Abstract
Carcinoma cells hijack the epithelial–mesenchymal transition (EMT) for tumor dissemination. Paired-related homeobox 1 (PRRX1) has been identified as a new EMT inducer. However, the function of PRRX1 in gastric cancer has not been elucidated. In this study, we observed that PRRX1 expression levels were upregulated and positively correlated with metastasis and EMT markers in human gastric cancer specimens. PRRX1 overexpression had distinct effects on the cell morphology, proliferation, migration and invasion of BGC823 and SGC7901 gastric cancer cells both in vitro and in xenografts. PRRX1 overexpression resulted in the regulation of the EMT molecular markers N-cadherin, E-cadherin and vimentin as well as the levels of intranuclear β-catenin and the Wnt/β-catenin target c-Myc. Furthermore, the inhibition of the Wnt/β-catenin pathway by XAV939 offset the effects of PRRX1 overexpression. These findings demonstrate that PRRX1 promotes EMT in gastric cancer cells through the activation of Wnt/β-catenin signaling and that PRRX1 upregulation is closely correlated with gastric cancer metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasion and metastasis are the hallmarks of malignant tumor progression [1]. The epithelial–mesenchymal transition (EMT), which was first recognized as a feature of embryogenesis, has also been shown to occur in wound healing, organ fibrosis and the initiation of metastasis [2]. There is increasing evidence that metastasis is initiated by EMT at the invasive front of primary carcinomas [3], and EMT is recognized as an important step in invasion and metastasis [4]. The invasive ability of cancer cells is acquired via transformation to the mesenchymal phenotype [5], and the expression of the EMT markers N-cadherin, E-cadherin and vimentin changes during this transition [6]. EMT is induced by genetic and epigenetic changes within the transforming cells as well as by signals from the tumor microenvironment [7]. EMT inducers, such as TWIST1 [8], SNAI1 [9], ZEB1 and ZEB2 [10, 11], are reported to be associated with EMT in cancer. Furthermore, EMT involves several related signaling pathways, such as TGF-β [12], NF-κB [13], Notch [14] and Wnt/β-catenin [15]. Wnt/β-catenin signaling has a major impact on EMT during cancer progression [16]. Paired-related homeobox 1 (PRRX1) was recently, reported as a new EMT inducer [17]. EMT is induced by overexpressing PRRX1 in breast cancer and colorectal cancer; however, the function of PRRX1 in these cell types is distinct. High PRRX1 expression levels were significantly associated with reduced metastasis and good prognosis in breast cancer [17]. But the opposite relationship was observed in colorectal cancer [18]. It is unknown whether PRRX1 induces EMT, through which pathway PRRX1 acts and what role PRRX1 may play in gastric cancer.
Materials and methods
Patient selection and tissue preparation
Between January 2005 and December 2008, 73 male and 52 female patients with gastric adenocarcinoma were selected in the Department of General Surgery of the First Affiliated Hospital of Chongqing Medical University (Chongqing, China) (see Table 2 for patients’ clinicopathological features). Radical resection was performed on these patients. Cancer tissues were excised, fixed in 10 % neutral-buffered formalin and embedded in paraffin blocks. The patients received neither chemotherapy nor radiotherapy before surgery. Informed consent for tissue specimens derived from patients, and the study was approved by the Research Ethics Committee of Chongqing Medical University.
Immunohistochemistry
Immunohistochemical staining was performed with the immunohistochemical SP kit to detect the expression of PRRX1, E-cadherin, N-cadherin, vimentin and β-catenin. Slides were deparaffinized, and antigen was retrieved by heating in a microwave oven for 15 min at 90 °C in citrate buffer, incubating in 3 % hydrogen peroxide for 20 min and blocking with normal goat serum for 30 min at room temperature. Slides were incubated with primary antibody overnight at 4 °C and then incubated with secondary antibodies at 37 °C for 30 min. Next, the slides were incubated with streptavidin–HRP for 30 min at 37 °C, rinsed with PBS, incubated for 15 min with the chromogen 3,3′-diaminobenzidine and then counterstained with hematoxylin. The staining results of targeted proteins were observed under the microscope. Negative controls were prepared by substituting primary antibody with non-immune rabbit serum. Two independent pathologists evaluated and scored the sections in 10 random visual fields for each section (double-blinded). Staining was graded semiquantitatively as described previously; the expression intensity scores (0 points for 0–5 %; 1 point for 6–25 %; 2 points for 26–50 %; 3 points for > 50 %) and positive staining cell scores (1 point = weak intensity; 2 points = moderate intensity; 3 points = strong intensity) were summed. The sum scores >3 were believed to represent significant overexpression and considered positive to simplify data analysis.
