Abstract
T-cell acute lymphoblastic leukemia (T-ALL) predominantly affects individuals in late childhood and young adulthood. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative modality particularly in the setting of poor risk genetics and/or persistent minimal residual disease. Limited studies have directly explored the impact of patient- and transplant-related factors on post-transplant outcomes in T-ALL. Using a large dataset from the European Society for Blood and Marrow Transplantation registry, we identified 1907 adult T-ALL patients (70% male) who underwent their first allo-HSCT in first complete remission (CR1) from matched sibling donors (MSD; 45%), unrelated donors (UD; 43%) or haploidentical donors (12%) between 2010 and 2021. The median age at transplant was 33.4 years (18.1–75). The median follow up was 2.9 years. Most patients underwent total body irradiation (TBI)-based myeloablative conditioning (69%). The 2-year overall survival (OS) was 69.4%, and leukemia -free survival (LFS) was 62.1%. In multivariate analysis, advanced age at transplant negatively affected LFS (for each 10-year increment, HR = 1.11, p = 0.004), GVHD-free, relapse-free survival (GRFS) (HR = 1.06, p = 0.04), OS (HR = 1.12, p = 0.002), and non-relapse mortality (NRM) (HR = 1.23, p < 0.001). More recent years of allo-HSCT were associated with improved GFRS (For each 3-year increment, HR = 0.89, p < 0.001), OS (HR = 0.9, p = 0.02), and decreased NRM (HR = 0.82, p = 0.008). TBI improved LFS. (HR = 0.79, p = 0.02), GRFS (HR = 0.83, p = 0.04), and relapse incidence (RI) (HR = 0.65, p < 0.001). Female-to-male transplant negatively affected GRFS (HR = 1.21, p = 0.02) and OS (HR = 1.23, p = 0.048). In vivo T-cell depletion significantly improved GFRS (HR = 0.74, p < 0.001). This large study identified prognostic factors, such as age at transplant conditioning regimen, in influencing post-transplant in adult T-ALL patients undergoing allo-HSCT. Importantly, a significant improvement over time was noted. These findings hold great promise for new adapted treatment strategies and can serve as a benchmark for future studies in that setting.
Similar content being viewed by others
Introduction
T-cell acute lymphoblastic leukemia (T-ALL), also known as precursor T-cell acute lymphoblastic leukemia, originates from T-lymphoblasts at various stages of differentiation and early maturation. It predominantly affects individuals in late childhood and young adulthood [1]. The presence of more than 20% bone marrow blasts, regardless of extra medullary involvement, is the defining criterion for the diagnosis of T-ALL as opposed to T-cell acute lymphoblastic lymphoma [2].
The current management of T-ALL has led to outcomes comparable to those of B-cell acute lymphoblastic leukemia (B- ALL) [3]. Notably, outcomes in adult patients are inferior to those in younger patients due to disparities in genetic expression, mutations, and the utilization of intensive treatments [4]. In the UKALL XII/ECOG 2993 trial involving 365 adult patients, 94% of the individuals achieved complete remission (CR), but the five-year overall survival (OS) rate was merely 48%. Prognostic factors, such as gender, age, and the type of stem cell transplant donor, exerted a significant influence on outcomes. Favorable prognostic indicators encompassed CD1a positivity, and the absence of CD13, while the presence of complex abnormalities was strongly associated with an unfavorable prognosis [5]. A study by Trinquand, et al. [5] in the Group for Research in Adult Acute Lymphoblastic Leukemia (GRAALL) on series of 212 adult patients with T-ALL included in the multicenter randomized GRAALL-2003 and -2005 trials, identified NOTCH1 and FBXW7 (N/F) mutations in 67% of the patients were associated with better outcomes (5-year OS: 75% vs 47% with patients without N/F). While K-RAS, N-RAS (N/K-RAS), and PTEN genes were associated with a poorer prognosis (CIR: 24% in patients with no N/K-RAS mutation or PTEN abnormalities vs 57% in patients with). Another Study by Bond, et. al. [6] in the same group showed that patients with Early thymic precursor (ETP) T-ALL were associated with higher rates of corticosteroid resistance and early bone marrow chemotherapy resistance than patients with non ETP T-ALL (63.8% and 87% respectively) wherein specifically ETP patients were more likely to have positive MRD post induction than non ETP patients (71.4% vs 20.9%).
