal growth factor (EGF) downregulation. It also increased the viral distribution within the tumor and elevation of pro-inflammatory cytokines such as M-CSF-1, monocyte chemoattractant protein-1 (MCP-1), MCP-5 and chemokine eotaxin [53]. In a larger context the oncolytic potential of vaccinia virus has been observed in pre-clinical studies with various cancers such as breast cancer including breast cancer stem-like cells [54], squamous cell carcinoma [55], salivary gland carcinoma [56], human sarcomas [57], etc. Cyclophosphamide has been used as a chemotherapeutic agent for the treatment of various carcinomas, and thus it can arguably be said that this combination, if successful in clinical settings, can emerge as a viable therapeutic model.
Histone deacetylase inhibitors (HDACis)
HDACis are a class of chemotherapeutic agents which have already been approved for lymphoma therapy [58]. In many instances carcinogenesis and tumor progression have been attributed to deregulation of HDACs. There has been a growing interest in the use of HDACis with OVs to enhance the oncolysis as they have been shown to hyperacetylate nucleosome core proteins to drive expression of anti-tumor genes and also acetylate non-histone proteins such as chaperones, regulators of DNA damage repair and transcription factors including p53 [59]. Many molecules are being investigated at clinical levels for the treatment of various malignancies out of which vorinostat (cutaneous T-cell lymphoma), romidepsin (cutaneous and peripheral T cell lymphoma) and belinostat (refractory peripheral T cell lymphoma) have been approved by the FDA [60].
VSV variant VSVΔ51 in combination with vorinostat has been found to increase viral replication, apoptosis, decrease interferon-mediated anti-viral response in xenograft models of refractory prostate, melanoma, colon, breast and ovarian tumors [61]. Replication-deficient adenoviral vector Ad.CMV-GFP administered in combination with romidepsin increased the expression of viral entry receptor CAR (Coxsackievirus and adenovirus receptor) in xenograft mouse model of melanoma causing increased infectivity with respect to virus internalization into tumor cells [62]. Patient-derived xenograft model of glioblastoma showed differential activation of multiple cell death pathways upon synergistic use of LBH589 and Scriptaid with Ad-Δ24-RGD vector [63]. HSV-1 variant G47Δ and trichostatin A decreased vascular endothelial growth factor secretion and angiogenesis in xenograft model of glioma and colorectal cancer [64]. Alternatively, this combination of OV and HDACi is not limited to a two-component therapy.
Pre-clinical testing of adenoviral vector bearing p73 gene and a small hairpin RNA against HDAC1 (OV.shHDAC1.p73) to target mice xenograft model of malignant melanoma exhibited increased apoptosis, induction of autophagy, complete regression of tumor and extended survival with no resurgence within 16 weeks of observation [65].
Immune checkpoint inhibitors
Emerging studies suggest that immunogenic cell death is a major component of OV-induced cell death. It establishes anti-tumor immunity by either secretion/release or exposure of DAMPs (danger-associated molecular patterns) and PAMPs (pathogen-associated molecular patterns) causing maturation of antigen-presenting cells leading to activation of antigen-specific CD4+ and CD8+ T cells [66, 67]. Antibodies such as Ipilimumab (CTLA-4), Nivolumab (PD1) and Penbrolizumab (PD1) have been approved by the FDA for treatment of advanced metastatic melanoma [68] due to observed reversal of tumor cell-mediated repression of T-cell response by blocking immune checkpoint proteins [60]. Examples can be found in pre-clinical studies with VSV and CTLA-4 inhibitor in Her2/neu-positive D2F2/E2 murine mammary tumor model showing complete remission and immunity towards tumor antigens [69]. Intra-tumoral NDV and anti-CTLA-4 antibody therapy caused tumor regression with increased survival rate in bilateral B16-F10 melanoma mouse model and a prostate adenocarcinoma transgenic mouse model, TRAMP C2 [70]. Similarly, phase I clinical trials with T-Vec and Ipilimumab or Penbrolizumab for metastatic melanoma therapy have shown encouraging results [71].
