Abstract
Background
Lymph node (LN) metastasis confers gastric cancer (GC) progression, poor survival and cancer-related death. Aberrant activation of Wnt/β-catenin promotes epithelial-mesenchymal transition (EMT) and LN metastasis, whereas the constitutive activation mutation of Wnt/β-catenin is rare in GC, suggesting that the underlying mechanisms enhancing Wnt/β-catenin activation need to be further investigated and understood.
Methods
Bioinformatics analyses and immunohistochemistry (IHC) were used to identify and detect LN metastasis-related genes in GC. Cellular functional assays and footpad inoculation mouse model illustrate the biological function of CCT5. Co-immunoprecipitation assays, western blot and qPCR elucidate the interaction between CCT5 and E-cadherin, and the regulation on β-catenin activity.
Results
CCT5 is upregulated in LN metastatic GCs and correlates with poor prognosis. In vitro assays prove that CCT5 markedly promotes GC cell proliferation, anti-anoikis, invasion and lymphatic tube formation. Moreover, CCT5 enhances xenograft GC growth and popliteal lymph node metastasis in vivo. Furthermore, CCT5 binds the cytoplasmic domain of E-cadherin and abrogates the interaction between E-cadherin and β-catenin, thereby releasing β-catenin to the nucleus and enhancing Wnt/β-catenin signalling activity and EMT.
Conclusion
CCT5 promotes GC progression and LN metastasis by enhancing wnt/β-catenin activation, suggesting a great potential of CCT5 as a biomarker for GC diagnosis and therapy.
Similar content being viewed by others
Background
Gastric cancer (GC) is one of the most commonly diagnosed malignant cancer types worldwide [1]. Notably, the morbidity and mortality of GC patients are higher in developing countries than that in developed countries, especially ~50% of newly diagnosed GC patients occur in China [2]. Despite advances in GC therapeutic strategies over the last decades, the 5-year survival rate of GC patients in China remains as low as about 30%; likewise, the 5-year survival rate of patients with metastatic GCs is less than 10% [3, 4]. Therefore, it is urgent to investigate the mechanisms of GC metastasis and to identify effective prognostic and therapeutic biomarkers for metastatic GCs.
Cancer metastasis involves series of sophisticated cascades procedures, including local invasion, lymph node (LN) metastasis, anti-anoikis in circulation flow and colonisation at metastatic sites [5]. Of note, epithelial-mesenchymal transition (EMT) has been well established as a key event to contribute cancer progression and metastasis in almost all types of somatic cancers, which is provoked by multiple signalling pathways, such as Wnt/β-catenin, TGF-β and PI3K/Akt [6, 7]. Therefore, exploring the molecules involving the regulation activity of the signalling pathways will provide an insight into understanding the mechanism of the cancer metastasis related EMT process [8].
The aberrant over-activated Wnt/β-catenin signalling is observed in various types of cancers and plays a crucial role in tumour initiation and malignant progression [9,10,11]. In quiescent status, most of β-catenin binds with E-cadherin, which is presented as the E-cadherin/β-catenin adhesion complex located in cell-cell adherent junctions on the membrane. Decreasing of E-cadherin level or disrupting the E-cadherin/β-catenin interaction facilitates Wnt/β-catenin signalling pathway activation through releasing β-catenin to the nucleus and functioning as a transcriptional co-factor [9, 12]. However, compared to that ~80% colorectal cancer patients are bearing oncogenic alteration of APC or CTNNB1 gene, the genomic alteration frequencies of APC and CTNNB1 in GC are less than 20% [13,14,15]. These suggest that the veiled mechanism enhancing Wnt/β-catenin signalling activation in GCs remains need to be further investigated, which may provide new diagnostic/prognostic markers and therapeutic targets for metastatic GCs.
CCT5 is a subunit of chaperonin containing TCP1 complex that is also known as the TCP1 ring complex (TRiC). The expression levels of CCT5 have been found to be upregulated in types of cancers, including breast cancer, colon cancer, lung cancer, glioblastoma, hepatocellular carcinoma and ovarian cancer [16,17,18,19,20,21]. Nonetheless, the functional significance of CCT5 in cancer development and progression remains indistinct, especially in GC. In the current study, we elucidate that CCT5 plays a pro-EMT and pro-LN metastasis role in GCs. Mechanistically, CCT5 interacts with E-cadherin to competitively abrogate the E-cadherin/β-catenin adhesion complex, enhancing β-catenin activation and conferring GC progression. Moreover, CCT5 is upregulated in LN metastatic GCs and correlated with poor prognosis of GC patients, suggesting the therapeutic, diagnostic, and prognostic potential of CCT5.
Methods
Cell lines
Gastric cell lines, including NCI-N87, SNU16, AGS, SNU1, MKN28, SNU5, MGC803 and HGC27, and non-cancerous HEK293FT and GES-1 cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) or National Collection of Authenticated Cell Cultures (SIBS; Shanghai, China) and maintained in RPMI-1640 medium (for GES-1, NCI-N87, SNU16, SNU1, MKN28, MGC803 and HGC27 cell lines), Iscove’s Modified Dulbecco’s Medium (for SNU5 cell line), Kaighn’s Modification of Ham’s F-12 Medium (for AGS cell line) or Dulbecco’s modified Eagle’s medium (for HEK293FT cell line) supplemented with 10% foetal bovine serum (FBS; Corning, Tewksbury, MA, USA) and 1% penicillin/streptomycin (penicillin 100 U/ml and streptomycin 10 μg/ml) (GIBCO). All cell lines were authenticated by short tandem repeat (STR) DNA profiling at Medicine Laboratory of Forensic Medicine Department of Sun Yat-Sen University (Guangzhou, China), and were verified to be mycoplasma-free.
