Abstract
Viruses are being researched as cutting-edge therapeutic agents in cancer due to their selective oncolytic action against malignancies. Immuno-oncolytic viruses are a potential category of anticancer treatments because they have natural features that allow viruses to efficiently infect, replicate, and destroy cancer cells. Oncolytic viruses may be genetically modified; engineers can use them as a platform to develop additional therapy modalities that overcome the limitations of current treatment approaches. In recent years, researchers have made great strides in the understanding relationship between cancer and the immune system. An increasing corpus of research is functioning on the immunomodulatory functions of oncolytic virus (OVs). Several clinical studies are currently underway to determine the efficacy of these immuno-oncolytic viruses. These studies are exploring the design of these platforms to elicit the desired immune response and to supplement the available immunotherapeutic modalities to render immune-resistant malignancies amenable to treatment. This review will discuss current research and clinical developments on Vaxinia immuno-oncolytic virus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is becoming the biggest cause of mortality worldwide [1]. Cancer is a serious worldwide problem. The number of people diagnosed with cancer is expected to increase by more than 50% globally. The number of new cancer cases diagnosed worldwide in 2020 was around 19.6 million, with an estimated 10 million deaths. It is predicted that by 2040 there will be approximately 28 million new cases and 16 million deaths globally [2]. The development of cancer is primarily attributed to DNA damage and genetic instability. This is because DNA lesions that remain unrepaired or are incorrectly repaired can result in mutations that initiate or drive cancer. Additionally, various forms of genomic instability can facilitate the progression of tumors through multiple stages and contribute to resistance against therapeutic interventions [3]. Despite numerous progress indicators, current cancer therapies exhibit limited efficacy against specific tumor types alongside potential drawbacks such as drug resistance, cancer recurrence, and significant side effects [4].
Most malignancies can evade the body’s natural defenses, making them difficult to treat [5]. An immune system is trained to destroy pathogens while protecting self-replicating cells. However, malignant tumor cells may manipulate the immune system in their own favor, helping them to proliferate and spread. The term “concomitant tumor immunity” describes this occurrence [6]. Immuno-editing is the term for this three-step procedure, including elimination, equilibrium, and escape [7, 8]. The immune system detects tumor cell antigens and eradicates them during the elimination phase [9]. Some cells may survive the first phase of elimination and move on to the second, rapidly modifying their antigens so the immune system cannot detect them [10, 11]. Tumor cells begin to multiply at this point resulting in a significant increase in mass. When the immune system cannot keep the tumor under control, it is referred to as the third phase of escape [12,13,14,15].
There are certain drawbacks associated with traditional cancer treatments. Toxicity is significant because chemotherapy and radiation cannot precisely target cancer cells [16]. Also, if chemo-resistance develops, surgery is the only option left. Another crucial factor that should not be disregarded is the insufficiency of conventional medicines to construct long-lasting immunity to prevent metastasis and the recurrence of cancer.
Over the last decade, immunotherapeutic strategies have gained traction in preclinical studies and clinical practice to combat cancer. In order to eliminate the tumor, the standard oncological approach involves destroying or eliminating the cancer cells themselves. Tumor regression, anti-tumor immunological memory formation, and persistent responses are the results of immunotherapy, which is undertaken to boost the immune system’s capacity to eliminate cancer cells [17].
Immuno-oncolytic viruses are a subcategory of anticancer treatments because they have natural features that allow viruses to efficiently infect, replicate, and destroy cancer cells [18]. Genetically modified oncolytic viruses are being researched as a potential treatment for many cancers [19]. Anticancer treatments that include oncolytic viral therapy are becoming more promising approaches. Tumors can be destroyed by oncolytic virus (OVs) due to their ability to multiply only in cancer cells and destroy them. In conjunction with its primary function, OVs also have the secondary effect of stimulating the immune system. As a defense mechanism, tumors develop an immunosuppressive microenvironment to prevent immune system attack. OVs have demonstrated potential as an agent for immunotherapy. The occurrence of viral infection and consequent induction of immunogenic cell death in tumors elicits innate and adaptive immune reactions that facilitate additional tumor eradication [20]. To determine the effectiveness of these immuno-oncolytic viruses, several clinical studies are now being conducted. The first dosage of the investigational anticancer medication Vaxinia was given to a human subject in a Phase 1 clinical trial recently. In this innovative treatment, an oncolytic Vaxinia virus is used. These viruses specifically target cancer cells for infection and destruction while leaving healthy cells alone. The genetically engineered smallpox virus Vaxinia has been found to be effective against a wide variety of malignancies in lab and animal models. The City of Hope, a cancer research and treatment facility in the United States, and Imugene, a biotech firm in Australia, are conducting a clinical study to evaluate the new oncolytic virus in patients with advanced solid tumors [21]. Evidence suggests that Vaxinia has the potential to be even more successful in shrinking tumors than the first generation of oncolytic viruses. This review will discuss current research and clinical developments on the Vaxinia immuno-oncolytic virus.
Vaxinia virus
Professor Yuman Fong discovered this virus vaccine at the renowned City of Hope Comprehensive Cancer Center in Los Angeles. It appears to be the most promising choice for treating diverse tumors [22]. To develop this virus, scientists combined the genomes of several pox virus species to develop a safer and more effective virus as shown in Fig. 1. The smallpox vaccine (Vaccinia virus) belonging to the member of the poxvirus is genetically modified to produce Vaxinia [21,22,23]. Vaccinia has a brief, well-defined life cycle, spreading swiftly from cell to cell without integrating into the host's DNA. Vaccinia belongs to the Poxviridae family and has very stable double-stranded DNA. It was the active therapeutic agent in the vaccine that successfully eradicated smallpox, one of the most devastating diseases ever known to humanity. It kills many kinds of cancer cells. It has the potential as a medication for treating cancer and as a vector for delivering genes in gene therapy [24, 25].