In many instances, the use of immune checkpoint blocking antibodies lead to systemic immune-related adverse effects and restriction of viral replication [60]. Insertion of checkpoint inhibitors into the viral genome ensures the safety of this therapy and localization of antibodies to tumor site. Recently, Western Reserve oncolytic vaccinia virus harboring hamster monoclonal IgG (J43) recognizing murine programmed cell death protein (mPD-1) was successfully generated by the insertion of three different forms of mPD-1 binders: the whole antibody (monoclonal antibody (mAb)), fragment antigen-binding (Fab) and single-chain variable fragment (scFv). Testing of this construct on B16-F10 melanoma model and MCA 205 fibrosarcoma model showed significantly enhanced localization of J43 antibody at the tumor site with reduced tumor growth and increased survival in case of the MCA 205 model [72]. Earlier studies also support the feasibility of OVs armed with antibodies against checkpoint inhibitors such as adenoviral vector Ad5/3-Δ24aCTLA4 expressing complete human mAb specific for CTLA-4 causing increased oncolysis in mouse xenograft models of prostate and lung cancer [73]. Similarly, measles virus coding for anti CTLA-4 (MV-aCTLA-4) and PD-L1 (MV-aPD-L1) antibodies also showed enhanced therapeutic benefits with antibody localization in B16-CD20 melanoma model with no immune-mediated toxicity [74].
A recent study involving Ad-Δ24-RGD armed with immune co-stimulator mouse OX40 ligand (OX40L) administered in combination with anti-PD-L1 antibody showed an effective example of potent in situ autologous cancer vaccination in immunocompetent mouse glioma models by enhancing the tumor-specific activation of lymphocytes and proliferation of TAA-specific CD8+T cells resulting in long-lasting immune memory and therapeutic efficacy [75].
Other modalities which can also be combined with OVs are radionucleotides, nucleotide analogs [76] and another OV. For example, intra-tumoral administration of reovirus and systemic delivery of VSV encoding complementary DNA library of melanoma antigens (VSV-ASMEL) in a B16-melanoma mice model showed significantly increased survival [77].
Conclusion
OVs as a tool of cancer therapy can be the missing link in the development of a comprehensive anti-cancer regimen. Earlier representative of anti-cancer virotherapy despite being mildly cancer-selective had limitations related to morbidity and low activity, making them unsuitable for the development of a viable therapeutic model. On the contrary, genetic alteration of viral genome has allowed researchers to generate candidate OVs to fill the lacunae of a specific and targeted anti-cancer therapeutic moiety with desirable safety, tumor toxicity and margin for alterations to target wide variety of cancers. The most successful example can be found in T-Vec, an HSV-1-based OV armed with GM-CSF [66] which has been approved for the treatment of melanoma. In addition, ongoing clinical trials with various candidate OVs has further augmented the hope for a viable anti-cancer therapy. However, there are many challenges still posed by the ever-changing nature of cancer and its microenvironment. Despite the promising results in pre-clinical settings, there have been host-dependant reactions with respect to anti-cancer, anti-viral immune response and the accessibility to all malignant cells, which have proven to be major limiting factors for OV-based anti-cancer therapy. In many instances, this interplay of tumor and host responsiveness towards the presence and activity of OVs pose a hindrance in the effectivity of OV-based monotherapy. However, many limiting host responses can be curbed by administering OV with pre-existing anti-cancer therapeutics. For effective translation of pre-clinical success of OVs to clinical settings, validation of OV is needed to be carried out in animal models considering the accessibility of OV to tumors and host immune response as selection criteria. Until the development of OVs as a single anti-cancer therapeutic, not bound by the above-mentioned shortcomings, virotherapy can be incorporated as an arm of anti-cancer regimen along with various pre-existing therapeutics such as chemotherapy, immunotherapy and radiation which supplement the viral activity and heighten the anti-tumor response.