Clinical specimens and ethical approval
All clinical tissue specimens used in this study were obtained from and histopathologically diagnosed at Jiangmen Central Hospital. 101 cases GC tissues and 5 cases non-cancerous gastric benign hyperplasia tissues were collected from surgery and frozen in liquid nitrogen until further use. For the use of these clinical materials for research purposes, prior patients’ consents and approval from the Institutional Research Ethics Committee of Jiangmen Central Hospital were obtained. The study is compliant with all relevant ethical regulations involving human participants.
Animal model
Male BALB/c-nu mice (5–6 weeks of age, 18–20 g) were housed in specific pathogen-free facilities for animal studies. At least five nude mice per group were used to ensure the adequate power and each mouse with different weight was randomly allocated. For establishment of lymph node metastasis model, indicated cells (1 × 106) were subcutaneously implanted into the left-side foot pad of lower extremity of nude mice (n = 5 per group). Tumour formation was monitored over a 4-week period with monitoring by bioluminescent imaging every 7 days. For bioluminescent imaging assay, five minutes prior to imaging, mice were injected intraperitoneally (i.p.) with 150 mg/kg luciferin. Following general anaesthesia, images were taken and analysed with Spectrum Living Image version 4.2 (Caliper Life Sciences, Waltham, MA, USA). At the experimental endpoint, nude mice were anaesthetised and sacrificed, and both the foot pad xenografted tumours and popliteal lymph node were resected, sectioned (5 μm in thickness) and histologically examined by H&E staining. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University. Priori sample size estimation was not done. Randomisation was not done because no xenograft experiments involving treatment was not done in this study. No blinding was done during xenograft experiments.
Immunohistochemistry (IHC) assay
IHC assays using anti-CCT5, anti-β-catenin, or anti-LYVE1 antibodies were separately conducted on paraffin-embedded specimens of GC patients. The immunostaining intensities of indicated proteins was evaluated and scored by two independent observers, scoring both the proportions of positive staining tumour cells and the staining intensities in 10 random fields. Scores representing the proportion of positively stained tumour cells was graded as: 0 (no positive tumour cells), 1 (<10%), 2 (10–50%), and 3 (>50%). The staining intensity was determined as: 0 (no staining); 1 (weak staining = light yellow), 2 (moderate staining = yellow brown), and 3 (strong staining = brown). The staining index (SI) was calculated as staining intensity × percentage of positive tumour cells, resulting in scores as 0, 1, 2, 3, 4, 6 and 9. Cutoff values for high- or low-expression of interested proteins were determined by the median of all samples.
Enzyme-linked immunosorbent assay (ELISA)
The Human VEGF-C Quantikine ELISA Kit was purchased from R&D Systems (DVEC00; Minneapolis, MN, USA). Cells were cultured in 6-well dishes with 2.5 ml medium for 48 h to reach proximately 80% confluence. Then the culture medium was collected to measure the secreted VEGFC levels according to the manufacturer’s instructions.
TCGA dataset analysis and sequencing data deposition
RNAseq data for mRNA expression of 32 pairs of GC tissues and paired adjacent non-cancerous gastric tissues, and 415 cases of GC tissues, as well as their corresponding clinical information, were mined from The Cancer Genome Atlas (TCGA) stomach adenocarcinoma (STAD) datasets [https://cancergenome.nih.gov/].
Statistical analysis
All statistical analyses except the sequencing data were performed using the PASW Statistics 18 version 18.0.0 (SPSS Inc., Chicago, IL, USA) software package and GraphPad Prism 8 version 8.3.0 (GraphPad Software, San Diego, CA, USA). Survival curves were analysed by the Kaplan–Meier method and compared by the log-rank test. Comparisons between two groups were performed using Student’s t-test (two-tailed), while analyses comparing multiple treatments with a control group were performed using two-way ANOVA with post hoc Dunnett’s multiple comparisons test. All error bars represent the mean ± SD derived from three independent experiments. In all cases, P < 0.05 was considered to be statistically significant.