While approximately 80% of adult patients can achieve CR, additional post-remission therapy is necessary [7]. Consolidation chemotherapy is generally preferred over allogeneic hematopoietic stem cell transplantation (allo-HSCT) for standard-risk patients in first CR (CR1). In high-risk patients, allo-HSCT is crucial, offering a 10-year survival of 45% compared to only 10% with consolidation chemotherapy [8, 9].
Despite the importance of allo-HSCT, the outcomes and prognostic factors after transplant in these patients remain unclear. A study conducted by the Late Effects Working Committee of the International Bone Marrow Transplant Registry (IBMTR), which analyzed 1458 patients alive for two years and free of disease post-transplant, revealed that age above 40 years at transplant, incomplete remission at transplant, and female donor to male recipient combination, were associated with poorer outcomes. More than 85% of surviving patients had a Karnofsky performance score of more than 90 [8]. The prognostic value of graft-versus-host disease (GVHD) in the study was complex, as it correlated with shorter post-transplant survival but a lower risk of relapse, potentially attributable to the graft-versus-leukemia (GVL) effect. The primary causes of death ranked in descending order were relapse, GVHD, new malignancies, and organ failure [8, 10,11,12,13].
Limited studies have directly investigated the impact of patient characteristics and transplant- related factors on long-term outcomes of allo-HSCT transplantation for adult patients with T-ALL. This is primarily due to the disease predominantly affecting children, and adult treatment strategies being extrapolated from pediatric chemotherapy regimens. The present study aims to address this gap by evaluating the impact of patient and transplant characteristics on post-transplant outcomes in T-ALL patients using a large dataset from the European Society for Blood and Marrow Transplantation (EBMT) registry.
Methods
Design and selection criteria
This is a retrospective registry-based analysis, approved by the EBMT acute leukemia working party (ALWP). The EBMT registry is a voluntary working group consisting of more than 600 transplant centers that are required to report annually, all consecutive stem cell transplantations and follow-ups. Audits are routinely performed to determine the accuracy of the data. All patients who proceeded to transplantation provided written informed consent for the use of their data for clinical research, in accordance with the local ethics committee and the modified Declaration of Helsinki.
Inclusion criteria were adult patients (>=18 years old) with T-ALL in first complete remission who underwent their first allo-HSCT between January 2010 and December 2021 using matched sibling donor (MSD), unrelated donor (UD), or haploidentical donors (haplo). Cord blood transplants and T LBL patients were excluded.
Parameters of interest
The measured outcomes of interest included leukemia-free survival (LFS), GVHD-free, and relapse-free survival (GRFS), OS, relapse incidence (RI), non-relapse mortality (NRM), acute GVHD (aGVHD) grades II-IV and III-IV, chronic GVHD (cGVHD), and extensive cGVHD. LFS was defined as survival without evidence of relapse or progression, with relapse defined as the reappearance of blasts in the blood or bone marrow (>5%) or any extra medullary site. NRM referred to death without evidence of relapse or progression. OS was defined as the probability of survival regardless of disease status. GRFS encompassed survival free from events including grade III-IV aGVHD, extensive cGVHD, relapse, or death. All the outcomes have been censored at last follow-up.
Statistical analyses
Standard demographic and transplant-related characteristics were summarized using median and range for continuous variables, and frequency and percentage for categorical variables. Associations between variables were assessed using appropriate statistical tests, such as Fisher’s exact test, χ² test, or Mann–Whitney test.
To estimate probabilities of OS, LFS, and GRFS, Kaplan-Meier estimation was employed. Cumulative incidence was used to estimate the endpoints of NRM, RI, aGVHD, and cGVHD, considering competing risks. RI and NRM were mutually competing events. Relapse and death were competing events for GVHD related outcomes. Proportional-hazards Cox regression was used to estimate hazard ratios (HR) and their corresponding 95% confidence intervals (CI). All statistical analyses were conducted using a two-sided α level of 0.05. All statistical analyses were performed with R 4.3.2 software packages.
Results
Patient characteristics
In this study, a total of 1907 patients met the inclusion criteria (Table 1). The median age at transplant was 33.4 years (18.1–75). Median time between the diagnosis of T-ALL and allo-HSCT was 5.9 months (1–23.8) (Table 1).