Cell culture and antibodies
The human gastric adenocarcinoma cell lines BGC823 and SGC7901 were obtained from the Key Laboratory of General Surgery. The cells were cultured in RPMI medium 1640 (RPMI-1640, HyClone, China) supplemented with 10 % fetal bovine serum (FBS, HyClone, China) in a humidified 5 % CO2 atmosphere at 37 °C.
The antibodies used in this study were as follows: PRRX1 antibodies (OriGene, Rockville, USA); E-cadherin, N-cadherin, vimentin, β-catenin and c-Myc antibodies (Abcam, USA); β-actin (Beyotime, Jiangsu, China); anti-LaminB1 (Sigma-Aldrich, USA); Alexa Fluor 549-conjugated goat anti-rabbit IgG (H + L); HRP-conjugated goat anti-mouse IgG; and HRP-conjugated goat anti-rabbit IgG (ZSGB-BIO, Beijing, China).
Lentiviral overexpression of PRRX1 in BGC823 and SGC7901 cells
The cDNA for human PRRX1 was cloned into pIRES2 by PCR (Invitrogen, Shanghai, China) via homologous recombination between pIRES2 and pLV–UbC–IRES2 by GeneChem Biomedical Co. Ltd (Shanghai, China). We obtained lentiviral vectors plasmids for overexpressing PRRX1 and then packaged into lentivirus particles according to manufacturer’s protocol (Invitrogen, Shanghai, China). A blank vector lentivirus gene delivery system was used as a negative control. A total of 5 × 105 BGC823 or SGC7901 cells were seeded into each well of a 6-well plate. When the cells reached 80–90 % confluence, PRRX1 or a blank vector lentivirus gene delivery system was transfected into the cells, which are hereafter referred to as the PRRX1 or MOCK groups. The PRRX1 group and Mock group were treated with 10 μM XAV939 (a Wnt/β-catenin signaling inhibitor) for 24 h to address the influence of Wnt/β-catenin signaling on the function of PRRX1.
Cell proliferation assay
After the indicated treatments, cells were incubated in 96-well plates at a density of 2 × 103 cells per well for 24 h. Cell proliferation was examined after 1, 2, 3, 4, 5, 6 and 7 days. Approximately 20 μl of MTT dye (5 mg/ml; Sigma-Aldrich) was added and incubated for another 4 h at 37 °C. Subsequently, 150 μl of dimethylsulfoxide was added to each well and mixed for 30 min. Spectrophotometric absorbance at 490 nm was determined with a microplate reader (Bio-Rad, Hercules, CA, USA). Each sample had three replicates.
Cell invasion and migration assays
Transwell invasion and migration assays were performed in 24-well 8-μm pore size transwell plates according to the manufacturer’s instructions (Corning, New York, NY, USA). The bottom of the transwell chamber was coated with BD Matrigel Basement Membrane Matrix (BD Biosciences, San Diego, CA, USA) in invasion assays, but no coating was applied for migration assays. The upper chamber was filled with 1 × 105 cells in RPMI 1640 containing 10 % fetal bovine serum. The lower chamber was filled with RPMI 1640 containing 15 % fetal bovine serum as a chemoattractant. After the chambers were incubated for 18 or 25 h at 37 °C, non-migrating or invading cells on the upper side of the chamber were removed from the surface of the membrane by scrubbing, and migrating or invading cells on the lower surface of the membrane were fixed with methanol, mounted, dried and stained with hematoxylin–eosin (HE). The number of cells invading through the Matrigel or migrating through the pores was counted by a technician blinded to the experimental settings in 10 randomly selected microscopic fields for each filter. The test was repeated three times.