Results
CCT5 is upregulated in GCs and correlates with LN metastasis and poor prognosis of GCs
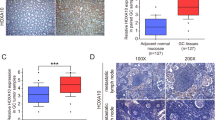
In light of that lymph node metastasis presents as an early stage of GC metastasis and is considered as an independent prognostic factor and a surgical operation indicator of GC [22]. To explore the molecules potentially regulating GC metastasis, we initially analysed the Cancer Genome Atlas (TCGA) stomach adenocarcinoma (STAD) dataset. As shown in the diagram, among the genes altered in patients bearing lymph node metastatic GC, CCT5, a subunit of chaperonin containing TCP1 complex, was identified significantly upregulating in lymph node metastatic GCs, as compared to GCs without lymph node metastasis (Fig. 1a, b). Subsequently, the clinical significance and the potential role of CCT5 in human GCs were further investigated in the STAD dataset of TCGA. The mRNA level of CCT5 was significantly upregulated in STAD tumour samples as compared with adjacent normal tissues, which include 32 pairs of gastric cancer/normal tissues, and 415 cases of gastric cancer vs. 35 cases of normal gastric tissues (Fig. 1c, d). Moreover, in our collected cohort containing 101 GC specimens and 5 benign hyperplasia specimens, IHC staining showed that CCT5 was significantly increased accompanied with tumour progression in GCs (Fig. 1e, f). In addition, the correlation between CCT5 expression and clinicopathological characteristics was further examined by χ2 test in our cohort, which showed that high CCT5 expression was positively correlated with stages of cancer (P < 0.01) and lymph node metastasis (P < 0.01), but not with the status of age, gender, tumour size and distant metastasis (Supplementary Table 1). Importantly, Kaplan–Meier plot survival analysis and log-rank test showed that GC patients bearing high CCT5 expression displayed a significantly shorter median survival time of 26.02 months and lower 5-year survival rate of 27.40%, compared with the 46.22 month median survival and 43.73% rate 5-year survival for those with low CCT5 expression; in parallel, high CCT5 level was positively correlated with shorter recurrence-free survival (CCT5 high: median survival 29.57 months and 34.49% 5-year survival rate, vs. CCT5 low: median survival 55.06 months and 49.12% 5-year survival rate) in the TCGA STAD dataset (Fig. 1g). Moreover, the protein and mRNA levels of CCT5 in GC cell lines was differentially overexpressed as compared with gastric epithelial cell line GES-1 (Fig. 1h, i). These analyses suggest that the aberrant upregulated CCT5 in GCs might play an important role in GC malignant progression.
a Heatmap shows the genes significantly altered in patients passed away bearing stomach adenocarcinoma tissues with lymph node metastasis of the TCGA STAD cohort. b Analysis of CCT5 expression in stomach adenocarcinoma tissues with or without LN metastasis in the TCGA STAD cohort. c, d Analysis of CCT5 expression in adjacent normal tissues and stomach adenocarcinoma tissues in the TCGA STAD cohort. e, f Representative images of immunohistochemical staining for CCT5 in benign gastric hyperplasia and stage I-IV gastric cancer tissues (e), and the staining index of CCT5 in our collected cohort (f). g Kaplan–Meier analysis (Log-rank test) of the 5-year overall survival (left panel) and disease-free survival (right panel) of gastric cancer patients in the TCGA STAD datasets, who were divided into low or high CCT5 expression subgroups. h, i CCT5 mRNA and protrin levels were detected in human gastric mucosal epithelial cell line GES-1 and series of human gastric cancer cell lines. Results are presented as mean ± SD.
CCT5 potently promotes GC cell survival, invasion and lymphangiogenesis in vitro
To elucidate the role of CCT5 in GC malignant progression, SNU16 and MKN28 cells with stably expressing CCT5 cDNA, MGC803 and HGC27 cells with stably silencing of CCT5 expression were constructed (Supplementary Fig. 1a, b). Following, we investigated the effects of CCT5 on GC cell survival and invasion. The colony formation assays and suspension-induced anoikis assays revealed that CCT5 significantly promoted the proliferation, survival and anti-anoikis abilities of GC cells, as compared to vector-control cells, while silencing CCT5 showed the opposite effects (Fig. 2a–d). Moreover, the transwell matrix penetration assays elucidated that CCT5-overexpressed GC cells exhibited higher invasive capacity than vector-control cells, whereas the invasive capacity was impaired in CCT5-suppressed cells (Fig. 2e–g). In addition, the tube formation assays revealed that overexpressing CCT5 strongly provoked; conversely, silencing CCT5 abrogated the ability of GC cell inducing tube formation of human lymphatic endothelial cells (HLECs) (Fig. 2h–j). These results clearly suggest a pro-survival, pro-invasion and pro-lymphangiogenesis role of CCT5 in GC cells, which have been implicated to importantly contribute to cancer cell survival and migration in the cascaded metastasis process.
a–c Representative images and quantification of cell colonies formed by indicated cells. d The sub-G1 DNA contents of detached cells for vector-control, CCT5-overexpressing or silencing cells are shown in the anoikis assay. e–g Representative images and quantification of invading cells in three random fields of Matrigel-coated transwell assays performed with indicated cells. h–j Representative images and quantification of tube lengths in five random fields formed by human lymphatic endothelial cells with indicated cell culture supernatant treatment. Scale bar: 200 μm (e, f), 400 μm (i, j). Results are presented as mean ± SD.