The majority (70.2%) of patients were male. In terms of cytomegalovirus (CMV) status, 66.8% were CMV-positive at the time of the transplant. Additionally, a substantial proportion (80.4%) of patients had a good functional status, as indicated by a Karnofsky performance score of 90 or higher.
Similarly, 64.4% of the donors were male with 23.4% of the transplants involving a female donor to a male patient. In terms of CMV status, 57% of the donors were CMV-positive at the time of transplant. The donor types were diverse, with the majority being MSD (45.4%), followed by MUD (31.9%), Haplo (11.8%), and MMUD (10.9%).
The most common (84%) stem cell source was PBSC, with the remainder (16%) being sourced from bone marrow. Most patients (86.2%) underwent a myeloablative conditioning treatment, with TBI-based regimens being the most common (68.7%). In terms of GVHD prophylaxis, a significant portion of patients received cyclosporine in combination with either methotrexate (54.4%) or mycophenolate mofetil (16.1%). Only 15.8% of the patients received post-transplant cyclophosphamide (PTCy). Additionally, while most of the transplants did not undergo in vitro T- cell depletion, a considerable proportion (44.3%) were treated with in vivo T-cell depletion, primarily using anti-thymocyte globulin (ATG) or alemtuzumab (CAMPATH-1H).
Patient outcomes following allogeneic hematopoietic stem cell transplantation
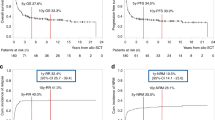
The median follow-up duration was 2.9 years (95% CI: 2.6–3.1). The 2-year OS was 69.4% (95% CI: 66.9–71.7), while the leukemia-free survival (LFS) was 62.1% (95% CI: 59.5–64.6) (Fig. 1, Table 2). The RI was 25.6% (95% CI: 23.3–27.9), and the NRM was 12.3% (95% CI:
10.7–14). The 2-year GFRS was 45.3% (95% CI: 42.7–47.9). Figure 1.
The incidence of aGVHD grades II-IV at 100 days was 32.6% (95% CI: 30.3–34.8), while the incidence of aGVHD grades III-IV at 100 days was 10% (95% CI: 8.6–11.6).
The 2-year cumulative incidence of cGVHD was 37.3% (95% CI: 34.8–39.9), and the cumulative incidence of extensive cGVHD was 16.8% (95% CI: 14.8–18.9) (Table 2).
Multivariate analysis: impact of prognostic factors on post-transplant outcomes
Overall survival and Leukemia free survival
The two years OS and LFS for patients younger than the median age of 33.4 years were 73% [69.6–76.2] vs. 65.8% [62.2–69.2] and 66% [62.4–69.4] vs. 58.1% [54.4–61.7], respectively. For every 10-year increase in age at allo-HSCT, there was a corresponding hazard ratio (HR) of 1.12 (p = 0.002) for OS and HR of 1.11 (p = 0.004) for LFS. (Table 3).
For patient who had undergone TBI based treatment the OS at 2 years was 71% vs 65.8% and LFS 64.8% vs 56.2% respectively. The use of TBI as part of the conditioning regimen was associated with better LFS outcome where the HR was 0.79 (p = 0.02) (Table 3).
For patients who had received Female to Male donation transplants the 2 years OS was 66% vs 70.5% and have a negative impact on OS (HR of 1.23 [p = 0.048]). Finally, 3-year increment in the year period of allo-HSCT was associated with a HR of 0.9 (p = 0.02) for OS. Figure 2.
Disease relapse incidence
The two years RI for use of TBI was 22.5% vs 32.3%. Use of TBI as part of the conditioning regimen was a factor significantly associated with lower RI with an HR of 0.65 (p < 0.001) (Table 3).
Non relapse mortality
The two years NRM for patients younger than the median age of 33.4 years was 9% [7.1–11.2] vs. 15.5% [13–18.2]. For each 10-year increase in age at allo-HSCT, there was a notable rise in the HR of 1.23 (p < 0.001) for NRM (Table 3).
The year period of allo-HSCT was also found to be a significant predictor for NRM. Each 3-year increment in the year period of allo-HSCT was associated with a HR of 0.82 (p = 0.008) Figure 2.