Vaxinia virus was developed through recombination between several vaccinia virus and other poxvirus species, making it more potent than viruses based on single strains. Shreds of evidence from animal studies and other preclinical studies establish the efficacy of this virus compared to its mother viruses and some other viruses [21,22,23]. This virus is engineered to specifically infect cancerous tumors, where they may replicate inside the tumor cells themselves and then cause their demise known as oncolysis. Transgene expression in tumors (intratumoral, or IT) or around tumors (peritumoral) may trigger oncolysis in cancer cells without affecting healthy tissue. In the process of developing the Vaxinia virus, homologous recombination was performed on the viral J2R gene, which resulted in the disruption and partial deletion of the J2R gene sequence and the introduction of the human sodium-iodide symporter (hNIS). The virus has been genetically modified to exhibit selectivity towards cancer cells, while disregarding healthy cells of the host organism. Following infection, CF33-hNIS enters the host cells and undergoes replication until lysis occurs, releasing numerous antigenic progenies into the systemic circulation. The expression of ATP and HGMB1 leads to a robust immune system response to these antigens. The upregulation of the human sodium-iodide symporter (hNIS) results in the recruitment of iodine to facilitate the process of apoptosis. The ultimate outcome is a modified immune response that is equipped to detect and combat malignant cells [24, 25]. Patients with malignancies that are difficult to treat with current therapies may get improved clinical outcomes and quality of life through the usage of this virus.
A single variable fragment targeting programmed death-ligand 1 (PD-L1) has been developed using a poxvirus CF33. hNIS and anti-programmed cell death-ligand 1 (anti-PD-L1) are loaded into CF33. It seeks out cancer cells with high expression of PD-L1 as PD-L1 is highly expressed in cancer cells and generates anti-PD-L1, which has the potential to further improve the function of anti-tumor immune cells. The hNIS gene allows the transport of iodine 123I will lead to the uptake of 124I by the cells and consequently detection by PET imaging (or 123I for SPECT) [26].
Vaxinia virus as an anticancer therapeutic agent
In cancer cells, the interferon pathway (IFN pathway), the cell’s primary antiviral response, is usually impaired [27]. A pro-inflammatory response is elicited when OVs invade tumor cells [28, 29]. The virus can induce immunogenic cell death (ICD) [30, 31]. In ICD, the apoptosis process occurs by stimulating the endoplasmic reticulum and thus releasing certain harmful compounds known as damage-associated molecular patterns (DAMPs), which initiate the process of apoptosis [32, 33].
The OVs infect cancer cells with the pathogen-associated molecular pattern (PAMPs), which includes aspects of DNA, RNA, viral capsids, and protein products. Dendritic cells (DCs) are recruited and activated, and then specific T lymphocytes are stimulated as a result, the death of cancer cells in this kind of apoptosis occurs [34]. DCs are recruited and activated, and then specific T lymphocytes are stimulated as a result, the death of cancer cells and apoptosis occurs [35].
Thus, OVs can readily infect and perform their intended role in the transformed cells [36]. OVs treatment is designed to target cancer cells. Furthermore, it aims to rebuild an immune system that has been compromised by the existence of a tumor microenvironment [37]. A pro-inflammatory response is elicited when OVs invade tumor cells [38].
Vaxinia virus targeting cancer cells
Vaxinia virus anticancer efficacy is assumed to be mediated by two separate mechanisms, i.e., lytic replication and development of immune response against tumor cells [39, 40]. Multiple signaling pathways work in healthy cells to identify and eliminate pathogenic viruses. The production of Toll-like receptors (TLRs), which are intracellular proteins, and the release of interferons are triggered by the presence of viral elements and hence activating these signaling pathways [41, 42].
The pattern recognition receptors known as TLRs are triggered by repeating sequences found on pathogenic viruses known as PAMPs. Virus capsid components, viral DNA, RNA, and viral protein products may all serve as PAMPs [43, 44]. Innate immunity and antiviral responses in host cells are activated by TLR signaling. Factors in host cells, such as retinoic acid-inducible gene 1 (RIG), TNF-related factor 3 (TRAF3), interferon-related factor 3 (IRF3), interferon-related factor 7 (IRF7), had been shown to have a role in the elimination of viruses [45]. Coordinating the antiviral mechanism in infected cells, these molecules activate JAK-STAT (Janus kinase-signal transducer and activator of transcription) pathway [46].
By bolstering local IFN release it stimulates protein kinase R (PKR) activation. The intracellular protein kinase PKR is responsible for recognizing virus double-stranded RNA and other elements [47, 48]. After being activated by viral components, PKR inhibits protein synthesis in the host cell, which leads to rapid apoptosis and virus clearance in normal cells.
The IFN receptor is a potential mediator of antiviral action mediated by the production of interferons IFN which generates innate immunity to viral infections (IFNR). TLRs induces the production of cytokines and type I IFNs via signaling through the TIR-domain-containing adapter-inducing IFN (TRIF), myeloid differentiation primary response protein MYD88, nuclear factor k-B (NF-kB), IRF3, IRF7 [49, 50]. The JAK-STAT signaling pathway is activated in response to type I IFNs, which causes upregulation of protein kinase R (PKR) and IRF7.