CCT5 promotes GC growth and LN metastasis in vivo
Subsequently, the effect of CCT5 on xenografted GC tumours was further investigated in vivo by using a footpad inoculation mouse model. The CCT5-overexpressed MKN28 cell, CCT5-silenced MGC803 cell and vector-control cells, which stably expressed firefly luciferase, were vaccinated into the footpads of nude mice (n = 5 per group). The resulting footpad tumours and popliteal lymph nodes were enucleated and analysed after 4 weeks of injection. Noticeably, the tumours formed by MKN28-CCT5 cells displayed larger volumes and abundant levels of microlymphatic vessel density (MLD) compared with the control tumours, as indicated by the LYVE-1-positive cells (LYVE-1 staining index) (Fig. 3a, b, d–f). Conversely, tumour volumes and MLDs were markedly reduced in the tumours formed by MGC803-CCT5 sh1 cell (Fig. 3a, c–f). Meanwhile, we found that popliteal lymph nodes in mice inoculated with CCT5-transduced cells presented larger volumes and displayed higher ratio of metastatic lymph nodes to total dissected popliteal lymph nodes in the MKN28-CCT5 group (100%, 6/6) than that in the vector control groups (33.3%, 2/6) (Fig. 3a, b, g–j). In contrast, the popliteal lymph nodes dissected from mice injected with CCT5-silenced cells showed smaller volumes than vector-control tumours, and the ratio of metastatic lymph nodes were drastically suppressed in the group (83.33%, 5/6 vs. 16.67%, 1/6) (Fig. 3a, c, g–j). Taken together, these findings indicate that CCT5 promotes tumour growth and lymphatic metastasis of GC in vivo.
a–c Represent bioluminescent and gross anatomy images of nude mice lower limbs with footpad inoculation indicated cells (a), and quantitation of bioluminescent intensities and tumour volumes of footpad xenografts in each group (b, c, n = 5 per group). d–f Representative images of immunohistochemical staining for LYVE-1 in footpad xenografts formed by indicated cells (d), the footpad tumour volume (e) and staining index of LYVE-1 in each group (f). g, h Images of popliteal lymph nodes dissected from nude mice with footpad inoculation indicated cells (g), and the volume of popliteal lymph nodes in each group (h). i, j H&E staining of popliteal lymph nodes dissected from nude mice with footpad inoculation indicated cells (i), and the ratio of metastatic lymph nodes in each group (j). Scale bar: 200 μm (d), 800 μm (i). Results are presented as mean ± SD.
CCT5 facilitates Wnt/β-catenin signalling pathway activation and epithelial-mesenchymal transition
To explore the underlying mechanism by which CCT5 promotes GC progression and metastasis, the gene set enrichment analysis (GSEA) was employed to identify the veiled cancer biology process and signalling pathway associated with CCT5 expression in TCGA STAD dataset. Of note, the results showed that CCT5 expression was positively correlated with gene signatures upregulated during metastasis and lymphatic angiogenesis, meanwhile inversely correlated with gene signatures downregulated in the processes (Fig. 4a, b). Moreover, CCT5 levels were significantly associated with the Wnt/β-catenin signalling regulated gene signatures in GCs, suggesting that CCT5 may involve cancer progression and metastasis through regulating Wnt/β-catenin activity (Fig. 4c).
a–c GSEA analysis of the TCGA STAD datasets shows positive correlation between CCT5 with gene signatures during metastasis, lymphatic angiogenesis and Wnt/β-catenin signalling activation. d–g qPCR analysis of EMT markers (d, e) and Wnt/β-catenin target genes’ expression (f, g) in indicated cells. h Western blot analysis of EMT markers and Wnt/β-catenin target genes’ protein levels in indicated cells. i ELISA analysis of VEGFC concentration in corresponding cell culture medium of indicated cells. j TOP/FOP-Flash luciferase activity analysis of indicated cells. Results are presented as mean ± SD.
Due to that EMT is a core event for epithelium-derived cancer cell migration and dissemination to metastatic organs, which is markedly influenced by the activity of Wnt/β-catenin signalling. Following, we tested whether EMT markers and the downstream target genes of β-catenin were altered in cells with CCT5 overexpression or silencing. The results showed that SNU16-CCT5 and MKN28-CCT5 cells expressed a significantly higher level of the mesenchymal markers N-cadherin (CDH2), Vimentin (VIM), Slug (SNAI2), ZEB2 and Cyclin D1 (CCND1), as compared with vector-control cells; moreover, the expression level of epithelial marker E-cadherin (CDH1) were drastically suppressed in CCT5 overexpressed cells (Fig. 4d, h). Conversely, the levels of mesenchymal markers were downregulated and the levels of epithelial markers were elevated in CCT5 silenced MGC803 and HGC27 cells compared to vector cells (Fig. 4e, h). Consistently, the expression levels of β-catenin downstream target genes were provoked by CCT5 ectopic overexpression, but restrained by CCT5 silencing, which include MYC, TCF4, LEF1, VEGFC, BMP4 and MMP7 (Fig. 4f–h). Notably, VEGFC, a downstream gene of Wnt/β-catenin signalling to regulate lymphatic angiogenesis, was significantly altered not only at the transcriptional level, but also at the secretory level in corresponding cell culture medium (Fig. 4i).
In addition, the transcriptional activity of β-catenin was measured by Top/Fop-Flash luciferase activity assays in GC cells, which showed that overexpression of CCT5 significantly increased the transcriptional activity of β-catenin in SNU16 and MKN28 cells, whereas silencing CCT5 suppressed the transcriptional activity of β-catenin in MGC803 and HGC27 cells (Fig. 4j). Taken together, these results indicate that CCT5 expression leads to Wnt/β-catenin signalling activation and epithelial-mesenchymal transition of GC cells.