Graft versus host disease AGVHD
For patients transplanted between (2010 and 2016) compared to those transplanted between (2017 and 2020) the 100 days cumulative incidence of aGVHD grade II-IV 35.2% [32.1–38.2] vs
29.1% [25.8–32.5], 2 years cGVHD 39.7% [36.3–43] vs. 34.4% [30.4–38.5], and of 2 years extensive cGVHD 18.6% [16-21.4] vs. 14.4% [11.5-17.7]. The year period of allo-HSCT was also found to be a significant predictor for several outcomes. Each 3-year increment in the year period of allo-HSCT was associated with a HR of 0.83 (p = 0.001) for aGVHD and a HR of 0.8 (p < 0.001) for cGVHD. Figure 2.
For patients receiving a graft from a female donor the 2 years incidence of cGVHD compared to those with a male donor was 42.3% [36.8-47.7] vs. 35.8% [32.9–38.8]. Female to Male donation transplants had a negative impact on the development of both global (HRs of 1.39 (p = 0.002)) and extensive cGVHD (HR 1.47 (p < 0.01)) (Table 3).
The use of in vivo T-cell depletion showed a 100 days incidence of aGVHD grade III-IV 8.3% [6.5–10.5] vs. 11.2% [9.2–13.4], 2 years cumulative incidence of cGVHD 29.7% [26.1–33.4] vs. 43.4% [39.7–46.9], and 2 years cumulative incidence of extensive cGVHD 10.1% [7.9–12.7] vs. 21.9% [18.9–25.1]. The use of in vivo T-cell depletion improved aGVHD grade III-IV (HR of 0.54 (p = 0.005)), cGVHD (HR 0.51 (p < 0.001)) and extensive cGVHD (HR of 0.36 (p < 0.001)), indicating a potential reduction in the risk of developing acute and extensive cGVHD (Table 3).
Factors, such as type of donor, were significantly associated with GVHD outcomes. For aGVHD grade II-IV, MUD, Haplo, and MMUD transplants yielded significant HRs of 1.5 (p = 0.02), 1.73 (p < 0.001), and 1.87 (p < 0.001), respectively. In the case of aGVHD grade III-IV, similarly, significant HRs were observed for MUD, Haplo, and MMUD transplants, with HRs of 2.04 (p = 0.01), 1.83 (p = 0.01), and 2.41 (p = 0.003), respectively (Table 3). These findings highlight the potential influence of donor type on the occurrence and severity of aGVHD.
GRFS
For patients transplanted before 2017 the 2 years GFRS was 43.1% [39.8–46.4] vs 48.2% [43.9–52.4]. Each 3-year increment in the year period of allo-HSCT was associated with a HR of 0.89 (p < 0.001) for GRFS. Figure 2.
The use of in vivo T-cell depletion showed a 2 years incidence of GRFS 49.7% [45.8–53.5] vs. 42.2% [38.6–45.7]. The use of in vivo T-cell depletion improved GFRS (HR of 0.74 (p = 0.001)) (Table 3). The 2 years GRFS for patients receiving TBI was 46.9% vs 41.7%, the use of TBI as part of the conditioning regimen was associated with better GFRS outcomes where the HR was 0.83 (p = 0.04). Female to Male donation transplants had a negative impact on GRFS (HR of 1.21 (p = 0.02)). Advanced age at transplant is associated with poorer outcomes in terms of GRFS. Specifically, for every 10-year increase in age at allo-HSCT, there was a corresponding HR of 1.06 (p = 0.04) for GRFS (Table 3).
Discussion
In this EBMT registry-based study we compared outcomes over time of allo-HSCT for adult patients with T-ALL in CR1, using different conditioning regimens and different types of donor. We showed a significant improvement over time in term of GFRS and the cumulative incidence of global and extensive cGVHD. We noticed over time better NRM, aGVHD, cGVHD, GRFS and OS. Furthermore, our study demonstrated that advanced age at transplant is associated with poorer outcomes in terms of NRM, LFS, GRFS, and OS.