By binding to viral particles, these cell cycle regulators initiate type I IFN transcriptional pathways that lead to the premature death of infected cells and the production of cytokines that alert the immune system to the presence of a viral infection, therefore restricting viral replication. This mechanism is aberrant in cancer cells. Some innate signaling components, such as RIG1, IRF7, and IRF3, may be downregulated in cancer cells. As a result, TLR and RIG1 are less able to identify viral particles, leaving cancer cells open to viral replication as shown in Fig. 2. In addition, cancer cells may dampen the pro-apoptotic and cell cycle-regulating actions of type I IFNs by down-regulating essential aspects of the type I IFN signaling pathway. Mutated PKR activity and aberrant IFN pathway signaling may prevent cancer cells from clearing viruses [51].
To prolong the time needed to complete its life cycle, virus may alter various aberrant signaling pathways inside tumor cells to halt apoptosis. Virus kills tumor cells by inducing cell death after viral replication, which eliminates tumor cells and prepares the body’s immune system to mount an attack. The mode of cell death and the production of danger signals from virus-infected cells may considerably induce the host’s immune responses. In contrast to apoptosis, the death of cells through necrosis or pyroptosis is more likely to provoke an immune response [52].
Development of immune response against tumor cells by Vaxinia virus
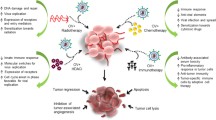
Virus relies on stimulating both innate immune and adaptive immune systems against the tumor. Oncolytic viruses cause tumor cell death after replication and thus release both tumor-associated antigens, cellular danger-associated molecular pattern signals (DAMPs) as well as virus pathogen-associated molecular patterns. This tends to the production of adaptive immunity [49,50,51,52]. These molecules recruit APCs, and their maturation is promoted, consequently activating antigen-specific CD8 + and CD8 + T cells and allowing CD8 + T cells to proliferate to cytotoxic effectors cells, thus promoting anti-tumor immunity [53]. The generation of chemokines, interferons, DAMP, and PAMP factors activates the tumor-infiltrating inflammatory responses and reverses the immunological suppression of the tumor microenvironment, allowing effective therapeutic responses [54].
Calreticulin, ATP, and HMGB1 are three substances that may be classified as DAMPs in ICD. Antigen-presenting cells (APCs) in the tumor microenvironment identify these essential molecules, prompting an immune response [55]. In addition, when virus infect and destroy cancer cells, they release tumor-associated specific antigens into the environment, which the immune system can recognize and respond to, disabling the immuno-editing process as shown in Fig. 3.
Tumor-associated specific antigens are released into the milieu, allowing the immune system to detect and elicit a reaction that breaks down the immuno-editing process [56]. Vaxinia virus-infected tumor cell produces an inflammatory site, producing cytokines that stimulate the immune system. A detrimental response is activating an immune system against the virus. It might build anti-tumor immunological memory with long-term effects to protect the host from recurrence [57].
Recent perspectives on Vaxinia virus targeting different cancers
The anticancer properties of the Vaxinia virus against various types of tumors are listed below:
Vaxinia virus against colon cancer
Various studies have been done to evaluate this virus's oncolytic activity and to image how it targets cancer cells. O’Leary et al. evaluated Vaxinia virus activity against colorectal cancer cells. A recombinant virus was produced by exchanging the thymidine kinase locus with one that encodes firefly luciferase (Fluc). Luciferase was used as an imageable agent attached to the virus for evaluating the movement and mechanism. In vitro, cytotoxicity assay and the viral replication test were carried out. Single doses of this virus were administered either intratumorally or intravenously to in vivo CRC flank xenografts. The luciferase imaging and organ titer methods were used to investigate viral biodistribution. In vitro studies established that virus infects, multiplies within, and ultimately kills CRC cells dose-dependently. In colorectal cancer cell lines, CF33-Fluc firefly luciferase effectively kills cells dose-dependently. At low doses, rapid tumor shrinkage occurred in the colorectal tumor xenograft model in vivo. Necroptotic process was found to be involved in the demise of CRC. Regardless of the route of administration, CF33-Fluc can replicate inside and kill colorectal cancer cells under in vitro and in vivo conditions. Luciferase expression paved the way for real-time monitoring of the replication of virus [58].
The purpose of this study was to evaluate Vaxinia ability to stimulate in vivo radioisotope uptake and to assess its efficacy towards colon cancer in vitro and in vivo. This work further delves into the application of radioactive isotopes for combinatorial tumor elimination and the cell death patterns of virus in preclinical colorectal cancer models. The resulting virus exhibits reproducible hNIS expression, and it replicates and kills immunogenic colon cancer cells in vitro. Regression of tumors in colorectal cancer xenograft models in vivo demonstrates the tumor-specific effectiveness of CF33-hNIS Vaxinia virus. When hNIS is expressed early during infection, positron emission tomography (PET) of I-124 uptake provides reliable imaging of viral replication. I-124 uptake activity was proportional to viral replication and tumor shrinkage. Finally, the addition of systemic administration of the radiotherapeutic isotope I-131 is done after virus infection of colon cancer. Xenografts improve the tumor shrinkage compared to virus treatment alone in HCT116 xenografts, suggesting the synergy of oncolytic viral therapy with radio ablation in vivo [59].
The recombinant orthopoxvirus is helpful in the treatment of colon cancer. Researchers used a mouse model of colon cancer to examine the effects of CF33 derivatives with and without immune checkpoint inhibition (anti-PD-L1). The expression of PD-L1 on tumor cells was elevated by CF33 infection, resulting in significantly more lymphocytes and macrophages infiltrating tumors. Tumors treated with virus also had more activated CD8 + (IFN +) T cells than tumors treated with the control virus. Moreover, resistant to tumor rechallenge, long-term survival was achieved when CF33 derivatives were combined with anti-PD-L1. Analysis of immune cells from treated mice demonstrated that antiviral T-cell activation was much greater than anti-tumor T-cell activation in an MHC-I-dependent way and that tumor-specific T-cell activation was robust in tumors treated with the virus [60].