Soft agar assays
A quantity of 5 × 103 cells was suspended in 0.35 % agarose (RPMI medium 1640 mixed with 0.7 % agarose) and poured onto a 0.6 % agarose (RPMI medium 1640 mixed with 1.2 % agarose) bed. Colonies were counted after 3 weeks. Colonies >100 μm diameter were counted, and the number of cells colony formation was counted by a technician blinded to the experimental settings in 10 randomly selected microscopic fields. The test was repeated three times.
Immunofluorescent analysis
Cells were grown in dishes and fixed for 30 min in 4 % paraformaldehyde. The cells were permeabilized with 0.04 % Triton X-100 for 10 min and blocked with 5 % bovine serum albumin for 40 min. The primary antibody, diluted in phosphate-buffered solution, was applied overnight at 4 °C. Following phosphate-buffered solution washing steps, the appropriate Alexa Fluor 549-linked secondary antibody was applied for 30 min at room temperature. Cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI), and images were acquired with a fluorescence microscope.
Animal studies
Five-week-old female BALB/c nude mice, purchased from the National Biological Industry Base, Laboratory Animal Center of Chongqing Medical University (Chongqing, China), were used to examine tumorigenicity. To evaluate the role of PRRX1 in tumor formation, four groups of gastric cancer cells, SGC7901 (MOCK and PRRX1) and BGC823 (MOCK and PRRX1), were propagated and inoculated subcutaneously into the flanks of nude mice (3 × 106 cells in 0.1 ml volume). Tumor volumes were determined according to the following formula: v(mm3) = length × width2/2. Tumor diameters were measured every week using vernier calipers. After 6 weeks, the mice were killed, and tumors were weighed. All experimental procedures and protocols were approved by the Animal Ethics Committee of Chongqing Medical University. All the procedures involving animals were conducted as indicated in the guidelines of the National Institutes of Health (NIH) for animal care (Guide for the Care and Use of Laboratory Animals, Department of Health and Human Services, NIH Publication No. 86-23, revised 1985).
Western blot analysis
Whole-cell extracts were prepared in lysis buffer containing 150 mM sodium chloride, 0.1 M Tris, 1 % Tween-20, 50 mM diethyldithiocarbamic acid, 1 mM ethylenediamine tetraacetic acid and protease inhibitors at pH 8.0. The lysates were centrifuged at 12,000 rpm for 15 min at 4 °C, and the supernatants were collected. To detect the cellular localization of β-catenin, nuclear and cytoplasmic fractions were isolated using the Nuclear and Cytoplasmic Protein Extraction kit (Beyotime, China). Fifty micrograms of proteins was loaded onto each well. The transferred membranes were subsequently incubated with primary antibodies overnight at 4 °C and then with secondary antibodies for 1 h. Bands were visualized and quantified using the ECL chemiluminescence detection system (ChemiDoc™ XRS imager, Bio-Rad, USA).
Real-time quantitative PCR analysis
Total RNA from cells was isolated using TRIzol as recommended by the manufacturer (TaKaRa, Dalian, China). The concentration and purity of the total RNA were assessed using a UV spectrophotometer (UltroSPec2100 Pro, Amersham, USA). Total RNA was reverse-transcribed using the PrimeScript RT Reagent kit (TaKaRa, Dalian, China). Quantitative PCR (RT-qPCR) was performed with the CFX96™ Real-Time System (Bio-Rad, USA) using SYBR Premix Ex Taq™ II (TaKaRa, Dalian, China). The relative levels of target gene mRNA are expressed as the ratio of target to tubulin and calculated from the standard curves using the \(2^{{ - \varDelta \varDelta C_{\text{t}} }}\) method as directed. The experiment was performed in triplicate. The primers were as follows: PRRX1 (forward 5′-GCA GGC TTT GGA GCG TGT CT-3′ and reverse 5′-TCC TGC GGA ACT TGG CTC TT-3′); c-Myc (forward 5′-TTC GGG TAG TGG AAA ACC AG-3′ and reverse 5′-CAG CAG CTC GAA TTT CTT CC-3′); N-cadherin (forward 5′-CAT CCT GCT TAT CCT TGT GCT G-3′ and reverse 5′-CTG GTC TTC TTC TCC TCC ACC TT-3′); E-cadherin (forward 5′-TCG TCA CCA CAA ATC CAG TG-3′ and reverse 5′-CAT TCA CAT CAA GCA CAT CC-3′); vimentin (forward 5′-TGA ATA CCA AGA CCT GCT CAA-3′ and reverse 5′-ATC AAC CAG AGG GAG TGA ATC-3′); and β-catenin (forward 5′-TGC AGT TCG CCT TCA CTA TG-3′ and reverse 5′-ACT AGT CGT GGA ATG GCA CC-3′).