References
Housman G, Byler S, Heerboth S, Longacre M, Snyder N, Sarkar S. Drug resistance in cancer: an overview. Cancers (Basel). 2014;6:1769–92.
Das SK, Menezes ME, Bhatia S, Wang X-Y, Emdad L, Sarkar D, et al. Gene therapies for cancer: strategies, challenges and successes. J Cell Physiol. 2015;230:259–71.
Bluming A, Ziegler J. Regression of Burkitt’s lymphoma in association with Measles infection. Lancet. 1971;298:105–6.
Hansen RM, Libnoch JA. Remission of chronic lymphocytic leukemia after smallpox vaccination. Arch Intern Med. 1978;138:1137–8.
Russell SJ, Peng KW. Viruses as anticancer drugs. Trends Pharmacol Sci. 2007;28:326–33.
Russell SJ, Peng K-W, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–70.
Toro Bejarano M, Merchan JR. Targeting tumor vasculature through oncolytic virotherapy: recent advances. Oncolytic Virother. 2015;4:169–81.
Loskog A. Immunostimulatory gene therapy using oncolytic viruses as vehicles. Viruses. 2015;7:5780–91.
Grote D, Russell SJ, Cornu TI, Cattaneo R, Vile R, Poland GA, et al. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood. 2001;97:3746–54.
Russell SJ, Peng KW. Measles virus for cancer therapy. Curr Top Microbiol Immunol. 2009;330:213–41.
Antonio Chiocca E. Oncolytic viruses. Nat Rev Cancer. 2002;2:938–50.
Peng Z. Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther. 2005;16:1016–27.
Wong HH, Lemoine NR, Wang Y. Oncolytic viruses for cancer therapy: overcoming the obstacles. Viruses. 2010;2:78–106.
Yang L, Pang Y, Moses HL. TGF-β and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220–7.
Naik S, Nace R, Federspiel MJ, Barber GN, Peng K-W, Russell SJ. Curative one-shot systemic virotherapy in murine myeloma. Leukemia. 2012;26:1870–8.
Hu JCC, Coffin RS, Davis CJ, Graham NJ, Groves N, Guest PJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12:6737 LP–6747.
Seubert CM, Stritzker J, Hess M, Donat U, Sturm JB, Chen N, et al. Enhanced tumor therapy using vaccinia virus strain GLV-1h68 in combination with a β-galactosidase-activatable prodrug seco-analog of duocarmycin SA. Cancer Gene Ther. 2011;18:42–52.
Wei D, Li Q, Wang X-L, Wang Y, Xu J, Feng F, et al. Oncolytic Newcastle disease virus expressing chimeric antibody enhanced anti-tumor efficacy in orthotopic hepatoma-bearing mice. J Exp Clin Cancer Res. 2015;34:153.
Nistal-Villan E, Bunuales M, Poutou J, Gonzalez-Aparicio M, Bravo-Perez C, Quetglas JI, et al. Enhanced therapeutic effect using sequential administration of antigenically distinct oncolytic viruses expressing oncostatin M in a Syrian hamster orthotopic pancreatic cancer model. Mol Cancer. 2015;14:210.
Mccart JA, Puhlmann M, Lee J, Hu Y, Libutti SK, Alexander HR, et al. Complex interactions between the replicating oncolytic effect and the enzyme / prodrug effect of vaccinia- mediated tumor regression. Cancer Gene Ther. 2000;7:1217–23.
Dingli D, Peng K-W, Harvey ME, Vongpunsawad S, Bergert ER, Kyle RA, et al. Interaction of measles virus vectors with Auger electron emitting radioisotopes. Biochem Biophys Res Commun. 2005;337:22–29.
Advani SJ, Sibley GS, Song PY, Hallahan DE, Kataoka Y, Roizman B, et al. Enhancement of replication of genetically engineered herpes simplex viruses by ionizing radiation: a new paradigm for destruction of therapeutically intractable tumors. Gene Ther. 1998;5:160–5.