CCT5 interacts with E-cadherin to dissociate E-cadherin/β-catenin adhesion complex
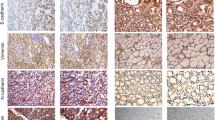
Subsequently, the subcellular location of β-catenin was investigated by the immunoblots performed using whole cell lysates or separated cytosolic and nuclear fractions of cell lysates, which showed that transient overexpressing CCT5 resulted in a decreased level of cytosolic β-catenin and an increased level of nuclear β-catenin in MKN28 cell with relatively constant total β-catenin levels; conversely, transient silencing CCT5 suppressed β-catenin nuclear translocation of MGC803 cell (Fig. 5a). In addition, immunofluorescence staining further revealed that the plasma membrane-localised β-catenin in MKN28-Vector cells was markedly relocated and accumulated to the nucleus in MKN28-CCT5 cell; on the contrary, the nucleus-localised β-catenin in MGC803-Vector cell was translocated to the plasma membrane in CCT5 silenced MGC803 cell, suggesting that CCT5 facilitates nuclear translocation and accumulation of β-catenin without effecting β-catenin protein level (Fig. 5b).
a Western blot analysis of β-catenin protein in the whole cell lysis, nucleus or cytoplasm with indicated treatment. b Immunofluorescence staining shows the subcellular localisation of β-catenin in indicated cells. Before performing assays, all cell lines were pretreated with recombinant Wnt3a (20 ng/ml) over night. Scale bar: 200 μm. c The effect of overexpressing Flag-tagged CCT5 in a dose-dependent manner abrogating the interaction between β-catenin and HA-tagged E-cadherin as evaluated by immunoprecipitation of HA in MKN28 cell. d The effect of silencing CCT5 on enhancing the interaction between β-catenin and HA-tagged E-cadherin as evaluated by immunoprecipitation of HA in MGC803 cell. e Endogenous and exogenous IP assays showed that CCT5 interacted with E-cadherin. f Schematic diagram of the E-cadherin protein domains and the truncated E-cadherin proteins. g Interaction between CCT5 and different truncated constructions of HA-tagged E-cadherin was evaluated by co-IP assays in 293FT cells.
Interestingly, we noticed that addition of CCT5 expression decreased the binding of β-catenin to E-cadherin in a dose-dependent manner of MKN28 cells (Fig. 5c). Meanwhile, the interaction between β-catenin and E-cadherin was enhanced in CCT5 silenced cells, as compared to control vector MGC803 cells (Fig. 5d). In light of that the E-cadherin/β-catenin adhesion complex restrained β-catenin nuclear translocation and involved in sustaining the epithelial phenotype of cancer cells [23], we further investigated the mechanism of CCT5 impeding the interaction between E-cadherin and β-catenin. The endogenous and exogenous immunoprecipitation (IP) assays revealed that CCT5 was identified as a binding partner of E-cadherin (Fig. 5e). Moreover, IP assays with truncated mature E-cadherin proteins showed that the cytoplasmic domain of E-cadherin specifically interacted with CCT5 (Fig. 5f, g), which was also previously reported as a binding domain of E-cadherin interacted with β-catenin. In summary, these data suggest that overexpressed CCT5 binds to the cytoplasmic domain of E-cadherin, resulting in disruption the E-cadherin/β-catenin adhesion complex, thereby facilitating β-catenin nuclear translocation and target genes’ transcriptional activation.
Activation of Wnt/β-catenin signalling is crucial for CCT5-induced GC progression and LN metastasis
To determine the functional significance of Wnt/β-catenin signalling activation in GC malignancies promoted by CCT5, we further constructed cells stably expressing the dominant-negative TCF4 (dnTCF4) in CCT5 overexpressed MKN28 cell. As shown in Fig. 6a–d, the promotion effects of CCT5 on GC cell proliferation, anti-anoikis, invasion and HLEC tube formation were dramatically abrogated by dnTCF4 expression (Fig. 6a–d). Moreover, the tumour volume and MLD of tumours formed by dnTCF4 and CCT5 co-expressing MKN28 cell were significantly lower than that of tumours formed by CCT5 overexpressing MKN28 cell (Fig. 6e, f). Consistently, ectopic overexpressed dnTCF4 markedly reduced the LN volumes and the ratio of popliteal LN metastasis occurrence compared to control cells (Fig. 6e, g). In addition, the expression levels of mesenchymal cell markers and β-catenin downstream target genes were markedly restrained by ectopic expressed dnTCF4 in MKN28-CCT5 cell, meanwhile the epithelial cell marker CDH1 level was restored by dnTCF4 as compared to control vector cell (Supplementary Fig. 2a, b).