Statistical analysis
The protein expression levels and clinicopathological parameters were compared by the λ 2-test. Continuous variables were evaluated by unpaired Student’s t test. Bivariate correlations between study variables were calculated by Spearman’s rank correlation coefficients. Statistical analyses were performed with the SPSS 11.0 software (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered to be statistically significant.
Results
PRRX1 expression regulated EMT-related proteins and associated with tumor metastasis in gastric cancer
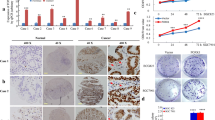
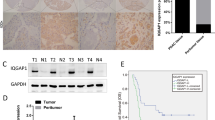
The PRRX1 expression levels in cancer tissue and adjacent normal gastric mucosa of gastric cancer patients were determined by immunohistochemical analysis. PRRX1 expression was significantly higher in gastric cancer tissues relative to adjacent normal gastric mucosa (Fig. 1). The expression levels in 125 gastric cancer specimens of the EMT-related proteins N-cadherin, E-cadherin, vimentin and β-catenin among the levels of the EMT-related proteins, β-catenin and PRRX1 were evaluated. As presented in Table 1, the expression levels of PRRX1, vimentin, N-cadherin and β-catenin were significantly higher in gastric cancer tissues than in adjacent normal gastric mucosa (P < 0.05); E-cadherin, in contrast, exhibited reduced expression levels in gastric cancer tissues with respect to adjacent normal mucosa (P < 0.05). The correlations between the clinicopathological features of gastric cancer patients and the expression levels of PRRX1, N-cadherin, E-cadherin, vimentin and β-catenin are summarized in Table 2. There were close correlations between the levels of PRRX1, N-cadherin, E-cadherin, vimentin or β-catenin and the depth of tumor invasion. In particular, the nuclear localization of β-catenin was increased in gastric cancer tissues and was also related to the depth of tumor invasion (Table 2). E-cadherin, vimentin and N-cadherin exhibited significant correlations with patients’ tumor metastasis status (P < 0.05). Table 3 demonstrates that the expression levels of PRRX1 were positively correlated in gastric cancer tissues with those of vimentin, N-cadherin and β-catenin but were inversely correlated with E-cadherin.
PRRX1 modulates invasion, migration and cell proliferation in gastric cancer cells
To demonstrate the effect of PRRX1 on the invasion and migration of gastric cancer cells, an in vitro cell invasion assay was performed based on the principle of the Boyden chamber assay. Cells that have invaded through the Matrigel are shown in Fig. 2b. When PRRX1 was overexpressed, the number of cancer cells migrating through the Matrigel increased significantly compared with the Mock group (P < 0.05). To provide further support for the positive effect of PRRX1 expression on cell migration, the migration assay was performed. As shown in Fig. 2a, in the PRRX1-overexpressing gastric cancer cells, the number of cancer cells migrating through the Boyden chamber pores increased significantly compared with the Mock group (P < 0.05). These results imply that PRRX1 promotes gastric cancer cell invasion and migration.