Blank SV, Rubin SC, Coukos G, Amin KM, Albelda SM, Molnar-Kimber KL. Replication-selective herpes simplex virus type 1 mutant therapy of cervical cancer is enhanced by low-dose radiation. Hum Gene Ther. 2002;13:627–39.
Stanziale SF, Petrowsky H, Joe JK, Roberts GD, Zager JS, Gusani NJ, et al. Ionizing radiation potentiates the antitumor efficacy of oncolytic herpes simplex virus G207 by upregulating ribonucleotide reductase. Surgery. 2002;132:353–9.
Adusumilli PS, Stiles BM, Chan MK, Chou TC, Wong RJ, Rusch VW, et al. Radiation therapy potentiates effective oncolytic viral therapy in the treatment of lung cancer. Ann Thorac Surg. 2005;80:409–16..
Adusumilli PS, Chan MK, Hezel M, Yu Z, Stiles BM, Chou TC, et al. Radiation-induced cellular DNA damage repair response enhances viral gene therapy efficacy in the treatment of malignant pleural mesothelioma. Ann Surg Oncol. 2007;14:258–69..
Advani SJ, Markert JM, Sood RF, Samuel S, Gillespie GY, Shao MY, et al. Increased oncolytic efficacy for high-grade gliomas by optimal integration of ionizing radiation into the replicative cycle of HSV-1. Gene Ther. 2011;18:1098–102.
Liu C, JN S, CA P, Paraskevakou G, PJ Z, Schroeder M. Combination of measles virus virotherapy and radiation therapy has synergistic activity in the treatment of glioblastoma multiforme. Clin Cancer Res. 2007;12:7155–65.
Idema S, Lamfers MLM, van Beusechem VW, Noske DP, Heukelom S, Moeniralm S, et al. AdΔ24 and the p53-expressing variant AdΔ24-p53 achieve potent anti-tumor activity in glioma when combined with radiotherapy. J Gene Med. 2007;9:1046–56.
Rogulski KR, Wing MS, Paielli DL, Gilbert JD, Kim JH, Freytag SO. Double suicide gene therapy augments the antitumor activity of a replication-competent lytic adenovirus through enhanced cytotoxicity and radiosensitization. Hum Gene Ther. 2000;11:67–76.
Portella G, Pacelli R, Libertini S, Cella L, Vecchio G, Salvatore M, et al. ONYX-015 enhances radiation-induced death of human anaplastic thyroid carcinoma cells. J Clin Endocrinol Metab. 2003;88:5027–32.
Geoerger B, Grill J, Opolon P, Morizet J, Aubert G, Lecluse Y, et al. Potentiation of radiation therapy by the oncolytic adenovirusdl1520 (ONYX-015) in human malignant glioma xenografts. Br J Cancer. 2003;89:577–84.
Chen Y, DeWeese T, Dilley J, Zhang Y, Li Y, Ramesh N, et al. CV706, a prostate cancer-specific adenovirus variant, in combination with radiotherapy produces synergistic antitumor efficacy without increasing toxicity. Cancer Res. 2001;61:5453 LP–5460.
Dilley J, Reddy S, Ko D, Nguyen N, Rojas G, Working P. Oncolytic adenovirus CG7870 in combination with radiation demonstrates synergistic enhancements of antitumor efficacy without loss of specificity. Cancer Gene Ther. 2005;12:715–22.
Ma G, Shimada H, Hiroshima K, Tada Y, Suzuki N, Tagawa M. Gene medicine for cancer treatment: commercially available medicine and accumulated clinical data in China. Drug Des Devel Ther. 2008;2:115–22.
Blanchard M, Shim KG, Grams MP, Rajani K, Diaz RM, Furutani KM, et al. Definitive management of oligometastatic melanoma in a murine model using combined ablative radiation therapy and viral immunotherapy. Int J Radiat Oncol Biol Phys. 2015;93:577–87.