a Representative images and quantification of cell colonies formed by MKN28-CCT5 cell with ectopic dnTCF4 expression and control vector cell. b The sub-G1 DNA contents of detached cells for indicated cells are shown in the anoikis assay. c Representative images and quantification of invading cells in three random fields of Matrigel-coated transwell assays performed with indicated cells. d Representative images and quantification of tube lengths in five random fields formed by human lymphatic endothelial cells with indicated cell culture supernatant treatment. e Represent bioluminescent and gross anatomy images of nude mice lower limbs with footpad inoculation indicated cells, and quantitation of bioluminescent intensities and the volumes of footpad xenografts tumours and popliteal lymph nodes in each group (n = 5 per group). f Representative images of immunohistochemical staining for LYVE-1 in footpad xenografts formed by indicated cells, and the staining index of LYVE-1 in each group. g H&E staining of popliteal lymph nodes dissected from nude mice with footpad inoculation indicated cells, and the ratio of metastatic lymph nodes in each group. h Representative images of immunohistochemical staining for CCT5, β-catenin and LYVE-1 in GC tissues. i The number of nuclear β-catenin positive and negative GC tissues in two subgroups divided by CCT5 expression in our collected cohort (n = 33). j The staining index of LYVE-1 in two subgroups divided by CCT5 expression in our collected GC cohort (n = 33). Scale bar: 200 μm (c, f, h), 400 μm (d) and 800 μm (g). Results are presented as mean ± SD.
To elucidate the clinical relevance between CCT5 level and Wnt/β-catenin signalling activation in GCs, IHC analyses of CCT5, β-catenin and LYVE-1 were further performed in 33 cases of GC tissue specimens. The results showed positive correlations between CCT5 expression and β-catenin nuclear localisation in our collected cohort. Specifically, 9/16 cases of GC tissues expressing high CCT5 levels showed β-catenin nuclear localisation; in parallel, only 3/17 cases of GC tissues expressing low CCT5 levels showed β-catenin nuclear localisation (Fig. 6h, i). Consistently, the MLD in GC tissues expressing high levels of CCT5 were markedly higher than that in GC tissues expressing low levels of CCT5 (Fig. 6h, j). Taken together, our data clearly support the notion that CCT5 overexpression promotes GC malignant progression via enhancing Wnt/β-catenin signalling activation.
Discussion
Aberrant activation of Wnt/β-catenin signalling drives tumour initiation and progression in various human cancer types [9]. The activation or inactivation status of Wnt/β-catenin signalling is dependent on and balanced by the cytosolic β-catenin levels. In the absence of Wnt signals, the β-catenin destruction complex (APC, Axin, GSK3β and CK1α) facilitates the serine phosphorylation of cytosolic β-catenin N-terminal, which leads to β-TrCP mediated β-catenin ubiquitination and proteasomal degradation. When Wnt ligands activate Frizzled receptors, the β-catenin destruction complex is ceased through interacting with phosphorylated LRP5/6 and DVL, therefore leading to cytosolic β-catenin accumulation and nuclear translocation [24]. Notably, as compared to the high frequency (~80%) of oncogenic mutation on APC or CTNNB1 gene in colorectal cancer, which leads to constitutive Wnt/β-catenin signal activation, the frequency of these mutations is relative lower in GC (<20%) [25]. Thus, exploring the molecular basis for the dysregulated Wnt/β-catenin signalling activity in GCs is of great value for our understanding the fundamental mechanism of GC initiation and progression, and developing new diagnostic and therapeutic strategies future. In the current study, we proved that CCT5, a subunit of chaperonin containing TCP1 complex, involves enhancing Wnt/β-catenin signalling activity in GCs through abrogating the interaction between E-cadherin and β-catenin, which unveils an important role of CCT5 in enhancing Wnt/β-catenin activation.
The E-cadherin/β-catenin adhesion complex regulates cell anchoring junctions, which functions in organising and tethering microfilaments to maintain cell adhesion and polarity. Uncoupling of E-cadherin/β-catenin interaction enhances cytosolic β-catenin accumulation and nuclear translocation, more than that, the complex also functionally involves epithelial-mesenchymal transition (EMT), which plays a pivotal role in tumour metastasis through markedly transforming both the internal signals and external morphology of cancer cells [12]. Decreasing of E-cadherin expression or loss-of-function mutations in CDH1 gene induces adhesion complex destruction, whereas the expression level of E-cadherin is dramatically upregulated in GCs as compared to normal tissues, and the mutation frequency of CDH1 is less than 10% in GC, suggesting that the E-cadherin/β-catenin interaction may be regulated by unknown molecules [25]. For example, PDLIM1 and Smad7 have been reported to stabilise the E-cadherin/β-catenin complex and inhibit metastasis by enhancing cell-cell adhesion in colorectal cancer and breast cancer cells [26, 27]. Differently, our data demonstrate that CCT5 specifically interacted with the cytoplasmic domain of E-cadherin that also interacts with β-catenin; and upregulated CCT5 in GCs competitively abrogates the interaction between E-cadherin and β-catenin, leading to β-catenin nuclear translocation and Wnt/β-catenin signal activation. Therefore, our study reveals an important role of CCT5 in modulating E-cadherin/β-catenin adhesion complex integrity and the related downstream signalling pathway cascades. Moreover, to identify the amino acids residues being responsible for CCT5-E-cadherin interaction is the key to understanding the molecular basis of the biological function of CCT5, as well as the potential targeting strategy for future therapeutic purpose. Because of the full length of CCT5 protein is identified as a conserved “TCP-1 chaperonin family, epsilon subunit” domain, further systemic and sophisticated protein structural and molecular biology studies based on traditional protein docking programs or novel artificial intelligent programs, like AlphaFold, are required for further unveiling the key amino acids and provide insight into the interaction between CCT5 and E-cadherin.