PRRX1 promotes migration, invasion, anchorage-independent growth and proliferation in gastric cancer cells. a Cellular migration was analyzed using a migration assay. The migration of various groups of BGC823 and SGC7901 cells was visualized by hematoxylin and eosin (HE) staining. b The invasive properties of the cells were analyzed using a Matrigel-coated plate. Cells were stained with HE. c The soft agar colony-formation assay. Representative images depicting colonies formed by various groups of migrating BGC823 and SGC7901 cells. d PRRX1 overexpression affected BGC823 and SGC7901 cells proliferation, based on the MTT assay (*P < 0.05 vs. MOCK groups)
The proliferation of BGC823 and SGC7901 cells was increased upon PRRX1 overexpression (Fig. 2d). The proliferation curves indicate that PRRX1 might enhance the proliferation rate of gastric cancer cells.
PRRX1 promotes EMT in gastric cancer cells
Ectopic PRRX1-expressing gastric cancer cells (BGC823 and SGC7901) were used to examine whether PRRX1 induced EMT in gastric cancer cells. After PRRX1 expression was induced using a lentivirus, BGC823 and SGC7901 cells exhibited a more spindle-like fusiform shape and a less sheet-like architecture than mock-transfected cells. The expression of EMT markers was quantified by RT-qPCR and Western blot 48 h after transfection. Significantly increased mRNA and protein levels of vimentin, N-cadherin and β-catenin were observed in PRRX1-overexpressing cells increased significantly. In contrast, the expression of E-cadherin in PRRX1-overexpressing cells decreased significantly (Fig. 3a, b). These changes were also observed by immunofluorescence staining (Fig. 4). These results suggest that PRRX1 is a driving force of EMT in gastric cancer cells.
PRRX1 regulates EMT markers and the Wnt/β-catenin pathway. a The mRNA expression levels of the EMT markers (E-cadherin, N-cadherin and vimentin), β-catenin and PRRX1 in various groups of BGC823 and SGC7901 cells were measured by quantitative real-time PCR. b The protein levels of the EMT markers, β-catenin, c-Myc and PRRX1 and the subcellular localization of β-catenin in various groups of cells were assayed by Western blotting
PRRX1 regulates the Wnt/β-catenin pathway in gastric cancer cells
We monitored the expression and localization of β-catenin and the expression of its downstream target c-Myc to determine whether PRRX1 regulated the Wnt/β-catenin pathway in BGC823 and SGC7901 gastric cancer cells. As shown in Fig. 3, the overexpression of PRRX1 significantly increased the mRNA and total protein levels of β-catenin and c-Myc as well as the intranuclear levels of β-catenin. The increased nuclear staining of β-catenin upon PRRX1 overexpression was also observed by immunofluorescence staining (Fig. 4). Moreover, we observed that the inhibition of Wnt/β-catenin signaling by XAV939 neutralized the EMT, migration and invasion promoted by PRRX1 overexpression (Fig. 5a–c). These results suggest that PRRX1 might be involved in activating the Wnt/β-catenin pathway in gastric cancer cells.
PRRX1 promotes EMT through the Wnt/β-catenin pathway. a The protein levels of the EMT markers, β-catenin, c-Myc and PRRX1 and the subcellular localization of β-catenin in different groups of BGC823 PRRX1 and SGC7901 PRRX1 cells (treated with 10 μM XAV939 or DMSO for 24 h) were assayed by Western blotting. b The migration of the cells (treated with 10 μM XAV939 or DMSO) was analyzed using a migration assay. c The invasive properties of the cells (treated with 10 μM XAV939 or DMSO) were analyzed using a Matrigel-coated plate (*P < 0.05 vs. treated with DMSO groups)
PRRX1 promoted anchorage-independent growth in gastric cancer cells
In soft agar colony-formation assays, the overexpression of PRRX1 in SGC7901 and BGC823 cells resulted in larger and more colonies, revealing the increased capacity for anchorage-independent growth compared with MOCK cells (Fig. 2c).
PRRX1 promotes gastric cancer cell proliferation in vivo
We next tested the role of PRRX1 in tumorigenesis using a nude mouse xenograft model. Nude mice xenografted with SGC7901 or BGC823 cells developed solid tumors after 6 weeks. Tumor volumes and weights were greater when PRRX1 was stably overexpressed compared to the control group (Fig. 6).