Twigger K, Vidal L, White CL, De Bono JS, Bhide S, Coffey M. et al. Enhanced in vitro and in vivo cytotoxicity of combined reovirus and radiotherapy. Clin Cancer Res. 2008;14:912–23.
McEntee G, Kyula JN, Mansfield D, Smith H, Wilkinson M, Gregory C, et al. Enhanced cytotoxicity of reovirus and radiotherapy in melanoma cells is mediated through increased viral replication and mitochondrial apoptotic signalling. Oncotarget. 2016;7:48517–32.
Wilkinson MJ, Smith HG, McEntee G, Kyula-Currie J, Pencavel TD, Mansfield DC, et al. Oncolytic vaccinia virus combined with radiotherapy induces apoptotic cell death in sarcoma cells by down-regulating the inhibitors of apoptosis. Oncotarget. 2016;7:81208–22.
Lee W-P, Tai D-I, Tsai S-L, Yeh C-T, Chao Y, Lee S-D, et al. Adenovirus type 5 E1A sensitizes hepatocellular carcinoma cells to gemcitabine. Cancer Res. 2003;63:6229 LP–6236.
Guan YS, Sun L, Zhou XP, et al. Combination therapy with recombinant adenovirus-p53 injection (rAd-p53) via transcatheter hepatic arterial chemoembolization for advanced hepatic carcinoma. Shijie Huaren Xiaohua Zazhi. 2005;13:125–7.
Xia Z-J, Chang J-H, Zhang L, Jiang W-Q, Guan Z-Z, Liu J-W, et al. [Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus]. Ai Zheng. 2004;23:1666–70.
Nemunaitis J, Clayman G, Agarwala SS, Hrushesky W, Wells JR, Moore C, et al. Biomarkers predict p53 gene therapy efficacy in recurrent squamous cell carcinoma of the head and neck. Clin Cancer Res. 2009;; 15:7719 LP–7725.
Kangasniemi L, Parviainen S, Pisto T, Koskinen M, Jokinen M, Kiviluoto T, et al. Effects of capsid-modified oncolytic adenoviruses and their combinations with gemcitabine or silica gel on pancreatic cancer. Int J Cancer. 2012;131:253–63.
Pesonen S, Diaconu I, Cerullo V, Escutenaire S, Raki M, Kangasniemi L, et al. Integrin targeted oncolytic adenoviruses Ad5-D24-RGD and Ad5-RGD-D24-GMCSF for treatment of patients with advanced chemotherapy refractory solid tumors. Int J Cancer. 2012;130:1937–47.
Heinemann L, Simpson GR, Boxall A, Kottke T, Relph KL, Vile R, et al. Synergistic effects of oncolytic reovirus and docetaxel chemotherapy in prostate cancer. BMC Cancer. 2011;11:221.
Roulstone V, Khan K, Pandha HS, Rudman S, Coffey M, Gill GM, et al. Phase I trial of cyclophosphamide as an immune modulator for optimizing oncolytic reovirus delivery to solid tumors. Clin Cancer Res. 2015;21:1305–12.
Lolkema MP, Arkenau H-T, Harrington K, Roxburgh P, Morrison R, Roulstone V, et al. A phase I study of the combination of intravenous reovirus type 3 Dearing and gemcitabine in patients with advanced cancer. Clin Cancer Res. 2011;17:581 LP–588.
Karapanagiotou EM, Roulstone V, Twigger K, Ball M, Tanay MA, Nutting C, et al. Phase I/II trial of carboplatin and paclitaxel chemotherapy in combination with intravenous oncolytic reovirus in patients with advanced malignancies. Clin Cancer Res. 2012;18:2080–9.
Adusumilli PS, Chan M-K, Chun YS, Hezel M, Chou T-C, Rusch VW, et al. Cisplatin-induced GADD34 upregulation potentiates oncolytic viral therapy in the treatment of malignant pleural mesothelioma. Cancer Biol Ther. 2006;5:48–53.