Vaxinia virus against pancreatic cancer
Vaxinia virus has more cytotoxicity effect and more superior to other existing viruses. The CF33 gene was selected for more research. This study demonstrates that virus has the potential to target and eliminate pancreatic tumors quickly after receiving only a single intratumoral dosage. Initially, the tumors grew more significantly during the first two weeks compared to the control group. The tumor development rate slowed to a plateau by the third week compared to the control group. Finally, a decrease in the amount of the tumor that had been vaccinia-injected was observed after 3 weeks. The researcher exhibited a compressed timeline using CF33-Fluc. By Day 4, there was a discernible size difference between the tumors and the controls. From Day 4 to Day 8, there was a plateau, and then beginning on Day 8, there was a regression. After a single intratumoral low dose, this virus promoted rapid cell death in six different pancreatic cancer cell lines, which resulted in the release of damage-associated molecular patterns and regression of PANC-1 injected and non-injected distant xenografts in vivo. The virus was found to preferentially multiply in tumors when luciferase imaging was used, which conforms to the low virus titers reported in solid organs [61].
Recent studies provide essential information on the in vitro multimodal cancer-killing efficacy of virus as anti-PD-L1. Zhang et al. describe the Vaxinia virus-anti-PD-L1 mechanism to eliminate pancreatic ductal adenocarcinoma (PDAC) cells by regulating the immune system dynamically and inhibiting the PD-L1 checkpoint that has eluded early viral oncolysis. To counteract the immunosuppressive effects of PD-L1, researchers demonstrate that a substantial quantity of functional anti-PD-L1 antibodies was delivered. Experiments in which BxPC-3 cells and actuated T cells were cocultured along with CF33-hNIS-anti-PD-L1 virus demonstrated that the virus-encoded anti-PD-L1 effectively inhibits PD-L1 binding on BxPC-3 cells, which resulted in an increase in B granzyme in perforin release. Granzyme B and perforin are two proteins secreted by CD8 + T cells as they go through the granule exocytosis pathway. These two proteins play a significant role in activating CD8 + T cells to destroy cancer cells. Target cell apoptosis is induced by the granule protein perforin, which also facilitates the delivery of the cytotoxic enzyme granzyme B to malignant neoplastic cells. This is accomplished mainly through the activation of caspase. As a single treatment, Vaxinia virus has the potential to activate anti-tumor T cells in PDAC in a manner that is time-dependently increased [62].
Malignant peritoneal metastases from gastrointestinal cancers continue to be deadly. A chimeric orthopox Vaxinia encoding human sodium-iodide symporter (hNIS) and anti-human programmed death-ligand 1 antibody has shown potent anti-pancreatic cancer action in preclinical models (PDAC). After injecting tumors under the skin, the researcher studied whether virus could travel to the abdominal cavity (peritoneum) and infect, identify, and destroy peritoneal cancers in vivo. Athymic mice were injected with human PDAC AsPC-1-ffluc cells subcutaneously and intraperitoneal routes. Following tumor engraftment, treatment was given with CF33-hNIS-anti-PD-L1 immuno-oncolytic virus. The tumor volume and size were measured, and the animal survival was recorded using bioluminescence imaging, PET/CT imaging, and 124I-based positron emission tomographic staining after administration of CF33-hNIS-anti-PD-L1. The expression of hNIS was verified by immuno histochemical labeling in both subcutaneous and abdominal tumors after viral therapy. Mice treated with CF33-hNIS-anti-PD-L1 had substantially lower subcutaneous and peritoneal tumor burden and better survival than control. Seven days after the initial intravenous dosage of CF33-hNIS-anti-PD-L1, uptake of 124I was detected by PET/CT in subcutaneous and peritoneal tumors. The researcher demonstrates that CF33-hNIS-anti-PD-L1 aids in detecting and eliminating both superficial and deep cancers in the peritoneal cavity after superficial intraperitoneal therapy [63].
Vaxinia virus against breast cancer
The oncolytic virus CF33-hNIS-F14.5 was studied by Chaurasiya et al. to investigate whether it affects the tumor’s immune environment. They discovered that viral infection increases the levels of PD-L1 on triple-negative breast cancer cells under in vitro and in vivo conditions. The virus promotes tumor infiltration by CD8 + T lymphocytes in the orthotopic triple-negative breast cancer mouse model. Similarly, large amounts of the pro-inflammatory cytokines IFN and IL-6 were discovered in the tumors of mice that were treated with CF33-hNIS-ΔF14.5. Even greater degrees of immune regulation were observed in mice with both the virus and the anti-PD-L1 antibody administered to them as treatment. A combination of CF33-hNIS-ΔF14.5 and anti-PD-L1 antibody injected intratumorally resulted in a substantial anti-tumor impact, with fifty percent of the animals having total tumor regression. Furthermore, the treated mice did not grow tumors after being rechallenged with the same cancer cells, suggesting that they gained immunity against them. These studies established that virus positively affects the tumor immunological milieu rendering the cancer cells susceptible to the immune checkpoint inhibitor anti-PD-L1 [64].