Over the past few years, significant advancements have been made in the management of patients diagnosed with B-ALL, encompassing both Philadelphia chromosome-positive (Ph + ) and Philadelphia chromosome-negative (Ph-) variants [14]. These notable advances in patient care can be attributed to the recent approval and integration of innovative therapeutic modalities such as monoclonal or bispecific antibodies, exemplified by inotuzumab ozogamicin, and blinatumomab [15], chimeric antigen receptor T-cell (CAR-T) therapy, and new-generation tyrosine kinase inhibitors [16]. On the other hand, targeted therapies in T-ALL is still lacking. Immunotherapeutic modalities are currently the subject of active investigation within the context of T-ALL, wherein specific antigens, notably CD5, CD7, and CD38, have emerged as prospective targets owing to their demonstrable expression patterns [17]. Nevertheless, the use of allo-HSCT as primary therapeutic intervention for adult patients with T-ALL remains a subject of deliberation, particularly in light of the ongoing endeavors to enhance outcomes achieved through conventional-dose chemotherapy [18].
TBI is considered as the backbone for conditioning in ALL. In our study, most patients (86.2%) underwent myeloablative conditioning treatment with TBI-based regimens. The use of TBI was associated with better LFS and GFRS outcomes, as well as reduced RI. Unfortunately, in our study, we did not have specific data related to the exact dose of TBI, due to its antileukemic activity which is dose-dependent and therefore the maximum tolerated dose should be preferentially used [19]. According to the survey performed among the EBMT centers, the total dose of 12 Gy is the most commonly used [20]. Clinical practice varies among centers with regard to many technical aspects of TBI including dose rate, organ shielding and methods of patient immobilization that may affect both safety and efficacy of the treatment [21, 22].
Our multivariate analysis revealed the role of other potentially modifiable factors such as donor type, such as female donors to male recipients, which had a negative impact on GRFS, OS, and the development of both global and extensive cGVHD. The use of in vivo T-cell depletion had potential benefits in terms of GFRS, aGVHD grade III-IV, cGVHD and extensive cGVHD indicating a potential reduction in the risk of developing acute and extensive cGVHD.
These findings correspond well with a recent report showing inferior outcomes when using PBSC as compared to bone marrow in allo-HSCT from haploidentical donors for patients with ALL [23]. Increased risk of cGVHD when using PBSC as source of stem cells may be diminished by administration of ATG as part of the conditioning regimen [24]. Indeed, the use of ATG is recommended in both UD and MSD [24]. Interestingly, recent results of retrospective analyses focusing separately on patients with both Ph+ and Ph- ALL demonstrated an increased risk of relapse when using ATG [25,26,27], but these findings were not confirmed in our study showing no increase in the risk of relapse but lower incidence in terms of acute and chronic GVHD among patients treated with in vivo T-cell depletion.
Other transplant characteristics, such as the use of Haplo and MMUD were associated with high risk of aGVHD grade II-IV but not different incidence in term of aGVHD grade III-IV compared to MSD and MUD. There is no increased risk of NRM, or reduced risk of relapse, which suggests a more effective Graft versus Leukemia reaction when using UD. These findings highlight the potential influence of donor type on the occurrence and severity of aGVHD. This observation corroborates well with previous reports published by our group [28] for adult patients treated between 1993 and 2012 with myeloablative allo-HSCT from MSD and MUD in CR1. Unfortunately, the results of this study cannot be translated to the Haplo setting.
Our study has some important limitations related to its retrospective and data registry nature. Limitations also include the heterogeneity of the conditioning regimen type and intensity, the dose of TBI, and the different types of donors. Also, data on minimal residual disease before transplantation were not available, which did not allow inclusion of this variable in multivariate analyses. Nevertheless, we believe that our findings highlighting the role of the intensity of conditioning regimens in adults with T-ALL referred for allo-HCT may be of clinical importance.
Conclusion
This large study has identified prognostic factors such as age at transplant, donor type, and conditioning regimen, in influencing key outcomes including OS, LFS, GVHD incidence, and NRM in adult T-ALL patients undergoing allo-HSCT. Importantly, a significant improvement over time in post-transplant outcomes was noted. These findings hold great promise for new adapted treatment strategies and can serve as a benchmark for future studies in that setting.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Raetz EA, Teachey DT. T-cell acute lymphoblastic leukemia. Hematol Am Soc Hematol Educ Program. 2016;2016:580–8.
Chiaretti S, Zini G, Bassan R. Diagnosis and subclassification of acute lymphoblastic leukemia. Mediterr J Hematol Infect Dis. 2014;6:e2014073.