Bourgeois-Daigneault M-C, St-Germain LE, Roy DG, Pelin A, Aitken AS, Arulanandam R, et al. Combination of paclitaxel and MG1 oncolytic virus as a successful strategy for breast cancer treatment. Breast Cancer Res. 2016;18:83.
Al-Shammari AM, Rameez H, Al-Taee MF. Newcastle disease virus, rituximab, and doxorubicin combination as anti-hematological malignancy therapy. Oncolytic Virother. 2016;5:27–34.
Hofmann E, Weibel S, Szalay AA. Combination treatment with oncolytic Vaccinia virus and cyclophosphamide results in synergistic antitumor effects in human lung adenocarcinoma bearing mice. J Transl Med. 2014;12:197.
Wang H, Chen NG, Minev BR, Szalay AA. Oncolytic vaccinia virus GLV-1h68 strain shows enhanced replication in human breast cancer stem-like cells in comparison to breast cancer cells. J Transl Med. 2012;10:167.
Yu Z, Li S, Brader P, Chen N, Yu YA, Zhang Q, et al. Oncolytic vaccinia therapy of squamous cell carcinoma. Mol Cancer. 2009;8:45.
Chernichenko N, Linkov G, Li P, Bakst RL, Chen C-H, He S, et al. Oncolytic vaccinia virus therapy of salivary gland carcinoma. JAMA Otolaryngol Head Neck Surg. 2013;139:173–82.
He S, Li P, Chen C-H, Bakst RL, Chernichenko N, Yu YA, et al. Effective oncolytic vaccinia therapy for human sarcomas. J Surg Res. 2012;175:e53–e60.
Garber K. HDAC inhibitors overcome first hurdle. Nat Biotech. 2007;25:17–19.
Bressy C, Hastie E, Grdzelishvili VZ. Combining oncolytic virotherapy with p53 tumor suppressor gene therapy. Mol Ther Oncolytics. 2017;5:20–40.
Marchini A, Scott EM, Rommelaere J. Overcoming barriers in oncolytic virotherapy with HDAC inhibitors and immune checkpoint blockade. Viruses. 2016;8:pii: E9
Nguyen TL-A, Abdelbary H, Arguello M, Breitbach C, Leveille S, Diallo J-S, et al. Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc Natl Acad Sci USA. 2008;105:14981–6.
Goldsmith ME, Aguila A, Steadman K, Martinez A, Steinberg SM, Alley MC, et al. The histone deacetylase inhibitor FK228 given prior to adenovirus infection can boost infection in melanoma xenograft model systems. Mol Cancer Ther. 2007;6:496 LP–505.
Berghauser Pont LM, Kleijn A, Kloezeman JJ, van den Bossche W, Kaufmann JK, de Vrij J, et al. The HDAC inhibitors scriptaid and LBH589 combined with the oncolytic virus Delta24-RGD exert enhanced anti-tumor efficacy in patient-derived glioblastoma cells. PLoS ONE. 2015;10:1–20.
Liu T-C, Castelo Branco P, Rabkin SD, Martuza RL. Trichostatin A and oncolytic HSV combination therapy shows enhanced antitumoral and antiangiogenic effects. Mol Ther. 2008;16:1041–7.
Schipper H, Alla V, Meier C, Nettelbeck DM, Herchenroder O, Putzer BM. Eradication of metastatic melanoma through cooperative expression of RNA-based HDAC1 inhibitor and p73 by oncolytic adenovirus. Oncotarget. 2014;5:5893–907.
Bartlett DL, Liu Z, Sathaiah M, Ravindranathan R, Guo Z, He Y, et al. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer. 2013;12:103.
Guo ZS, Liu Z, Bartlett DL. Oncolytic Immunotherapy: dying the right way is a key to eliciting potent antitumor immunity. Front Oncol. 2014;4:74.
