Abstract
Ceramic materials have rapidly become the material of choice for indirect restorations. There are a variety of material types available for use such as feldspathic ceramics, glass ceramics and many types of zirconia. Advances in digital dentistry led to a rapid switch from porcelain fused to metal restorations to all-ceramic restorations. Variations in composition, microstructure and processing affect mechanical properties and use of these materials. Having a better understanding of their differences is important for proper clinical selection. Ceramic materials may be classified several ways including by composition, microstructure, processing technique and clinical application. This article reviews the various types of ceramics based on structure and properties that relate to clinical selection. After reading this article, the reader should be able to: explain the types of ceramics in use in dentistry; understand clinical selection based on properties; and discuss the differences in zirconia-based ceramics.
Key points
-
Reviews the types of ceramics available for clinical use.
-
Classifies ceramics based on an ISO standard that provides guidelines for clinical use centred on flexural strength.
-
Describes the differences between zirconia materials and their clinical use.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Ceramics of various compositions are in widespread use as restorative materials in dentistry. Dental restorations must fulfil several criteria in order to be used successfully clinically. A material must have adequate fracture resistance to withstand the rigours of the oral cavity. The repeated stress application during chewing combined with rapid thermal changes in a watery environment place a significant stress on the restoration. The fit of the restoration must be such that there is no impingement on the surrounding tissue and should be sufficiently sealed to inhibit leakage and secondary decay. Appearance is often the major concern of the patient and the dentist. A natural appearance of the restoration has been of the principal driving forces behind the rapid expansion in aesthetic restorative materials and ceramics in particular. Ceramic materials may best be able to mimic natural teeth with respect to colour and light interaction. In addition to strength, fit and tooth-like appearance, restorative systems must be biocompatible. Ceramic materials are generally considered to be among the most biocompatible of all the restorative materials used. They are oxidised and resistant to corrosion, and generally do not engender an allergic or toxic reaction.
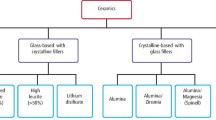
Ceramics are classified as non-metallic inorganic materials. These materials consist of metal oxides, borides, carbides and nitrides.1 Their structure is crystalline, displaying a regular periodic arrangement of the component atoms and may exhibit ionic or covalent bonding. Ceramics possess a wide range of strength values. These materials are brittle in that they exhibit extremely low plasticity, in which cracks can initiate and propagate without plastic deformation leading to fracture.
Ceramics may encompass glasses, glasses with crystal components and crystalline materials. Dental porcelain is a mixture of glass and crystal components. Glass has an amorphic structure. Ceramics may be classified in a variety of ways. These include by their microstructure, processing technique (powder/liquid, pressed, machined, or printed) and their clinical application.2,3,4,5 The International Standards Organisation (ISO) brings dentists, manufacturers and scientists together to write standards designed to aid the dental community in production and use of materials as well as to meet perceived minimum clinical requirements for a given material. This standard classifies materials using a combination of strength values and clinical applications. This article groups ceramics into categories based on the ISO standard.
It is important to evaluate the clinical requirements for the restoration and try to match these to the mechanical and physical properties of the restorative materials being considered for the patient. The properties of a material may derive from the interaction of fabrication method (processing), composition (material type) and microstructure. In particular, this interaction is well demonstrated in the use of machinable ceramics that have the same microstructure and chemical composition but are processed differently, leading to improvement in properties such as flexural strength and clinical success rates. Dental all-ceramic systems fall into these major categories: feldspar-based, castable and machinable glass-ceramics, interpenetrating phase ceramics, and high-strength machinable polycrystalline materials.
Feldspar-based
Feldspar-based materials for all-ceramic restorations are primarily fabricated by one of three methods: 1) a powder-liquid mixture where powder is mixed with a water-based liquid to form a useable mass that is shaped by hand and is subsequently fired in a furnace to produce the dense restoration; 2) pressing a pre-formed fully dense ingot that is heated to produce flow under pressure into a mould created with a lost wax technique; and 3) machined from a fully dense block.
Feldspars used in dental ceramics are aluminosilicates containing potassium, sodium, or calcium. These materials were first used in dentistry to make porcelain dentures. Flexural strength values usually range from 60 MPa to 70 MPa. The ISO standard identifies these materials as a Class I ceramic. They tend to be employed as veneering materials for metal or ceramic substructures, as well as for all-ceramic veneers, inlays, onlays and other single unit anterior restorations that are adhesively cemented.