Westbrook CA. Molecular subsets and prognostic factors in acute lymphoblastic leukemia. Leukemia 1997;11:S8–10.
Novel immunotherapies for adult patients with B-lineage acute lymphoblastic leukemia - PubMed [Internet]. [cited 2023 May 27]. Available from: https://pubmed.ncbi.nlm.nih.gov/28821272/
Trinquand A, Tanguy-Schmidt A, Ben Abdelali R, Lambert J, Beldjord K, Lengliné E, et al. Toward a NOTCH1/FBXW7/RAS/PTEN-based oncogenetic risk classification of adult T-cell acute lymphoblastic leukemia: a Group for Research in Adult Acute Lymphoblastic Leukemia study. J Clin Oncol J Am Soc Clin Oncol. 2013;31:4333–42.
Bond J, Graux C, Lhermitte L, Lara D, Cluzeau T, Leguay T, et al. Early response-based therapy stratification improves survival in adult early thymic precursor acute lymphoblastic leukemia: a group for research on adult acute lymphoblastic leukemia study. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35:2683–91.
Short NJ, Kantarjian H, Jabbour E. Optimizing the treatment of acute lymphoblastic leukemia in younger and older adults: new drugs and evolving paradigms. Leukemia 2021;35:3044–58.
Socié G, Stone JV, Wingard JR, Weisdorf D, Henslee-Downey PJ, Bredeson C, et al. Long- term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341:14–21.
Bazarbachi A, Boumendil A, Finel H, Castagna L, Dominietto A, Blaise D, et al. Influence of donor type, stem cell source and conditioning on outcomes after haploidentical transplant for lymphoma - a LWP-EBMT study. Br J Haematol. 2020;188:745–56.
El-Cheikh J, El Dika I, Massoud R, Charafeddine M, Mahfouz R, Kharfan-Dabaja MA, et al. Hyper-CVAD compared with BFM-like chemotherapy for the treatment of adult acute lymphoblastic leukemia. a retrospective single-center analysis. Clin Lymphoma Myeloma Leuk. 2017;17:179–85.
Uzunel M, Mattsson J, Jaksch M, Remberger M, Ringdén O. The significance of graft- versus-host disease and pretransplantation minimal residual disease status to outcome after allogeneic stem cell transplantation in patients with acute lymphoblastic leukemia. Blood. 2001;98:1982–4.
Champlin R, Gale RP. Acute lymphoblastic leukemia: recent advances in biology and therapy. Blood. 1989;73:2051–66.
Lee S, Kim D-W, Cho B, Kim Y-J, Kim Y-L, Hwang J-Y, et al. Risk factors for adults with Philadelphia-chromosome-positive acute lymphoblastic leukaemia in remission treated with allogeneic bone marrow transplantation: the potential of real-time quantitative reverse- transcription polymerase chain reaction. Br J Haematol. 2003;120:145–53.
Hefazi M, Litzow MR. Recent advances in the biology and treatment of B-cell acute lymphoblastic leukemia. Blood Lymphat Cancer Targets Ther. 2018;8:47–61.
Fracchiolla NS, Sciumè M, Papayannidis C, Vitale A, Chiaretti S, Annunziata M, et al. Blinatumomab and inotuzumab ozogamicin sequential use for the treatment of relapsed/refractory acute lymphoblastic leukemia: a real-life campus all study. Cancers. 2023;15:4623.
Malczewska M, Kośmider K, Bednarz K, Ostapińska K, Lejman M, Zawitkowska J. Recent advances in treatment options for childhood acute lymphoblastic leukemia. Cancers 2022;14:2021.
Leong S, Inglott S, Papaleonidopoulou F, Orfinada K, Ancliff P, Bartram J, et al. CD1a is rarely expressed in pediatric or adult relapsed/refractory T-ALL: implications for immunotherapy. Blood Adv. 2020;4:4665–8.
Sun W, Huang X. Role of allogeneic haematopoietic stem cell transplantation in the treatment of adult acute lymphoblastic leukaemia in the era of immunotherapy. Chin Med J (Engl). 2022;135:890–900.
Corvò R, Lamparelli T, Bruno B, Barra S, Van Lint MT, Vitale V, et al. Low-dose fractionated total body irradiation (TBI) adversely affects prognosis of patients with leukemia receiving an HLA-matched allogeneic bone marrow transplant from an unrelated donor (UD- BMT). Bone Marrow Transpl. 2002;30:717–23.