Clinical studies
Various clinical trials which are undergoing are enlisted in Table 1. An innovative CHECKvacc has been shown to have potent anticancer effects in TNBC xenografts. Both hNIS and anti-PD-1 proteins were expressed in CHECKvacc-infected cells and proved functional against various tumors. In preclinical studies, researchers found that cancer cells infected with CHECKvacc release functional hNIS and anti-PD-L1 against tumor cells. A low dose of CHECKvacc, in comparison to other OVs used in xenograft models, can reliably detect, and kill TNBC. In this phase 1 clinical trial, researcher assessed the safety and tolerability of the intratumoral injection of the CHECKvacc vaccine in patients having metastatic triple-negative breast cancer. To qualify this clinical study, patients must have an advanced and metastatic illness and have either progressed during or been intolerant to at least two previous treatment regimens. CHECKvacc administered intratumorally at one of eight assigned dose levels (1 × 105 PFU, 3 × 105 PFU, 1 × 106 PFU, 3 × 106 PFU, 1 × 107 PFU, 3 × 107 PFU, 1 × 108 PFU, 3 × 108 PFU) is administered on Days 1 and 15 of each 28-day cycle for a total of three cycles of treatment. Phase II dose recommendation, optimal biological dose, and response rate are important secondary goals in this study. The first three individuals at level 1 dosage were recruited consecutively for safety monitoring. Following sequential treatment of the first three participants, the research will use the Phase I Queue 3 + 3 (IQ 3 + 3) design, which increases the dosage level to 8 subjects if a single DLT has been recorded. The final recommended phase 2 dose study may involve as many as 12 participants to measure effectiveness. The expected number of patients to be accrued ranges from 33 to 78. Additional goals include studying viral dynamics, 99mTc SPECT imaging for virus tracking and tracking peripheral blood and tumor tissue to activate antiviral immunity, and tumor microenvironment alterations in conjunction with treatment response. NCT05081492 is the clinical trial number. Imugene is the company funding this study [65].
The immunological alterations in the tumor microenvironment and the safety of the treatment regimens will be evaluated when CF33-hNIS, a new chimeric orthopoxvirus, is delivered alone or in conjunction with pembrolizumab. Patients with any stage IV solid tumor with radiographic progression as measured by the Response Evaluation Criteria in Solid Tumors are candidates for treatment. These patients must have had at least two previous lines of therapy, one of which may have been an immune checkpoint inhibitor. Treatment with CF33-hNIS will begin on Day 1 of Cycle 1 and Day 8 of Cycle 1 and will continue Day 1 of each subsequent cycle for all enrolled patients. Pembrolizumab will be added to the combined regimen on the first day of each cycle, starting with Cycle 2. NCT05346484 is the clinical trial number [66].
Conclusion
Oncolytic viral therapy for cancer is a relatively new area of research. Vaxinia virus has the potential against various pancreatic, breast, and colon cancer. The various studies of Vaxinia virus against these kinds of tumors which are done by investigators are enlisted in this manuscript. Furthermore, there is an ongoing need and desire to use biomarkers to identify cancers that will respond to oncolytic immune treatment. Hopefully, these novel vaccinations will play a pivotal role in treating cancer patients. Research on human clinical trials based on Vaxinia virus therapy is currently ongoing. To improve outcomes, it may be necessary to identify and include more people who might benefit from these innovative vaccinations. Recent advances in molecular biotechnology have provided scientists with new opportunities to use the immune system to combat cancer.
Additionally, OVs therapy is developing, and we have a far greater understanding of how they function today than ever before. Combining oncolytic viruses with other medicines, especially immunotherapy, seems to be a promising area of research. In addition, clinical studies are needed to prove oncolytic virotherapy’s biosafety, and new OVs should be developed as soon as possible for use in clinical practice. Researchers in the future will try out other kinds of pharmacological combinations, new kinds of OVs that have been genetically modified, and different kinds of drug delivery systems. The use of oncolytic virotherapy to achieve targeted and specific immunotherapy is a promising area of research. However, most OVs are in the research and development phase and need more development before they can be introduced to the market.
Data availability
This submission does not require any availability of data and materials as this is a review paper.
References
Cancer in 2022—CPR22. (n.d.). Cancer Progress Report., https://cancerprogressreport.aacr.org/progress/cpr22-contents/cpr22-cancer-in-2022/. Accessed 30 April 2023
Cancer. (n.d.)., https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed 23 Aug 2022
Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149(4):778–89. https://doi.org/10.1002/ijc.33588.
Lauer UM, Beil J. Oncolytic viruses: challenges and considerations in an evolving clinical landscape. Future Oncol. 2022;18(24):2713–32. https://doi.org/10.2217/fon-2022-0440.
Kayode AA, Eya IE, Kayode OT. A short review on cancer therapeutics. Phys Sci Rev. 2022. https://doi.org/10.1515/psr-2021-0169.
Nenclares P, Harrington KJ. The biology of cancer. Medicine. 2020;48(2):67–72. https://doi.org/10.1016/j.mpmed.2019.11.001.
Cattley RC, Radinsky BR. Cancer therapeutics: understanding the mechanism of action. Toxicol Pathol. 2004;32(1_suppl):116–21. https://doi.org/10.1080/01926230490426507.
Vesely MD, Schreiber RD. Cancer immunoediting: Antigens, mechanisms, and implications to cancer immunotherapy: tumor antigens and cancer immunoediting. Ann N Y Acad Sci. 2013;1284(1):1–5. https://doi.org/10.1111/nyas.12105.
Gubin MM, Vesely MD. Cancer immunoediting in the era of immuno-oncology. Clin Cancer Res. 2022. https://doi.org/10.1158/1078-0432.CCR-21-1804.
Wilczyński JR, Nowak M. Cancer Immunoediting Elimination, Equilibrium, and Immune Escape in Solid Tumors. In: Klink M, Szulc-Kielbik I, editors. Interaction of Immune and Cancer Cells, vol. 113. Cham: Springer International Publishing; 2022. p. 1–57. https://doi.org/10.1007/978-3-030-91311-3_1.
Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, Kolahian S, Javaheri T, Zare P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 2020;18(1):59. https://doi.org/10.1186/s12964-020-0530-4.
Atsou K, Khou S, Anjuère F, Braud VM, Goudon T. Analysis of the equilibrium phase in immune-controlled tumors provides hints for designing better strategies for cancer treatment. Front Oncol. 2022;12:878827. https://doi.org/10.3389/fonc.2022.878827.