The pro-metastatic role of Wnt/β-catenin signalling has been strengthened by accumulated studies, which is determined by the unique transcriptome of β-catenin/TCF nuclear complex. Of note, Wnt/β-catenin signalling plays a critical role in lymphatic metastasis of multiple types of cancers, including lung cancer, breast cancer, laryngeal cancer, pancreatic adenocarcinoma, nasopharyngeal carcinoma and gastric cancer [28,29,30,31,32]. And lymphatic metastasis presents as an important indicator of tumour progression, especially for GCs, which serves as a prognostic marker and determines the treatment strategy for GC patients. However, the therapeutic effect of current practice remains suboptimal and options for targeted therapy towards metastatic GC is limited. Our study unveils a pro-LN metastasis role of CCT5 in GCs through modulating Wnt/β-catenin signalling activation. Despite the most well-established Wnt target genes, such as MYC, CCND1, MMP7 and LEF1, our study demonstrates that VEGFC, a pivotal cytokine in lymphangiogenesis, is significantly unregulated in both mRNA and protein secretory level by CCT5 overexpression in the GCs, further supporting the notion of that the outcome of Wnt/β-catenin activation is highly context-dependent [33]. In addition, we prove that both the VEGFC and other Wnt target genes’ expression are regulated by CCT5 in a Wnt/β-catenin pathway-dependent manner by reverse assays with ectopic dnTCF4 overexpression, demonstrating a tightly regulatory axis of CCT5/E-cadherin/β-catenin.
LN metastasis presents as an important clinical symptom of GC progression, which has been considered as an independent prognostic factor and a surgical operation indicator of GCs [22]. Evaluation of LN metastasis status preoperatively guides appropriate treatment strategy. In most cases, surgery is the primary intervention for GCs, which mainly contains the surgical procedure of gastrectomy and LN dissection. However, no obvious benefits on patients’ prognosis or quality of life could be obtain from surgery for GC patients with systemic symptoms, like LN metastasis, whose disease will rapidly progress to tumour relapse, multiple organs’ metastasis and drug resistance in a short period of time [34, 35]. Despite the importance of LN metastasis in GC development, the understanding of the molecular mechanisms and targeted therapy strategy for LN metastasis remains suboptimal, suggesting that LN metastasis of GC still needs to be further investigated.
Our research illustrates that CCT5 is correlated with poor prognosis and LN metastasis in clinical GC patients; moreover in vitro and in vivo assays prove that CCT5 markedly promotes GC progression and lymphatic metastasis through CCT5/E-cadherin/β-catenin axis. Therefore, the current findings provide a foundation for targeting CCT5 and E-cadherin/β-catenin as potential biomarkers and targets for the individualised management of GC patients.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Data and materials are available upon reasonable request if applicable.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32.
Chen W, Sun K, Zheng R, Zeng H, Zhang S, Xia C, et al. Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30:1–12.
Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8.
Wadhwa R, Song S, Lee JS, Yao Y, Wei Q, Ajani JA. Gastric cancer-molecular and clinical dimensions. Nat Rev Clin Oncol. 2013;10:643–55.
Pastushenko I, Brisebarre A, Sifrim A, Fioramonti M, Revenco T, Boumahdi S, et al. Identification of the tumour transition states occurring during EMT. Nature. 2018;556:463–8.
Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–205.
Noordhuis MG, Fehrmann RS, Wisman GB, Nijhuis ER, van Zanden JJ, Moerland PD, et al. Involvement of the TGF-beta and beta-catenin pathways in pelvic lymph node metastasis in early-stage cervical cancer. Clin Cancer Res. 2011;17:1317–30.
Zhang Y, Wang X. Targeting the Wnt/beta-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13:165.
Tian X, Liu Z, Niu B, Zhang J, Tan TK, Lee SR, et al. E-cadherin/beta-catenin complex and the epithelial barrier. J Biomed Biotechnol. 2011;2011:567305.
Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–7.
Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–56.
Liu Y, Sethi NS, Hinoue T, Schneider BG, Cherniack AD, Sanchez-Vega F, et al. Comparative Molecular Analysis of Gastrointestinal Adenocarcinomas. Cancer Cell. 2018;33:721–35 e728.
Engqvist H, Parris TZ, Kovacs A, Ronnerman EW, Sundfeldt K, Karlsson P et al. Validation of novel prognostic biomarkers for early-stage clear-cell, endometrioid and mucinous ovarian carcinomas using immunohistochemistry. Front Oncol. 2020;10:162.
Gao HJ, Zheng M, Sun SJ, Wang HW, Yue ZG, Zhu Y, et al. Chaperonin containing TCP1 subunit 5 is a tumor associated antigen of non- small cell lung cancer. Oncotarget. 2017;8:64170–9.
Hallal S, Russell BP, Wei H, Lee MYT, Toon CW, Sy J et al. Extracellular vesicles from neurosurgical aspirates identifies chaperonin containing TCP1 subunit 6A as a potential glioblastoma biomarker with prognostic significance. Proteomics 2019;19:e1800157.
He JC, McLaughlin RP, van der Beek L, Canisius S, Wessels L, Smid M, et al. Integrative analysis of genomic amplification-dependent expression and loss-of-function screen identifies ASAP1 as a driver gene in triple-negative breast cancer progression. Oncogene. 2020;39:4118–31.