These materials have also been developed into fine-grain machinable blocks such as the Vitablocs Mark II family (Vita Zahnfabrik, Bad Sackingen, Germany) and with CAD/CAM systems. There are numerous other manufacturers of this category of block. MKII has been in use for over 30 years for the fabrication of inlays, onlays and crowns with numerous studies showing a less than 1% per year failure rate, which compares favourably with metal-ceramic survival data.6,7,8,9 A pre-manufactured block has minimal residual porosity that could act as a weak point and lead to a sudden failure compared to hand-fabricated powder-liquid and pressable ceramics. These materials are Class 2 with minimum flexural strength values of 100 MPa for single unit anterior and posterior monolithic ceramics and are adhesively cemented.
In general, for any ceramic material, it is important to meet minimum thickness values to have sufficient load-bearing capacity to withstand stresses in the mouth. Furthermore, attention must be paid to the luting method recommended for these restorations. Minimum occlusal thickness value for crowns fabricated from this material is about 2.0 mm.
Feldspathic with leucite
These materials are feldspar-based porcelains with a leucite crystal content of about 10-25%. Leucite may alter the coefficient of thermal expansion (CTE), as well as inhibit crack propagation, thereby improving the material's strength. These may also be fabricated by mixing powder with a liquid and used to veneer metal and ceramic substructures as well as fabricate all-ceramic porcelain veneers, inlays and onlays. The original materials had a fairly random size and distribution of leucite crystals, with the average particle size being approximately several hundred micrometres. The large particle size in part contributed to the low fracture resistance and high wear rate of opposing tooth structure. Improvements in both flexural strength and abrasiveness were achieved by producing materials with much finer leucite crystals (10 μm to 20 μm). These materials are less abrasive and have much higher flexural strengths.10 These materials may be Class 1 or Class 2 according to the ISO standard, depending upon the flexural strength value of the specific ceramic.
Glass ceramics
According to Deubener et al. who worked on glass ceramic classification, glass ceramics are 'inorganic, non-metallic materials prepared by controlled crystallisation of glasses via different processing methods. They contain at least one type of functional crystalline phase and a residual glass'.11
Historically, the first widespread use of glass-ceramics and full ceramic restorations was a fluoromica-based material called Dicor developed by Corning Glass Works.12 Although this system is no longer available, much was learned from it regarding the handling of glass-ceramic materials. Dicor suffered from a high failure rate when cemented with zinc-phosphate cement, especially in the posterior region - as high as 70% within five years.13 However, when the Dicor was acid-etched, silane-treated and then adhesively cemented, Dicor fracture rates dropped to roughly half those experienced with zinc phosphate.14 Dental glass-ceramics with an occlusal thickness of 1.5 mm may be cemented without bonding (glass-ionomers and resin-ionomers). However, it is recommended that restorations of approximately 1.0 mm occlusal thickness should be adhesively cemented. Recent research investigating load to failure of glass-ceramic crowns demonstrated significantly higher failure loads for crowns adhesively cemented as opposed to those cemented with glass-ionomer.15
The addition of a second phase to a glass may improve flexural strength and fracture resistance by a method called dispersion strengthening. In order for a crack to propagate from a defect created in the material, the crack must go through or around the crystals. This requires additional energy and so makes the restoration more resistant to damage and failure in the mouth. In materials where crystals are grown in a glassy matrix, compressive stress around the crystals may further help to prevent crack propagation.
Glass matrix ceramics with leucite content of approximately 50 volume% were developed to improve flexural strength and expand the use of all-ceramic materials for single unit crowns throughout the mouth. The material starts as a homogeneous glass. A secondary heat treatment nucleates and grows crystals, which gives these materials increased flexural strength, wear, thermal shock and corrosion resistance due to the physical presence of the crystals and generation of compressive stress around the crystals.16 Therefore, glass-ceramics may be ideally suited for use as dental restorative materials. Improvements in properties depend on the interaction of the crystals and glassy matrix, as well as on the crystal size and amount. Finer crystals generally produce stronger materials. Glass-ceramics are in widespread use for cookware, missile nose cones, and even heat shields on space vehicles. They may be opaque or translucent depending on the chemical composition and the percentage of crystalline material.