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: From immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–8. https://doi.org/10.1038/ni1102-991.
Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–48. https://doi.org/10.1016/j.immuni.2004.07.017.
Whiteside T. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol. 2006;16(1):3–15. https://doi.org/10.1016/j.semcancer.2005.07.008.
Printezi MI, Kilgallen AB, Bond MJG, Štibler U, Putker M, Teske AJ, Cramer MJ, Punt CJA, Sluijter JPG, Huitema ADR, May AM, van Laake LW. Toxicity and efficacy of chronomodulated chemotherapy: a systematic review. Lancet Oncol. 2022;23(3):e129–43. https://doi.org/10.1016/S1470-2045(21)00639-2.
Alfarouk KO, Stock C-M, Taylor S, Walsh M, Muddathir AK, Verduzco D, Bashir AHH, Mohammed OY, Elhassan GO, Harguindey S, Reshkin SJ, Ibrahim ME, Rauch C. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 2015;15(1):71. https://doi.org/10.1186/s12935-015-0221-1.
Garmaroudi GA, Karimi F, Naeini LG, Kokabian P, Givtaj N. Therapeutic Efficacy of oncolytic viruses in fighting cancer: recent advances and perspective. Oxid Med Cell Longev. 2022;2022:1–14. https://doi.org/10.1155/2022/3142306.
Bommareddy PK, Shettigar M, Kaufman HL. Integrating oncolytic viruses in combination cancer immunotherapy. Nat Rev Immunol. 2018;18(8):498–513. https://doi.org/10.1038/s41577-018-0014-6.
de Graaf JF, de Vor L, Fouchier RAM, van den Hoogen BG. Armed oncolytic viruses: a kick-start for anti-tumor immunity. Cytokine Growth Factor Rev. 2018;41:28–39. https://doi.org/10.1016/j.cytogfr.2018.03.006.
Yuan Y, Zhang J, Kessler J, Rand J, Modi B, Chaurasiya S, Murga M, Tang A, Martinez N, Meisen H, Yamauchi D, Yost SE, Chong LMO, Seiz A, Nixon B, Ede N, Waisman JR, Stewart DB, Mortimer JE, Fong Y. Phase I study of intratumoral administration of CF33-HNIS-antiPDL1 in patients with metastatic triple negative breast cancer. J Clin Oncol. 2022;40(16_suppl):e13070–e13070. https://doi.org/10.1200/JCO.2022.40.16_suppl.e13070.
Imugene (ASX: IMU). (n.d.). https://www.imugene.com/. Accessed 23 Aug 2022
Greseth MD, Traktman P. The life cycle of the vaccinia virus genome. Ann Rev Virol. 2022. https://doi.org/10.1146/annurev-virology-091919-104752.
Zhang Z, Dong L, Zhao C, Zheng P, Zhang X, Xu J. Vaccinia virus-based vector against infectious diseases and tumors. Hum Vaccin Immunother. 2021;17(6):1578–85. https://doi.org/10.1080/21645515.2020.1840887.
Mackett M, Smith GL, Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci. 1982;79(23):7415–9. https://doi.org/10.1073/pnas.79.23.7415.
Woo Y, Zhang Z, Yang A, Chaurasiya S, Park AK, Lu J, Kim S-I, Warner SG, Von Hoff D, Fong Y. Novel chimeric immuno-oncolytic virus CF33-hNIS-antiPDL1 for the treatment of pancreatic cancer. J Am Coll Surg. 2020;230(4):709–17. https://doi.org/10.1016/j.jamcollsurg.2019.12.027.
Mojic M, Takeda K, Hayakawa Y. The dark side of IFN-γ: its role in promoting cancer immunoevasion. Int J Mol Sci. 2017;19(1):89. https://doi.org/10.3390/ijms19010089.
Schirrmacher V. Molecular Mechanisms of anti-neoplastic and immune stimulatory properties of oncolytic newcastle disease virus. Biomedicines. 2022;10(3):562. https://doi.org/10.3390/biomedicines10030562.
Esfahani K, Roudaia L, Buhlaiga N, Del Rincon SV, Papneja N, Miller WH. A Review of cancer immunotherapy: from the past, to the present, to the future. Curr Oncol. 2020;27(12):87–97. https://doi.org/10.3747/co.27.5223.
Davola ME, Mossman KL. Oncolytic viruses: how “lytic” must they be for therapeutic efficacy. OncoImmunology. 2019;8(6):e1581528. https://doi.org/10.1080/2162402X.2019.1596006.
Rojas-Domínguez A, Arroyo-Duarte R, Rincón-Vieyra F, Alvarado-Mentado M. Modeling cancer immunoediting in tumor microenvironment with system characterization through the ising-model Hamiltonian. BMC Bioinform. 2022;23(1):200. https://doi.org/10.1186/s12859-022-04731-w.
Nirmal AJ, Maliga Z, Vallius T, Quattrochi B, Chen AA, Jacobson CA, Pelletier RJ, Yapp C, Arias-Camison R, Chen Y-A, Lian CG, Murphy GF, Santagata S, Sorger PK. The Spatial landscape of progression and immunoediting in primary melanoma at single-cell resolution. Cancer Discov. 2022;12(6):1518–41. https://doi.org/10.1158/2159-8290.CD-21-1357.
Christie JD, Chiocca EA. Treat and repeat: oncolytic virus therapy for brain cancer. Nat Med. 2022;28(8):1540–2. https://doi.org/10.1038/s41591-022-01901-4.