Bauzon M, Hermiston T. Armed therapeutic viruses - a disruptive therapy on the horizon of cancer immunotherapy. Front Immunol. 2014;5:1–10.
Gao Y, Whitaker-Dowling P, Griffin JA, Barmada MA, Bergman I. Recombinant vesicular stomatitis virus targeted to Her2/neu combined with anti-CTLA4 antibody eliminates implanted mammary tumors. Cancer Gene Ther. 2008;16:44–52.
Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6:226ra32
Puzanov I, Milhem M, Andtbacka R, Minor D, Hamid O, Li A, et al. Phase 1 results of a phase 1b/2, multicenter, open-label trial to evaluate safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) vs ipi alone in previously untreated, unresected stage IIIB-IV melanoma. J Immunother Cancer. 2013;1:P84–P84.
Kleinpeter P, Fend L, Thioudellet C, Geist M, Sfrontato N, Koerper V, et al. Vectorization in an oncolytic vaccinia virus of an antibody, a Fab and a scFv against programmed cell death -1 (PD-1) allows their intratumoral delivery and an improved tumor-growth inhibition. Oncoimmunology. 2016;5:e1220467.
Dias JD, Hemminki O, Diaconu I, Hirvinen M, Bonetti A, Guse K, et al. Targeted cancer immunotherapy with oncolytic adenovirus coding for a fully human monoclonal antibody specific for CTLA-4. Gene Ther. 2012;19:988–98.
Engeland CE, Grossardt C, Veinalde R, Bossow S, Lutz D, Kaufmann JK, et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol Ther. 2014;22:1949–59.
Jiang H, Rivera-Molina Y, Clise-Dwyer K, Bover L, Vence L, Yuan Y, et al. Oncolytic adenovirus and tumor-targeting immune modulatory therapy improve autologous cancer vaccination. Cancer Res. 2017;77:3894–3907.
Ottolino-Perry K, Diallo J-S, Lichty BD, Bell JC, McCart JA. Intelligent design: combination therapy with oncolytic viruses. Mol Ther. 2010;18:251–63.
Ilett E, Kottke T, Thompson J, Rajani K, Zaidi S, Evgin L, et al. Prime-boost using separate oncolytic viruses in combination with checkpoint blockade improves anti-tumor therapy. Gene Ther. 2017;24:21–30.
Suzuki K, Fueyo J, Krasnykh V, Reynolds PN, Curiel DT, Alemany R. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin Cancer Res. 2001;7:120 LP–126.
Yang Y, Xu H, Shen J, Yang Y, Wu S, Xiao J, et al. RGD-modifided oncolytic adenovirus exhibited potent cytotoxic effect on CAR-negative bladder cancer-initiating cells. Cell Death Dis. 2015;6:e1760.
Yamamoto Y, Hiraoka N, Goto N, Rin Y, Miura K, Narumi K, et al. A targeting ligand enhances infectivity and cytotoxicity of an oncolytic adenovirus in human pancreatic cancer tissues. J Control Release. 2014;192:284–93.
Yamamoto Y, Nagasato M, Rin Y, Henmi M, Ino Y, Yachida S, et al. Strong antitumor efficacy of a pancreatic tumor-targeting oncolytic adenovirus for neuroendocrine tumors. Cancer Med. 2017;6:2385–97.
Nokisalmi P, Pesonen S, Escutenaire S, Ristimki A, Joensuu T, Guse K, et al. Clinical data from cancer patients treated with triple modified oncolytic adenovirus Ad5/3-Cox2L-D24. Hum Gene Ther. 2008;19:1076.
Pesonen S, Nokisalmi P, Escutenaire S, Sarkioja M, Raki M, Cerullo V, et al. Prolonged systemic circulation of chimeric oncolytic adenovirus Ad5/3-Cox2L-D24 in patients with metastatic and refractory solid tumors. Gene Ther. 2010;17:892–904.