The most widely used type is based on the original pressable ceramic Empress (Ivoclar, Liechtenstein); there are many other brands in this category due to the clinical success of this material. A number of pressable materials with properties and microstructure similar to Empress are available. The machinable version Empress CAD (Ivoclar) has also performed well clinically when used for posterior inlays and onlays, as well as anterior veneer and crown restorations. Minimum thickness for crowns is 2.0 mm and should also be adhesively cemented. Machinable and pressable systems have much higher fracture resistance than powder-liquid systems and have shown excellent clinical results for posterior inlay and onlay applications and anterior veneer and crown restorations.17,18,19,20,21,22 These materials are Class 2 according to the ISO standard.
Glass ceramics - lithium silicate-based
Materials with a different chemistry than the Empress material and with a higher fracture resistance were developed based on a lithium disilicate chemistry. Ivoclar introduced this material initially as Empress II and it is now marketed as IPS e.max pressable and machinable ceramics. Increasing the crystal content to approximately 70% and refining the crystal size improved mechanical and physical properties. According to the manufacturer, the glass phase is composed of mainly SiO2, Li2O, P2O5, ZrO2, ZnO and K2O.23 The glass matrix surrounds micron-size lithium disilicate crystals with sub-micron lithium orthophosphate crystals in between the lithium disilicate (Fig. 1).
The shape and volume of crystals increased the flexural strength to approximately 360 MPa or about three times that of Empress.24,25,26,27 This material can be translucent even with the high crystalline content due to the relatively low refractive index of the lithium disilicate crystals. The material is translucent enough that it can be used for full-contour restorations anywhere in the mouth and can be veneered with a matched porcelain. The machinable version is provided in a partially crystallised block to enable more rapid machining. After machining, the restoration is subjected to a secondary heat treatment to crystallise the material and obtain the final shade and mechanical properties.
Additional glass-ceramics with a different microstructure and chemistry have been developed to improve the crystal structure. The innovations involve changes in the chemistry and crystal structure of lithium silicate-based glass ceramics modified with approximately 10 mass% zirconium oxide. Machinable products include Celtra Duo (Dentsply-Sirona) that is pre-crystallised and Suprinity (Vita Zahnfabrik) that requires a crystallisation cycle after machining the restoration. The mean crystal size is approximately 1.5 μm, with even finer sub-micron crystals between the larger ones. A further refined version of this chemistry involves the production of sub-micron Virgilite crystals to increase overall crystal content and mechanical properties. This is marketed as Tessera by Denstply Sirona. A recent article by Lubauer et al. provides a detailed analysis of the crystal structure and composition of these materials.28 The minimum occlusal thickness values are 1.0 mm if adhesively cemented or 1.5 mm if cemented with non-adhesive materials such as glass-ionomers. The flexural strength of this type of glass ceramic is above 300 MPa and falls into Class 3. This class includes monolithic ceramics for single unit anterior or posterior prostheses and for threeunit prostheses not involving a molar restoration adhesively or non-adhesively cemented as well as fully covered substructures for single unit anterior or posterior prostheses and for threeunit prostheses not involving molar restoration adhesively or non-adhesively cemented.
Interpenetrating phase ceramics
Interpenetrating phase ceramics are characterised by two phases that are each intact three-dimensionally (intertwined) throughout the fully dense material (Fig. 2).29,30 This material class may have improved fracture resistance relative to the individual components due to the geometrical and physical constraints that are placed on the path that a crack must follow to cause fracture. A tortuous route through at the interface of each phase or through each phase is required in order for a crack to propagate through the entire restoration. Interpenetrating phase materials are generally fabricated by first creating a porous matrix - a ceramic 'sponge'. The pores are then filled by a second-phase material. It is important to note that both the ceramic and second phase are continuous and connected to each other. This is unlike glass-ceramics which have two separate phases - a glass matrix and individual crystals.
There are two interpenetrating phase materials that have been used for dental ceramic restorations. These are InCeram (Vita Zahnfabrik) that consisted of alumina or alumina and zirconia ceramic matrix infiltrated with a lanthanum glass and Enamic (Vita Zahnfabrik) that consists of a feldspathic ceramic matrix with a polymer second phase. In-Ceram (Vita Zahnfabrik) consisted of a family of all-ceramic restorative materials based on the same principle introduced in 1988. The system was developed as an alternative to conventional metal-ceramics and was the first system for all-ceramic posterior bridges, and met with great clinical success.31,32 The InCeram alumina and alumina-zirconia materials possessed a flexural strength of about 500-650 MPa and thus are a Class 4 ceramic.33 This category includes monolithic ceramics for three-unit prostheses involving a molar restoration and a fully covered substructure for three-unit prostheses involving a molar restoration.