Chen L, Zhou C, Chen Q, Shang J, Liu Z, Guo Y, Li C, Wang H, Ye Q, Li X, Zu S, Li F, Xia Q, Zhou T, Li A, Wang C, Chen Y, Wu A, Qin C, Man J. Oncolytic Zika virus promotes intratumoral T cell infiltration and improves immunotherapy efficacy in glioblastoma. Mol Ther—Oncolytics. 2022;24:522–34. https://doi.org/10.1016/j.omto.2022.01.011.
Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat Rev Cancer. 2014;14(8):559–67. https://doi.org/10.1038/nrc3770.
Prestwich RJ, Harrington KJ, Pandha HS, Vile RG, Melcher AA, Errington F. Oncolytic viruses: a novel form of immunotherapy. Expert Rev Anticancer Ther. 2008;8(10):1581–8. https://doi.org/10.1586/14737140.8.10.1581.
Tang C, Li L, Mo T, Na J, Qian Z, Fan D, Sun X, Yao M, Pan L, Huang Y, Zhong L. Oncolytic viral vectors in the era of diversified cancer therapy: from preclinical to clinical. Clin Transl Oncol. 2022;24(9):1682–701. https://doi.org/10.1007/s12094-022-02830-x.
Cejalvo JM, Falato C, Villanueva L, Tolosa P, González X, Pascal M, Canes J, Gavilá J, Manso L, Pascual T, Prat A, Salvador F. Oncolytic viruses: a new immunotherapeutic approach for breast cancer treatment. Cancer Treat Rev. 2022;106:102392. https://doi.org/10.1016/j.ctrv.2022.102392.
Haseley A, Alvarez-Breckenridge C, Chaudhury A, Kaur B. Advances in oncolytic virus therapy for glioma. Recent Pat CNS Drug Discov. 2009;4(1):1–13. https://doi.org/10.2174/157488909787002573.
Hemminki O, dos Santos JM, Hemminki A. Oncolytic viruses for cancer immunotherapy. J Hematol Oncol. 2020;13(1):84. https://doi.org/10.1186/s13045-020-00922-1.
Garg AD, Dudek-Peric AM, Romano E, Agostinis P. Immunogenic cell death. Int J Dev Biol. 2015;59(1-2–3):131–40. https://doi.org/10.1387/ijdb.150061pa.
Schmidt SV, Nino-Castro AC, Schultze JL. Regulatory dendritic cells: there is more than just immune activation. Front Immunol. 2012. https://doi.org/10.3389/fimmu.2012.00274.
Couzin-Frankel J. Cancer immunotherapy. Science. 2013;342(6165):1432–3. https://doi.org/10.1126/science.342.6165.1432.
Mostafa A, Meyers D, Thirukkumaran C, Liu P, Gratton K, Spurrell J, Shi Q, Thakur S, Morris D. Oncolytic reovirus and immune checkpoint inhibition as a novel immunotherapeutic strategy for breast cancer. Cancers. 2018;10(6):205. https://doi.org/10.3390/cancers10060205.
Gholami S, Marano A, Chen NG, Aguilar RJ, Frentzen A, Chen C-H, Lou E, Fujisawa S, Eveno C, Belin L, Zanzonico P, Szalay A, Fong Y. A novel vaccinia virus with dual oncolytic and anti-angiogenic therapeutic effects against triple-negative breast cancer. Breast Cancer Res Treat. 2014;148(3):489–99. https://doi.org/10.1007/s10549-014-3180-7.
Mardi A, Shirokova AV, Mohammed RN, Keshavarz A, Zekiy AO, Thangavelu L, Mohamad TAM, Marofi F, Shomali N, Zamani A, Akbari M. Biological causes of immunogenic cancer cell death (ICD) and anti-tumor therapy; combination of oncolytic virus-based immunotherapy and CAR T-cell therapy for ICD induction. Cancer Cell Int. 2022;22(1):168. https://doi.org/10.1186/s12935-022-02585-z.
Nutter Howard FH, Iscaro A, Muthana M (2022) Oncolytic viral particle delivery. In Systemic Drug Delivery Strategies (pp. 211–230). Elsevier, https://doi.org/10.1016/B978-0-323-85781-9.00008-7
Burgess HM, Pourchet A, Hajdu CH, Chiriboga L, Frey AB, Mohr I. Targeting Poxvirus decapping enzymes and mRNA decay to generate an effective oncolytic virus. Mol Ther—Oncolytics. 2018;8:71–81. https://doi.org/10.1016/j.omto.2018.01.001.
Gal-Ben-Ari S, Barrera I, Ehrlich M, Rosenblum K. PKR: a kinase to remember. Front Mol Neurosci. 2019;11:480. https://doi.org/10.3389/fnmol.2018.00480.
Ripp J, Hentzen S, Saeed A. Oncolytic viruses as an adjunct to immune checkpoint inhibition. Front Biosci-Landmark. 2022;27(5):151. https://doi.org/10.31083/j.fbl2705151.
Li D, Wu M. Pattern recognition receptors in health and diseases. Signal Transduct Target Ther. 2021;6(1):291. https://doi.org/10.1038/s41392-021-00687-0.
Sitta J, Claudio PP, Howard CM. Virus-Based Immuno-Oncology Models. Biomedicines. 2022;10(6):1441. https://doi.org/10.3390/biomedicines10061441.
Ahrends T, Borst J. The opposing roles of CD4+ T cells in anti-tumour immunity. Immunology. 2018;154(4):582–92. https://doi.org/10.1111/imm.12941.
Marchini A, Daeffler L, Pozdeev VI, Angelova A, Rommelaere J. Immune Conversion of tumor microenvironment by oncolytic viruses: the protoparvovirus H-1PV case study. Front Immunol. 2019;10:1848. https://doi.org/10.3389/fimmu.2019.01848.