Discussion
EMT is a process by which epithelial cells lose their cell polarity and cell–cell adhesion [19]. EMT endows cells with migratory and invasive properties, induces stem cell properties, prevents apoptosis and senescence and contributes to immunosuppression [20]. Epithelial cells express high levels of E-cadherin, whereas mesenchymal cells express high levels of N-cadherin, fibronectin and vimentin and lose E-cadherin expression [2]. These genetic changes are associated with tumor progression to metastasis and are accompanied by the activation of signaling networks associated with the migration and survival of mesenchymal cells in an anchorage-independent environment [21].
PRRX1 functions as a transcription coactivator, enhancing the DNA-binding activity of serum response factor [22]. PRRX1 regulates the differentiation of mesenchymal precursors [23] and plays a central role in pancreatic regeneration and carcinogenesis [24]. It has been reported that PRRX1 induces EMT [17, 18, 25]. PRRX1 may promote EMT by regulating the expression of E-cadherin, N-cadherin and vimentin. Our study demonstrates that PRRX1 expression is higher in gastric cancer tissues than in adjacent normal gastric mucosa and is significantly correlated with EMT markers and the metastasis of gastric cancer. PRRX1-expressing gastric cancer cells exhibit a more spindle-like shape and display upregulated N-cadherin and vimentin and downregulated E-cadherin. More importantly, we observed that PRRX1 could promote invasion, migration and cell proliferation in gastric cancer cells in vitro and could promote the proliferation of gastric cancer in vivo. These results suggest that PRRX1 promotes EMT and that EMT could be a mechanism of PRRX1-mediated gastric cancer invasion and metastasis [18, 25].
The mechanism underlying PRRX1 overexpression and EMT in cancer remains unclear [18]. The Wnt/β-catenin signaling pathway regulates EMT in gastrulation, cardiac valve formation and cancer [26, 27]. Activation of the Wnt/β-catenin pathway contributes to carcinogenesis in a subset of gastric cancers, [28] and activation of the Wnt/β-catenin signaling pathway has an important impact on the EMT in gastric cancer [29]. In this study, we found that Wnt/β-catenin signaling and β-catenin expression and localization were correlated with gastric cancer and PRRX1 expression in gastric cancer patients. Upon PRRX1 overexpression, β-catenin and c-Myc were upregulated, and the translocation of β-catenin into the nucleus was increased. When the Wnt/β-catenin pathway is activated, the binding of Wnt molecules to frizzled receptors inhibits the activity of the destruction complex and allows β-catenin to accumulate and translocate to the nucleus; the translocation of β-catenin into the nucleus may result in the loss of E-cadherin [30, 31]. Thus, overexpressed PRRX1 may activate the Wnt/β-catenin pathway. Furthermore, XAV939, a selective inhibitor of the Wnt/β-catenin pathway [29], inhibited Wnt/β-catenin signaling, neutralized the effect of EMT induced by PRRX1 overexpression, and suppressed the invasion and migration of gastric cancer cells. These results reveal that PRRX1 promotes EMT via Wnt/β-catenin signaling in gastric cancer cells and that Wnt/β-catenin is essential to the function of PRRX1. Furthermore, the Wnt/β-catenin pathway is also linked to the maintenance of stem cell properties [32, 33]. We observed a greater capacity for anchorage-independent growth in PRRX1-expressing cells compared to MOCK cells, suggesting that PRRX1-overexpressing cells exhibit a more stem-like phenotype [18, 34]. Our findings suggest that PRRX1 expression likely induces the stemness phenotype via the Wnt/β-catenin pathway in gastric cancer.