Enamic is a unique material in that it is a combination of interconnected polymer (20-25% by volume) and interconnected ceramic (75-80% by volume). The polymer component provides improved fracture resistance, resilience and machinability. It is provided in a block form; machining times and damage both are decreased when using this material. The ceramic component provides colour stability, improved modulus values and stress resistance. This material has widespread use for inlays, onlays, tooth-born and implant crowns.34,35,36,37 Enamic minimum thickness for crowns is 1.0 mm and is acid-etched, silane-treated and bonded with a resin cement.
Polycrystalline ceramics
Solid-sintered monophase ceramics are formed by directly sintering starting powders together without any intervening matrix to form a dense polycrystalline structure. Several processing techniques allow the fabrication of either solid-sintered aluminium oxide (Al2O3) or zirconium dioxide (ZrO2) frameworks and full contour restorations. One of the first all-ceramic CAD/CAM fabricated crowns was a polycrystalline alumina, called Procera AllCeram (Nobel Biocare, Kloten, Switzerland) with a strength of approximately 600 MPa.38 An oversized die was machined to compensate for the alumina firing shrinkage of about 20%. Alumina powder was pressed on the die, machined to form the crown form, and then fully sintered to form the final restoration.
Zirconia-based ceramic materials have unique properties that allow them to sustain damage and prevent fracture of the dental restoration. Many natural teeth survive a lifetime even though there are visible cracks in the tooth structure. Materials that can replicate that ability to survive with damage are ideal dental restorations. Zirconia-based ceramics have been used in other industries for many years; dental applications present unique problems in that custom parts must be made and the part should look like a tooth.39 Computer-aided design/computer-aided manufacturing (CAD/CAM) has enabled materials to be used that ordinarily cannot be fabricated conventionally. One of the most important of these materials is yttria partially stabilised tetragonal zirconia. Zirconia (ZrO2) is the oxidised form of zirconium (Zr) just as alumina (Al2O3) is an oxide of aluminium (Al).
Zirconia exists in three major phases: monoclinic, tetragonal and cubic. Monoclinic is room-temperature stable. Above 1,170 °C, zirconia transforms into the tetragonal intermediate phase; at 2,370 °C, the material changes into a cubic phase. In pure zirconia ceramics, the cubic-to-monoclinic phase transformation occurs during cooling with about a 5% volumetric expansion (causes cracks), which may then fracture zirconia at room temperature. Therefore, biomedical and structural/functional applications of zirconia typically do not use pure zirconia. The addition of other ceramic components may alter the presence and stability of these phases at room temperature. Zirconia (ZrO2) may exist primarily in the tetragonal phase at room temperature by adding components such as calcia (CaO), magnesia (MgO), yttria (Y2O3) and ceria (CeO2). If the right amount of component is added, then a fully stabilised cubic phase can be created, cubic zirconia 'diamond' jewellery. The addition of smaller amounts (3 mol% to 5 mol%) produces a partially stabilised zirconia. Although stabilised at room temperature, the tetragonal zirconia phase may change under stress to monoclinic with a subsequent 3% volumetric increase.40 This dimensional change diverts energy from the crack and creates compressive stress that may stop crack propagation. This is called 'transformation toughening'.41,42 This helps resist catastrophic failure - even though a crack may exist in the material, the phase change prevents it from proceeding throughout the restoration. Yttria tetragonal partially stabilised zirconia (Y-TZP) may be a 'universal' ceramic restorative material in that it has sufficient properties to withstand stresses in all regions of the mouth, as well as the ability to support multiple-unit restorations.43,44
Most zirconia restorations are fabricated by machining a porous or partially fired block of zirconia. The framework is milled oversized by about 25% and then fired at approximately 1,500 °C to fully densify the zirconia, producing a material with micron and submicron crystals with strength values from 900 MPa to 1,200 MPa.45 Most blocks have barcodes or inserts that tell the computer the density in order to properly mill the framework oversized. It is critically important that the density is homogeneous and known for each block to allow for proper oversizing during milling to produce an accurate and well-fitting dense restoration. Furthermore, the density must be uniform throughout the block, otherwise differential shrinkage occurs leading to warping and poor fit of the restoration. An alternate approach involves milling a fully dense block; this method was used on some early machining systems and is available now in a chairside system (Glidewell). However, due to the nature of zirconia, this approach initially required approximately two hours of milling time per unit out of a large zirconia block whereas milling of the porous block necessitates only about 30 to 45 minutes for a three-unit bridge.