Kielbik M, Szulc-Kielbik I, Klink M. Calreticulin—multifunctional chaperone in immunogenic cell death: potential significance as a prognostic biomarker in ovarian cancer patients. Cells. 2021;10(1):130. https://doi.org/10.3390/cells10010130.
Das K, Belnoue E, Rossi M, Hofer T, Danklmaier S, Nolden T, Schreiber L-M, Angerer K, Kimpel J, Hoegler S, Spiesschaert B, Kenner L, von Laer D, Elbers K, Derouazi M, Wollmann G. A modular self-adjuvanting cancer vaccine combined with an oncolytic vaccine induces potent antitumor immunity. Nat Commun. 2021;12(1):5195. https://doi.org/10.1038/s41467-021-25506-6.
Lemos de Matos A, Franco LS, McFadden G. Oncolytic viruses and the immune system: the dynamic duo. Mol Ther—Methods Clin Dev. 2020;17:349–58. https://doi.org/10.1016/j.omtm.2020.01.001.
O’Leary MP, Warner SG, Kim S-I, Chaurasiya S, Lu J, Choi AH, Park AK, Woo Y, Fong Y, Chen NG. A novel oncolytic chimeric orthopoxvirus encoding luciferase enables real-time view of colorectal cancer cell infection. Mol Ther—Oncolytics. 2018;9:13–21. https://doi.org/10.1016/j.omto.2018.03.001.
Warner SG, Kim S-I, Chaurasiya S, O’Leary MP, Lu J, Sivanandam V, Woo Y, Chen NG, Fong Y. A novel chimeric poxvirus encoding hnis is tumor-tropic, imageable, and synergistic with radioiodine to sustain colon cancer regression. Mol Ther—Oncolytics. 2019;13:82–92. https://doi.org/10.1016/j.omto.2019.04.001.
Kim S-I, Park AK, Chaurasiya S, Kang S, Lu J, Yang A, Sivanandam V, Zhang Z, Woo Y, Priceman SJ, Fong Y, Warner SG. Recombinant orthopoxvirus primes colon cancer for checkpoint inhibitor and cross-primes T cells for antitumor and antiviral immunity. Mol Cancer Ther. 2021;20(1):173–82. https://doi.org/10.1158/1535-7163.MCT-20-0405.
O’Leary MP, Choi AH, Kim S-I, Chaurasiya S, Lu J, Park AK, Woo Y, Warner SG, Fong Y, Chen NG. Novel oncolytic chimeric orthopoxvirus causes regression of pancreatic cancer xenografts and exhibits abscopal effect at a single low dose. J Transl Med. 2018;16(1):110. https://doi.org/10.1186/s12967-018-1483-x.
Zhang Z, Yang A, Chaurasiya S, Park AK, Lu J, Kim S-I, Warner SG, Yuan Y-C, Liu Z, Han H, Von Hoff D, Fong Y, Woo Y. CF33-hNIS-antiPDL1 virus primes pancreatic ductal adenocarcinoma for enhanced anti-PD-L1 therapy. Cancer Gene Ther. 2021. https://doi.org/10.1038/s41417-021-00350-4.
Chaurasiya S, Yang A, Kang S, Lu J, Kim S-I, Park AK, Sivanandam V, Zhang Z, Woo Y, Warner SG, Fong Y. Oncolytic poxvirus CF33-hNIS-ΔF14.5 favorably modulates tumor immune microenvironment and works synergistically with anti-PD-L1 antibody in a triple-negative breast cancer model. OncoImmunology. 2020;9(1):1729300. https://doi.org/10.1080/2162402X.2020.1729300.
Zhang Z, Yang A, Chaurasiya S, Park AK, Kim S-I, Lu J, Olafsen T, Warner SG, Fong Y, Woo Y. PET imaging and treatment of pancreatic cancer peritoneal carcinomatosis after subcutaneous intratumoral administration of a novel oncolytic virus, CF33-hNIS-antiPDL1. Mol Ther—Oncolytics. 2022;24:331–9. https://doi.org/10.1016/j.omto.2021.12.022.
CF33-hNIS-antiPDL1 for the Treatment of Metastatic Triple Negative Breast Cancer—Full Text View—ClinicalTrials.gov. (n.d.) https://clinicaltrials.gov/ct2/show/NCT05081492.
A Study of CF33-hNIS (VAXINIA), an Oncolytic Virus, as Monotherapy or in Combination with Pembrolizumab in Adults with Metastatic or Advanced Solid Tumors ClinicalTrials.gov. (n.d.). https://clinicaltrials.gov/ct2/show/NCT05346484. Acccessed 23 Aug 2022,
Acknowledgements
The authors are grateful to Shoolini University for providing junior research scholarship to Simran Deep Kaur and also to all the researchers who discovered Vaxinia virus and immuno-oncolytic drug delivery system that was helpful for framing this review paper.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors were involved in the study’s conception and initiation. The paper’s outline and structure were designed by SDK. All the figures and tables in the original draft were made by SDK. DNK and ADS read and commented on earlier versions of the paper. The manuscript is edited by SDK and DNK. The manuscript was edited under DNK’s direction. The final version, which was the result of multiple rounds of editing, was approved by all of the authors.
Corresponding author
Ethics declarations
Conflict of interest
Simran Deep Kaur, Deepak N Kapoor, Aman Deep Singh declare that they have no conflict of interest.
Ethical approval and consent to participations
Not applicable.
Consent for publications
We agreed with the journal policy and provided our consent for the publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, S.D., Singh, A.D. & Kapoor, D.N. Current perspectives on Vaxinia virus: an immuno-oncolytic vector in cancer therapy. Med Oncol 40, 205 (2023). https://doi.org/10.1007/s12032-023-02068-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02068-9