Yu D, Jin C, Leja J, Majdalani N, Nilsson B, Eriksson F, et al. Adenovirus with hexon Tat-protein transduction domain modification exhibits increased therapeutic effect in experimental neuroblastoma and neuroendocrine tumors. J Virol. 2011;85:13114–23.
Hsiao W-C, Sung S-Y, Liao C-H, Wu H-C, Hsieh C-L. Vitamin D3-inducible mesenchymal stem cell-based delivery of conditionally replicating adenoviruses effectively targets renal cell carcinoma and inhibits tumor growth. Mol Pharm. 2012;9:1396–408.
Lu C-S, Hsieh J-L, Lin C-Y, Tsai H-W, Su B-H, Shieh G-S, et al. Potent antitumor activity of Oct4 and hypoxia dual-regulated oncolytic adenovirus against bladder cancer. Gene Ther. 2015;22:305–15.
Fajardo CA, Guedan S, Rojas LA, Moreno R, Arias-Badia M, de Sostoa J, et al. Oncolytic adenoviral delivery of an EGFR-targeting T-cell engager improves antitumor efficacy. Cancer Res. 2017;77:2052 LP–2063.
Friedrich K, Hanauer JR, Prüfer S, Münch RC, Völker I, Filippis C, et al. DARPin-targeting of measles virus: unique bispecificity, effective oncolysis, and enhanced safety. Mol Ther. 2013;21:849–59.
Paraskevakou G, Allen C, Nakamura T, Zollman P, James CD, Peng KW, et al. Epidermal growth factor receptor (EGFR)– retargeted measles virus strains effectively target EGFR- or EGFRvIII expressing gliomas. Mol Ther J Am Soc Gene Ther. 2007;15:677–86.
Hanauer JR, Gottschlich L, Riehl D, Rusch T, Koch V, Friedrich K, et al. Enhanced lysis by bispecific oncolytic measles viruses simultaneously using HER2/neu or EpCAM as target receptors. Mol Ther Oncolytics. 2016;3:16003.
Guillerme JB, Gregoire M, Tangy F, Fonteneau JF. Antitumor virotherapy by attenuated measles virus (MV). Biology (Basel). 2013;2:587–602.
Amagai Y, Fujiyuki T, Yoneda M, Shoji K, Furukawa Y, Sato H, et al. Oncolytic activity of a recombinant measles virus, blind to signaling lymphocyte activation molecule, against colorectal cancer cells. Sci Rep. 2016;6:24572.
Fujiyuki T, Yoneda M, Amagai Y, Obayashi K, Ikeda F, Shoji K, et al. A measles virus selectively blind to signaling lymphocytic activation molecule shows anti-tumor activity against lung cancer cells. Oncotarget. 2015;6:24895–903.
Jing Y, Bejarano MT, Zaias J, Merchan JR. In vivo anti-metastatic effects of uPAR retargeted measles virus in syngeneic and xenograft models of mammary cancer. Breast Cancer Res Treat. 2015;149:99–108.
Miest TS, Yaiw K-C, Frenzke M, Lampe J, Hudacek AW, Springfeld C, et al. Envelope-chimeric entry-targeted measles virus escapes neutralization and achieves oncolysis. Mol Ther. 2011;19:1813–20.
Leoni V, Gatta V, Palladini A, Nicoletti G, Ranieri D, Dall’Ora M, et al. Systemic delivery of HER2-retargeted oncolytic-HSV by mesenchymal stromal cells protects from lung and brain metastases. Oncotarget. 2015;6:34774–87.
Gatta V, Petrovic B, Campadelli-Fiume G. The engineering of a novel ligand in gH confers to HSV an expanded tropism independent of gD activation by its receptors. PLoS Pathog. 2015;11:e1004907.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lal, G., Rajala, M.S. Recombinant viruses with other anti-cancer therapeutics: a step towards advancement of oncolytic virotherapy. Cancer Gene Ther 25, 216–226 (2018). https://doi.org/10.1038/s41417-018-0018-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-018-0018-1
- Springer Nature America, Inc.