The first generation of zirconia restorative materials contained about 0.25 wt% alumina, and while these materials have high strength, translucency was relatively low.46,47 Improvements in translucency were first made by keeping the same 3 mol% yttria concentration but decreasing the alumina content to about 0.05%. Additional generations of zirconia were fabricated to improve translucency by increasing yttria content to 4 and 5 mol%. In general, as yttria content increased, translucency increased but mechanical properties decreased from the 1,200 MPa range down to 500-800 MPa. The transformation toughening property is mostly lost in 4 and 5 mol% zirconia materials as the cubic crystal content increases. These materials become much more susceptible to surface damage with decreased fracture resistance. This is of particular importance when occlusal adjustment of the restoration is needed. The aesthetic quality, particularly translucency, enables these materials to be used anteriorly and even as veneers.48,49,50,51 Zirconia materials may fall into two different classes depending upon the type of zirconia. Materials with a flexural strength of 800 MPa and above are Class 5. This class includes monolithic ceramics for prostheses involving four or more units or fully covered substructures for prostheses involving four or more units. Other zirconia materials under 800 MPa are Class 4 ceramics.
Zirconia shades may be developed in several ways. Acid- or water-based solutions with dissolved metal salts may infuse into the porous zirconia before sintering to full density. The salt concentration and application time may be used to vary the amount of metal salt deposited in the pores and change the final shade. Alternatively, starting zirconia powder may be mixed with metal oxides to provide a uniform shade throughout the zirconia.
There are blocks with multiple layers that vary chroma from the 'cervical' to the 'incisal' regions in order to better match the gradation seen in natural teeth. There are also zirconia blocks with this chroma gradient and a translucency gradient with different amounts of yttria added to alter translucency, typically 3-6 layers going from a 3Y low translucency/high strength to an intermediate mixture and ending with a 5Y high translucent layer at the incisal region. At the time of writing, there is little long-term clinical or laboratory data to validate the overall properties of these materials. Overall, strength will vary depending upon the ratios of the types of different yttria layers and the processing of these materials.
Sintering schedules may also affect the optical and mechanical properties of zirconia. Initial firing protocols required about six hours, then one hour, and finally some chairside zirconia blocks may be fired in as little as 20-30 minutes depending upon the size of the restoration. It is important to note that different zirconia materials have specific cycles designed for the exact particle size and composition as produced by the manufacturer. Altering the firing schedule can decrease strength and alter the final chroma and translucency of the restoration.
Zirconia is most often luted to the tooth structure using glass-ionomer or other type of cement. However, several primers and cements containing the chemical 10 methacryloyloxydecyl dihydrogen phosphate (10-MDP) may aid in creating a chemical bond with zirconia and the corresponding resin cement.52 Glass-ionomers bond weakly or not at all.53 Zirconia bonding might improve retention to the tooth structure that is particularly helpful with minimum reduction and short crown preparations. When zirconia restorations are non-adhesively cemented, a low-pressure sandblasting of the internal surface is recommended using about 25-50 psi (2 bar) with 25-50 μm alumina. This provides for some mechanical retention of the cement. Some studies have demonstrated a potential problem in sandblasting the internal surface with respect to crack growth, while others have shown an improvement in properties.54,55
The surface finish for any ceramic is important as surface defects can create sites for crack propagation and failure but a rough surface also may result in excessive wear of the opposing dentition or any opposing restoration. Most of the ceramic materials in use have a fine microstructure with particle sizes in the 5-20 μm range. Zirconia is micron and submicron in most cases; however, Vickers hardness of zirconia is 1,350 as compared to natural tooth structure of 300-500 or feldspathic ceramics of 400-500.56,57,58,59 Therefore, if it is rough, the hardness may become the dominant factor resulting in excessive wear.60,61,62,63 There are numerous polishing systems consisting of silicone or other rubber wheels embedded with diamond particles to polish ceramics. It is important to follow the recommended sequential polishing steps with low speed and light pressure using water to cool the ceramic. Final polishing may be accomplished using a felt wheel with a micron-sized diamond paste. This will provide a smooth and wear-kind surface.