In summary, this study reports that PRRX1 regulates EMT by enhancing the Wnt/β-catenin pathway and induces stem cell properties in gastric cancer. PRRX1 stimulates gastric cancer invasion and metastasis, thereby contributing to poor prognosis [35, 36]. Our results concerning the relationship between PRRX1 expression and cancer metastasis differ from those seen in breast cancer [17], but are consistent with results observed in colorectal and pancreatic cancer. Thus, these data suggest that PRRX1 expression is associated with different phenotypes in different cancers. Transforming growth factor-β and microRNAs can regulate the expression of PRRX1 [17, 37]. Recently, it was reported that miR-124 could increase the radiosensitivity of CRC cells by blocking the expression of PRRX1 [38]. The inhibition of Wnt/β-catenin signaling could block EMT downstream of PRRX1. Thus, PRRX1-induced EMT is associated with the capacity of gastric cancer to migrate to distant organs and maintain stemness. Given the importance of PRRX1, EMT and the Wnt/β-catenin signaling pathway in gastric cancer, our findings not only provide an improved understanding of the molecular mechanisms underlying PRRX1 and tumor metastasis but also suggest an additional target for therapeutic intervention [24].
References
Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–64. doi:10.1126/science.1203543.
Kalluri R, Weinberg RA. The basics of epithelial–mesenchymal transition. J Clin Investig. 2009;119(6):1420.
Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial–mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22(6):725–36. doi:10.1016/j.ccr.2012.09.022.
Tsai JH, Yang J. Epithelial–mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27(20):2192–206. doi:10.1101/gad.225334.113.
Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66(23):11271–8.
Busch EL, McGraw KA, Sandler RS. The potential for markers of epithelial–mesenchymal transition to improve colorectal cancer outcomes: a systematic review. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1164–75.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–96.
Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, et al. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19(3):372–86. doi:10.1016/j.ccr.2011.01.036.
Fan XJ, Wan XB, Yang ZL, Fu XH, Huang Y, Chen DK, et al. Snail promotes lymph node metastasis and Twist enhances tumor deposit formation through epithelial–mesenchymal transition in colorectal cancer. Hum Pathol. 2013;44(2):173–80. doi:10.1016/j.humpath.2012.03.029.
Sanchez-Tillo E, Lazaro A, Torrent R, Cuatrecasas M, Vaquero E, Castells A, et al. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene. 2010;29(24):3490–500.
Wiles ET, Bell R, Thomas D, Beckerle M, Lessnick SL. ZEB2 represses the epithelial phenotype and facilitates metastasis in Ewing sarcoma. Genes Cancer. 2013;4(11–12):486–500.
Chaudhury A, Hussey GS, Ray PS, Jin G, Fox PL, Howe PH. TGF-beta-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. Nat Cell Biol. 2010;12(3):286–93. doi:10.1038/ncb2029.
Cai X, Brophy M, Hahn S, McManus J, Chang B, Pasula S, et al. The role of epsin in promoting epithelial–mesenchymal transition and metastasis by activating NF-κB signaling in breast cancer. Cancer Res. 2012;72(24 supplement 3):3292.
Timmerman LA, Grego-Bessa J, Raya A, Bertrán E, Pérez-Pomares JM, Díez J, et al. Notch promotes epithelial–mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18(1):99–115.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–96. doi:10.1038/nrm3758.
Howard S, Deroo T, Fujita Y, Itasaki N. A positive role of cadherin in Wnt/beta-catenin signalling during epithelial–mesenchymal transition. PLoS ONE. 2011;6(8):e23899. doi:10.1371/journal.pone.0023899.
Ocana OH, Corcoles R, Fabra A, Moreno-Bueno G, Acloque H, Vega S, et al. Metastatic colonization requires the repression of the epithelial–mesenchymal transition inducer Prrx1. Cancer Cell. 2012;22(6):709–24. doi:10.1016/j.ccr.2012.10.012.
Takahashi Y, Sawada G, Kurashige J, Uchi R, Matsumura T, Ueo H, et al. Paired related homoeobox 1, a new EMT inducer, is involved in metastasis and poor prognosis in colorectal cancer. Br J Cancer. 2013;109(2):307–11. doi:10.1038/bjc.2013.339.
Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial–mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Investig. 2009;119(6):1438.
Saitoh M, Miyazawa K. Transcriptional and post-transcriptional regulation in TGF-β-mediated epithelial–mesenchymal transition. J Biochem. 2012;151(6):563–71.
Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–54.
Grueneberg DA, Natesan S, Alexandre C, Gilman MZ. Human and Drosophila homeodomain proteins that enhance the DNA-binding activity of serum response factor. Science. 1992;257(5073):1089–95.
Lu M-F, Cheng H-T, Kern MJ, Potter SS, Tran B, Diekwisch T, et al. prx-1 functions cooperatively with another paired-related homeobox gene, prx-2, to maintain cell fates within the craniofacial mesenchyme. Development. 1999;126(3):495–504.
Reichert M, Takano S, von Burstin J, Kim S-B, Lee J-S, Ihida-Stansbury K, et al. The Prrx1 homeodomain transcription factor plays a central role in pancreatic regeneration and carcinogenesis. Genes Dev. 2013;27(3):288–300.
Takano S, Reichert M, Heeg S, Bakir B, Rhim AD, Stanger B, et al. 339 The Prrx1 homeobox transcription factor regulates invasion and EMT in pancreatic cancer. Gastroenterology. 2013;144(5):S-71.
Micalizzi DS, Farabaugh SM, Ford HL. Epithelial–mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15(2):117–34.
De Wever O, Pauwels P, De Craene B, Sabbah M, Emami S, Redeuilh G, et al. Molecular and pathological signatures of epithelial–mesenchymal transitions at the cancer invasion front. Histochem Cell Biol. 2008;130(3):481–94.
Clements WM, Wang J, Sarnaik A, Kim OJ, MacDonald J, Fenoglio-Preiser C, et al. β-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res. 2002;62(12):3503–6.
Huang J, Xiao D, Li G, Ma J, Chen P, Yuan W, et al. EphA2 promotes epithelial–mesenchymal transition through the Wnt/beta-catenin pathway in gastric cancer cells. Oncogene. 2014;33(21):2737–47. doi:10.1038/onc.2013.238.
Jing Y, Han Z, Zhang S, Liu Y, Wei L. Epithelial–mesenchymal transition in tumor microenvironment. Cell Biosci. 2011;1:29.
Malanchi I, Peinado H, Kassen D, Hussenet T, Metzger D, Chambon P, et al. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature. 2008;452(7187):650–3. doi:10.1038/nature06835.
Mao J, Fan S, Ma W, Fan P, Wang B, Zhang J, et al. Roles of Wnt/beta-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis. 2014;5:e1039. doi:10.1038/cddis.2013.515.
Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, et al. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327(5973):1650–3. doi:10.1126/science.1186624.
Shimozaki K, Clemenson GD Jr, Gage FH. Paired related homeobox protein 1 is a regulator of stemness in adult neural stem/progenitor cells. J Neurosci. 2013;33(9):4066–75. doi:10.1523/JNEUROSCI.4586-12.2013.
Otsuki S, Inokuchi M, Enjoji M, Ishikawa T, Takagi Y, Kato K, et al. Vimentin expression is associated with decreased survival in gastric cancer. Oncol Rep. 2011;25(5):1235–42.
Dicken BJ, Graham K, Hamilton SM, Andrews S, Lai R, Listgarten J, et al. Lymphovascular invasion is associated with poor survival in gastric cancer: an application of gene-expression and tissue array techniques. Ann Surg. 2006;243(1):64.
Yang D, Sun Y, Hu L, Zheng H, Ji P, Pecot CV, et al. Integrated analyses identify a master microRNA regulatory network for the mesenchymal subtype in serous ovarian cancer. Cancer Cell. 2013;23(2):186–99.
Zhang Y, Zheng L, Huang J, Gao F, Lin X, He L, et al. MiR-124 radiosensitizes human colorectal cancer cells by targeting PRRX1. PLoS ONE. 2014;9(4):e93917.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (No. 81172295).
Conflict of interest
The authors declare no financial or other conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, J., Fu, Z., Wei, J. et al. PRRX1 promotes epithelial–mesenchymal transition through the Wnt/β-catenin pathway in gastric cancer. Med Oncol 32, 393 (2015). https://doi.org/10.1007/s12032-014-0393-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0393-x