Giebel S, Miszczyk L, Slosarek K, Moukhtari L, Ciceri F, Esteve J, et al. Extreme heterogeneity of myeloablative total body irradiation techniques in clinical practice: a survey of the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer. 2014;120:2760–5.
Belkacemi Y, Labopin M, Giebel S, Loganadane G, Miszczyk L, Michallet M, et al. Single-dose daily fractionation is not inferior to twice-a-day fractionated total-body irradiation before allogeneic stem cell transplantation for acute leukemia: a useful practice simplification resulting from the SARASIN study. Int J Radiat Oncol Biol Phys. 2018;102:515–26.
Gao RW, Weisdorf DJ, DeFor TE, Ehler E, Dusenbery KE. Influence of total body irradiation dose rate on idiopathic pneumonia syndrome in acute leukemia patients undergoing allogeneic hematopoietic cell transplantation. Int J Radiat Oncol Biol Phys. 2019;103:180–9.
Nagler A, Dholaria B, Labopin M, Savani BN, Angelucci E, Koc Y, et al. Bone marrow versus mobilized peripheral blood stem cell graft in T-cell-replete haploidentical transplantation in acute lymphoblastic leukemia. Leukemia. 2020;34:2766–75.
Baron F, Mohty M, Blaise D, Socié G, Labopin M, Esteve J, et al. Anti-thymocyte globulin as graft-versus-host disease prevention in the setting of allogeneic peripheral blood stem cell transplantation: a review from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2017;102:224–34.
Li S-Q, Fan Q-Z, Xu L-P, Wang Y, Zhang X-H, Chen H, et al. Different effects of pre- transplantation measurable residual disease on outcomes according to transplant modality in patients with philadelphia chromosome positive ALL. Front Oncol. 2020;10:320.
Giebel S, Labopin M, Czerw T, Socié G, Blaise D, Ghavamzadeh A, et al. Impact of anti- thymocyte globulin on results of allogeneic peripheral blood stem cell transplantation for patients with Philadelphia-positive acute lymphoblastic leukaemia: an analysis by the Acute Leukemia Working Party of the EBMT. Eur J Cancer. 2019;106:212–9.
Czerw T, Labopin M, Giebel S, Socié G, Volin L, Fegueux N, et al. Anti-thymocyte globulin improves survival free from relapse and graft-versus-host disease after allogeneic peripheral blood stem cell transplantation in patients with Philadelphia-negative acute lymphoblastic leukemia: An analysis by the Acute Leukemia Working Party of the EBMT. Cancer. 2018;124:2523–33.
Giebel S, Labopin M, Socié G, Beelen D, Browne P, Volin L, et al. Improving results of allogeneic hematopoietic cell transplantation for adults with acute lymphoblastic leukemia in first complete remission: an analysis from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2017;102:139–49.
Author information
Authors and Affiliations
Contributions
Jean El Cheikh designed the protocol, wrote the protocol and report, conducted the search, interpreted results, updated reference lists, and wrote the manuscript. Maud Ngoya and Jacques-Emmanuel Galimard, extracted and analyzed data. Péter Reményi, Alexander Kulagin, Mahmoud Aljurf, Ashrafsadat Mousavi, Depei Wu, Tulay Ozcelik, Urpu Salmenniemi, Cristina Castilla-Llorente, Gerard Socie, Grzegorz Helbig, Thomas Schroeder, Ioanna Sakellari, Alessandro Rambaldi, Richard Burt, Alessandro Busca, Marie Balsat, Matthias Stelljes, Eolia Brissot, Sebastien Giebel, Zinaida Peric, Arnon Nagler, Ali Bazarbachi, and Fabio Ciceri contributed to the design of the review protocol, writing the report, and providing feedback. Mohamad Mohty was responsible for supervising, following up, and providing feedback.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El Cheikh, J., Ngoya, M., Galimard, JE. et al. Prognostic factors impacting post-transplant outcomes in adult T-cell acute lymphoblastic leukemia: a registry-based study by the EBMT acute leukemia working party. Bone Marrow Transplant 59, 1239–1246 (2024). https://doi.org/10.1038/s41409-024-02300-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-024-02300-8
- Springer Nature Limited