One additional critical thickness value comes into play when using all-ceramic bridges - this is the connector region. The height and cross-sectional area recommended for each material type must not be violated as these areas are under high stress and are the most likely failure site for all-ceramic bridges. The connector sizes vary depending on the material type and location in the mouth. It is critically important not to adjust connector size using a diamond disc after it has been fabricated and designed for a specific clinical case. The disc can create sharp notches that concentrate stress and lead to failure in these brittle materials - no matter how strong they may be.
Summary
The use of all-ceramic materials for indirect restorations grew rapidly as digital dentistry and CAD/CAM systems became more advanced and easier to use. There is now a wide variety of ceramic materials to choose from for a given clinical need. Clinical selection is often based on a combination of aesthetics, quality and stress resistance. While there are very high-strength materials available, minimum thickness values must be adhered to in order to have a restoration with sufficient load-bearing capacity. It is also important to realise that strength is not static; this property tends to decline as the material is subjected to the rigours of the oral cavity. Therefore, tooth preparation, luting procedures and proper occlusion are still of paramount importance and improper preparations cannot be masked by 'lots of MPa'. Table 1 summarises properties and uses of the ceramic armamentarium.
References
Kingery W D, Bowen H K, Uhlmann D R. Introduction to Ceramics. 2nd ed. New York: John Wiley and Sons, 1976.
Talibi M, Kaur K, Patanwala H S, Parmar H. Do you know your ceramics? Part 1: classification. Br Dent J 2022; 232: 27-32.
Talibi M, Kaur K, Parmar H. Do you know your ceramics? Part 2: feldspathic ceramics. Br Dent J 2022; 232: 80-83.
Gracis S, Thompson V P, Ferencz J L, Silva N R, Bonfante E A. A new classification system for all-ceramic and ceramic-like restorative materials. Int J Prosthodont 2015; 28: 227-235.
ISO 6872. Dentistry - Ceramic materials. 2015.
Otto T. CEREC restorations. CEREC inlays and onlays: the clinical results and experiences after 6 years of use in private practice. Schweiz Monatsschr Zahnmed 1995; 105: 1038-1046.
Reiss B, Walther W. Überlebensanalyse und klinische Nachuntersuchungen von zahnfarbenen Einlagefüllungen nach dem CEREC-Verfahren. Zahnaerztl Welt 1992; 100: 329-332.
Heymann H O, Bayne S C, Sturdevant J R et al. The clinical performance of CADCAMgenerated ceramic inlays: a four-year study. J Am Dent Assoc 1996; 127: 1171-1181.
Reiss B, Walther W. Clinical long-term results and 10-year Kaplan-Meier analysis of Cerec restorations. Int J Comput Dent 2000; 3: 9-23.
McLaren E A, Giordano R A. Zirconia-based ceramics: material properties, esthetics, and layering techniques of a new veneering porcelain, VM9. Quintessence Dent Technol 2005; 28: 99-111.
Deubener J, Allix M, Davis M et al. Updated definition of glass-ceramics. J Non Crystall Solids 2018; 501: 3-10.
Grossman D G. Tetrasilicic mica glass-ceramic material. U.S. Patent No. 3732087. 1973.
Ellison J A, Lugassy A A, Setcos J C, Moffa J P. Clinical trial of cast glass ceramic crowns, seven year findings. J Dent Res 1992; 71: 207.
Malament K A, Grossman DG: Bonded versus non-bonded Dicor crowns. J Dent Res 1992; 71: 321.
Vohra F, Altwaim M, Alshuwaier A S et al. Influence of Bioactive, Resin and Glass Ionomer luting cements on the fracture loads of dentin bonded ceramic crowns. Pak J Med Sci 2020; 36: 416-421.
Serbena F C, Zanotto E D. Internal residual stresses in glass-ceramics: A review. J Non Crystall Solids 2012; 358: 975-984.
Wagner J, Hiller K A, Schmalz G. Long-term clinical performance and longevity of gold alloy vs ceramic partial crowns. Clin Oral Investig 2003; 7: 80-85.
Brochu J F, El-Mowafy O. Longevity and clinical performance of IPS-Empress ceramic restorations - a literature review. J Can Dent Assoc 2002; 68: 233-237.
Kraemer N, Frankenberger R. Clinical performance of bonded leucite-reinforced glass ceramic inlays and onlays after 8 years. Dent Mater 2005; 21: 262-271.