Li WL, Liu J, Zhao HT. Prognostic power of a chaperonin containing TCP-1 subunit genes panel for hepatocellular carcinoma. Front. Genet. 2021;12:668871.
Nibbe RK, Markowitz S, Myeroff L, Ewing R, Chance MR. Discovery and scoring of protein interaction subnetworks discriminative of late stage human colon cancer. Mol Cell Proteom. 2009;8:827–45.
Akagi T, Shiraishi N, Kitano S. Lymph node metastasis of gastric cancer. Cancers (Basel). 2011;3:2141–59.
Huber AH, Weis WI. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell. 2001;105:391–402.
MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26.
Nanki K, Toshimitsu K, Takano A, Fujii M, Shimokawa M, Ohta Y, et al. Divergent routes toward Wnt and R-spondin Niche independency during human gastric carcinogenesis. Cell. 2018;174:856–69.e817.
Chen HN, Yuan KF, Xie N, Wang K, Huang Z, Chen Y, et al. PDLIM1 Stabilizes the E-cadherin/beta-catenin complex to prevent epithelial-mesenchymal transition and metastatic potential of colorectal cancer cells. Cancer Res. 2016;76:1122–34.
Tang Y, Liu ZY, Zhao L, Clemens TL, Cao X. Smad7 stabilizes beta-catenin binding to E-cadherin complex and promotes cell-cell adhesion. J Biol Chem. 2008;283:23956–63.
Liang TS, Zheng YJ, Wang J, Zhao JY, Yang DK, Liu ZS. MicroRNA-506 inhibits tumor growth and metastasis in nasopharyngeal carcinoma through the inactivation of the Wnt/beta-catenin signaling pathway by down-regulating LHX2. J Exp Clin Cancer Res. 2019;38:97.
Maftouh M, Belo AI, Avan A, Funel N, Peters GJ, Giovannetti E, et al. Galectin-4 expression is associated with reduced lymph node metastasis and modulation of Wnt/beta-catenin signalling in pancreatic adenocarcinoma. Oncotarget. 2014;5:5335–49.
Wang N, Yan H, Wu D, Zhao Z, Chen X, Long Q, et al. PRMT5/Wnt4 axis promotes lymph-node metastasis and proliferation of laryngeal carcinoma. Cell Death Dis. 2020;11:864.
Xu X, Zhang M, Xu F, Jiang S. Wnt signaling in breast cancer: biological mechanisms, challenges and opportunities. Mol Cancer. 2020;19:165.
Yu J, Tao S, Hu P, Wang R, Fang C, Xu Y, et al. CCR7 promote lymph node metastasis via regulating VEGF-C/D-R3 pathway in lung adenocarcinoma. J Cancer. 2017;8:2060–8.
Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001;61:6050–4.
Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N. Engl J Med. 2008;359:453–62.
Yasuda K, Adachi Y, Shiraishi N, Inomata M, Takeuchi H, Kitano S. Prognostic effect of lymph node micrometastasis in patients with histologically node-negative gastric cancer. Ann Surg Oncol. 2002;9:771–4.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82173225, 82172927, 81802274); the Science and Technology Program of Guangzhou City (202102020022); the Natural Science Foundation of Guangdong Province (2019A1515011174); the Fundamental Research Funds for the Central Universities (19ykpy162).
Author information
Authors and Affiliations
Contributions
Project planning was done by HT, YL and CL. YL, CL, XH and SL performed the majority of experiments and analysed data. XZ provided the subjects, technical assistance and expertise in the clinical samples’ analysis. YL and HT wrote the manuscript. HT and FX edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The clinical tissue specimens used in this study were obtained from and histopathologically diagnosed at Jiangmen Central Hospital. For the use of these clinical materials for research purposes, prior patients’ consents and approval from the Institutional Research Ethics Committee of Jiangmen Central Hospital were obtained. The study is compliant with all relevant ethical regulations involving human participants.
Consent to publish
All contributing authors agree to the publication of this article. All subjects have written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, Y., Liu, C., Zhang, X. et al. CCT5 induces epithelial-mesenchymal transition to promote gastric cancer lymph node metastasis by activating the Wnt/β-catenin signalling pathway. Br J Cancer 126, 1684–1694 (2022). https://doi.org/10.1038/s41416-022-01747-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01747-0
- Springer Nature Limited
This article is cited by
-
The clustering status of detached gastric cancer cells inhibits anoikis-induced ferroptosis to promote metastatic colonization
Cancer Cell International (2024)
-
Cytosolic Cadherin 4 promotes angiogenesis and metastasis in papillary thyroid cancer by suppressing the ubiquitination/degradation of β-catenin
Journal of Translational Medicine (2024)
-
Glioblastoma biomarkers in urinary extracellular vesicles reveal the potential for a ‘liquid gold’ biopsy
British Journal of Cancer (2024)
-
Rutin suppresses the malignant biological behavior of gastric cancer cells through the Wnt/β-catenin pathway
Discover Oncology (2024)
-
The truncated AXIN1 isoform promotes hepatocellular carcinoma metastasis through SRSF9-mediated exon 9 skipping
Molecular and Cellular Biochemistry (2024)