Manhart J, Chen H Y, Neuerer P et al. Thre-year clinical evaluation of composite and ceramic inlays. Am J Dent 2001; 14: 95-99.
van Dijken J W, Hasselrot L, Ormin A et al. Restorations with extensive dentin/enamel-bonded ceramic coverage. A 5-year follow-up. Eur J Oral Sci 2001; 109: 222-229.
Höland W, Rheinberger V, Apel E et al. Clinical applications of glass-ceramics in dentistry. J Mater Sci Mater Med 2006; 17: 1037-1042.
Lien W, Roberts H W, Platt J A, Vandewalle K S, Hill T J, Chu T-M G. Microstructural evolution and physical behaviour of a lithium disilicate glass ceramic. Dent Mater 2015; 31: 928-940.
Guazzato M, Albakry M, Ringer S P et al. Strength, fracture toughness and microstructure of a selection of all-ceramic materials. Part I. Pressable and alumina glass-infiltrated ceramics. Dent Mater 2004; 20: 441-448.
Albakry M, Guazzato M, Swain M V. Biaxial flexural strength, elastic moduli, and x-ray diffraction characterization of three pressable all-ceramic material. J Prosthet Dent 2003; 89: 374-380.
Hoeland W, Schweiger M, Frank M et al. A comparison of the microstructure and properties of the IPS Empress 2 and the IPS Empress glass ceramics. J Biomed Mater Res 2000; 53: 297-303.
Della Bona A, Mecholsky Jr J J, Anusavice K J. Fracture behaviour of Lithia disilicate and leucite based ceramics. Dent Mater 2004; 20: 956-962.
Lubauer J, Belli R, Peterlik H, Hurle K, Lohbauer U. Grasping the Lithium hype: Insights into modern dental Lithium Silicate glass-ceramics. Dent Mater 2022; 38: 318-332.
Clarke D. Interpenetrating phase composites. J Am Ceram Soc 1992; 75: 739-759.
Denry I, Kelly J R. Emerging ceramic-based materials for dentistry. J Dent Res 2014; 93: 1235-1242.
Proebster L. Survival rate of In-Ceram restorations. Int J Prosthodont 1993; 6: 259-263.
Scotti R, Catapano S, D'Elia A. A clinical evaluation of In-Ceram crowns. Int J Prosthodont 1995; 8: 320-323.
Guazzato M, Albakry M, Swain M V, Ironside J. Mechanical properties of In-Ceram Alumina and In-Ceram Zirconia. Int J Prosthodont 2002; 15: 339-346.
Coldea A, Swain M V, Thiel N. Mechanical properties of polymerinfiltratedceramic-network materials. Dent Mater 2013; 29: 419-426.
Coldea A, Swain M V, Thiel N. In-vitro strength degradation of dental ceramics and novel PICN material by sharp indentation. J Mech Behav Biomed Mater 2013; 26: 34-42.
Finelle G, Lee S J. Guided Immediate Implant Placement with Wound Closure by Computer-Aided Design/Computer-Assisted Manufacture Sealing Socket Abutment: Case Report. Int J Oral Maxillofac Implants 2017; DOI: 10.11607/jomi.4770.
He L H, Swain M. A novel polymer infiltrated ceramic dental material. Dent Mater 2011; 27: 527-534.
Hegenbarth E A. Procera aluminium oxide ceramics: a new way to achieve stability, precision, and esthetics in all-ceramic restorations. Quintessence Dent Technol 1996; 20: 21-34.
Green D J, Hannink R, Swain M V. Transformation Toughening of Ceramics. Boca Raton: CRC Press, 1989.
Piconi C, Maccauro G. Zirconia as a ceramic material. Biomaterials 1999; 20: 1-25.
Lange F F. Transformation toughening: Part 3, experimental observations in the ZrO2-Y2O3 system. J Mat Sci 1982; 17: 240-246.
Denry I, Kelly J R. State of the art of zirconia for dental applications. Dent Mater 2008; 24: 299-307.
Giordano R, Sabrosa C E. Zirconia: Material Background and Clinical Application. Compend Contin Educ Dent 2010; 31: 710-715.
Giordano R, McLaren E A. Ceramics Overview: Classification by Microstructure and Processing Methods. Compend Contin Educ Dent 2010; 31: 682-684.
Guazzato M, Albakry M, Ringer S P et al. Strength, fracture toughness and microstructure of a selection of all-ceramic materials. Part II. Zirconia-based dental ceramics. Dent Mater 2004; 20: 449-456.
Chevalier J, Cales B, Drouin M. Low-temperature aging of Y-TZP ceramics. J Am Ceram Soc 1999; 82: 2150-2154.
Lawson S. Environmental degradation of Zirconia ceramics. J Eur Ceram Soc 1995; 15: 485-502.
Clarke I C, Manaka M, Green S M et al. Current status of zirconia used in total hip implants. J Bone Joint Surg Am 2003; 85: 73-84.
Chevalier J, Gremillard L, Virkar A V, Clarke D R. The Tetragona-Monoclinic Transformation in Zirconia: Lessons Learned and Future Trends. J Am Ceram Soc 2009; 92: 1901-1920.
Klimke J, Trunec M, Krell A. Transparent tetragonal yttria-stabilized zirconia ceramics: influence of scattering caused by birefringence. J Am Ceram Soc 2011; 94: 1850-1858.
Inokoshi M, Shimizubata M, Nozaki K et al. Impact of sandblasting on the flexural strength of highly translucent zirconia. J Mech Behav Biomed Mater 2021; 115: 104268.
Mao L, Kaizer M, Zhao M, Guo B, Song Y, Zhang Y. Graded ultra-translucent zirconia (5Y-PSZ) for strength and functionalities. J Dent Res 2018; 97: 1222-1228.
Blatz M, Vonderheide M, Conejo J. The effect of resin bonding on long-term success of high-strength ceramics. J Dent Res 2018; 97: 132-139.
Kang S-H, Chang J, Son H-H. Flexural strength and microstructure of two lithium disilicate glass ceramics for CAD/CAM restoration in the dental clinic. Restor Dent Endod 2013; 38: 134.
Campos F, Valandro L F, Feitosa S A et al. Adhesive cementation promotes higher fatigue resistance to zirconia crowns. Oper Dent 2017; 42: 215-224.
Bulot D, Sadan A, Burgess J O, Blatz M B. Bond Strength of a Self-adhesive Universal Resin Cement to Lava Zirconia after Two Surface Treatments. J Dent Res 2005; 84(Spec Iss A): Abstract 0578.
Papanagiotou H P, Morgano S M, Giordano R A et al. In vitro evaluation of low-temperature aging effects and finishing procedures on the flexural strength and structural stability of Y-TZP dental ceramics. J Prosthet Dent 2006; 96: 154-164.
Pittayachawan P, McDonald A, Young A, Knowles J C. Flexural strength, fatigue life, and stress-induced phase transformation study of -TZP dental ceramic. J Biomed Mater Res B Appl Biomater 2009; 88: 366-377.
Shijo Y, Shinya A, Gomi H et al. Studies on mechanical strength, thermal expansion of layering porcelains to alumina and zirconia ceramic core materials. Dent Mater J 2009; 28: 352-361.
Park S, Quinn J B, Romberg E, Arola D. On the brittleness of enamel and selected dental materials. Dent Mater 2008; 24: 1477-1485.
Lohbauer U, Reich S. Antagonist wear of monolithic zirconia crowns after 2 years. Clin Oral Investig 2017; 21: 1165-1172.
Passos S P, Torrealba Y, Major P et al. In vitro wear behaviour of zirconia opposing enamel: a systematic review. J Prosthodont 2014; 23: 593-601.
Solá-Ruíz M F, Baima-Moscardó A, Selva-Otaolaurruchi E et al. Wear in Antagonist Teeth Produced by Monolithic Zirconia Crowns: A Systematic Review and Meta-Analysis. J Clin Med 2020; 9: 997.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Giordano II, R. Ceramics overview. Br Dent J 232, 658–663 (2022). https://doi.org/10.1038/s41415-022-4242-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41415-022-4242-6
- Springer Nature Limited
This article is cited by
-
Fracture resistance and fractographic analysis of pressable glass-ceramics with different partial coverage designs for maxillary premolars
BMC Oral Health (2024)
-
Quantum mechanical analysis of yttrium-stabilized zirconia and alumina: implications for mechanical performance of esthetic crowns
European Journal of Medical Research (2024)
-
Patient-specific implants made of 3D printed bioresorbable polymers at the point-of-care: material, technology, and scope of surgical application
3D Printing in Medicine (2024)
-
The effect of combining primers and cements from different cement systems on the bond strength between zirconia and dentin
BDJ Open (2024)
-
Does glaze firing affect the strength of advanced lithium disilicate after simulated defects?
Clinical Oral Investigations (2023)