Abstract
Relapse is a significant barrier to allogeneic hematopoietic stem cell transplantation (allo-HSCT) success. To explore the prognosis of patients who underwent relapse after allo-HSCT, we retrospectively examined 740 consecutive acute leukemia patients in our single center transplanted between January 2013 and December 2018, of which 178 relapsed. The median survival was 204 days (95%CI, 160.7–247.3) from relapse, and the 3-year post-relapse overall survival (prOS) rate was 17.8% (95%CI, 12.5–25.3%). Overall complete remission (CR) or CR with incomplete hematologic recovery (CRi) was achieved in 32.1% for the acute myeloid leukemia and 45.3% for acute lymphoblastic leukemia patients after salvage therapy, respectively. Grade III-IV acute graft-versus-host disease (GVHD) after transplantation and >20% bone marrow blasts at relapse were associated with worse prOS, while patients with chronic GVHD after transplantation, relapse later than 1 year after transplantation, and solitary extramedullary disease had better prOS. Therefore, we developed a concise risk scoring system for prOS based on the number of risk factors affecting prOS. This scoring system was validated with another cohort of post-transplant relapsed acute leukemia patients who received allo-HSCT between 2019 and 2020. Identifying relapse risk factors and providing personalized care for patients with poor prognoses is crucial for improving survival.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the most effective treatment for acute leukemia (AL). The overall effectiveness of allo-HSCT has been continuously promoted and the non-relapse mortality (NRM) has significantly decreased in recent years as a result of advancements in graft manipulation, conditioning regimens, and pharmacological graft-versus-host disease (GVHD) prophylaxis [1]. However, relapse after hematopoietic stem cell transplantation (HSCT) remains the most important factor limiting the success of HSCT, with an incidence as high as 30–40% [2]. Post-transplant relapse has surpassed infection, organ toxicity, and GVHD as the leading cause of post-transplant death. According to an analysis of data collected by the Center for International Blood and Marrow Transplant Research (CIBMTR) in 2018, the proportion of patients who died within 100 days and after 100 days after allogeneic transplantation due to relapse was 27% and 61%, respectively [3].
Withdrawing immunosuppressants, salvage chemotherapy, donor lymphocyte infusion (DLI), and secondary transplantation are all common therapeutic options; yet, their effects are still concerning, and the survival rates of patients who relapse after transplantation are incredibly low. Most patients died within 1 year after relapse. New approaches have progressively evolved, including monoclonal antibodies, chimeric antigen receptor T cells (CART) therapy, small molecule targeted medicines, demethylation medications, and immune checkpoint inhibitors [4]. However, there is no universal standard treatment for relapse due to factors such as disease heterogeneity.
Determining the prognostic factors influencing post-relapse survival, identifying the population with a poor prognosis, and exploring secure and efficient therapeutic options for relapse are therefore of utmost importance. This study screened out the population of AL patients who received allo-HSCT from January 2013 to December 2018, evaluated the data of AL patients who relapsed after transplantation, and reported the clinical outcomes of relapsed patients. By examining disease characteristics, transplantation parameters, relapse characteristics, and treatment options, we aimed to investigate the prognostic factors affecting post-relapse overall survival (prOS), post-relapse disease-free survival (prDFS), and post-relapse non-relapse mortality (prNRM). Thus, a concise risk scoring system for prOS was established, and the validity of this scoring system was further confirmed in relapsed patients transplanted between January 1, 2019, and December 31, 2020 (validation group).
Methods
Patients
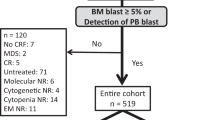
We conducted a retrospective review of 740 consecutive patients diagnosed with AL who underwent allo-HSCT at the Bone Marrow Transplant Center of the First Affiliated Hospital of Zhejiang University School of Medicine between January 1, 2013, and December 31, 2018. The inclusion criteria were: (1) diagnosed with AL; (2) age ≥18 years; (3) received their first allo-HSCT; (4) hematologic and/or extramedullary relapse after transplantation. Of the total patients, 178 were ultimately included in this study, with the last follow-up conducted on June 30, 2021. To validate the post-relapse risk score, we utilized a second cohort of 333 patients who underwent transplantation between January 1, 2019, and December 31, 2020, 54 of whom experienced relapse (Fig. 1). Characteristics of relapsed patients including pretransplant characteristics, transplant characteristics, relapse characteristics and post-relapse treatments were summarized in Table 1. The study protocol complied with the Declaration of Helsinki and was approved by the Ethics Review Committees of the First Affiliated Hospital of Zhejiang University. Written informed consent was obtained from each patient. The authors had full access to the data and assume responsibility for its authenticity.
Transplant regimens
Of all 178 analyzed patients, 174 received myeloablative conditioning (MAC) regimen and 4 patients received reduced-intensity conditioning (RIC) regimen. The MAC regimen consisted of Cytarabine (Ara-C 4 g/m2/day, intravenous infusion, –10 to –9 days), Busulfan (Bu 3.2 mg/kg/day, intravenous infusion, –8 to –6 days), Cyclophosphamide (Cy 1.8 g/m2/day, intravenous infusion, –5 to –4 days), and Methyl-N-(2-chloroethyl)-N-cyclohexyl-N-nitrosourea (Me-CCNU 250 mg/m2, orally, –3 days). Patients undergoing haploidentical-HSCT (haplo-HSCT) and unrelated HSCT received an in vivo T cell depletion regimen before transplantation, specifically: haplo-HSCT patients received anti-thymocyte globulin ATG-F (Grafalon, 2.5 mg/kg/day, intravenous infusion, –5 to –2 days) or ATG-G (Genzyme, 1.5 mg/kg/day, intravenous infusion, –5 to –2 days); unrelated HSCT patients received ATG-G (1.5 mg/kg/day intravenous infusion, -5 or -4 to -2 days). The RIC regimen consists of Fludarabine (Flu 30 mg/m2/day, intravenous, –10 to –5 days), Busulfan (Bu 3.2 mg/kg/day, intravenous, –5 to –6 days), and ATG-F (Grafalon, 5 mg/kg/day, intravenous, –4 to –1 days). All patients received peripheral blood stem cell transplantation mobilized by recombinant human granulocyte-colony stimulating factor (G-CSF) (donors used G-CSF 7.5-10 μg/ (kg × d) continuous subcutaneous injection for 4–5 days and then collected peripheral blood stem cells). GVHD prophylaxis regimen consisted of cyclosporine, short-course methotrexate, and mycophenolate mofetil.
Treatment for relapse post-transplantation
Relapsed patients should first withdraw immunosuppressive agents according to the GVHD situation, and then select optimal treatment that may be effective for the patient according to the pre-transplant treatment and disease characteristics, such as chemotherapy, DLI, targeted therapy and immunotherapy, and some patients were infused with donor stem cells that were cryopreserved at the time of transplantation.
Definitions and assessments
According to the Disease Risk Index (DRI), patients were categorized as low/intermediate risk or high/very high risk [5]. Relapse was defined as the re-emergence of leukemic cells in peripheral blood, blasts in bone marrow (BM) ≥ 5%, and/or extramedullary infiltration in patients once achieving complete remission (CR) after transplantation [6]. Patients who showed only measurable residual disease (MRD) were not defined as relapse. Early relapse was defined as relapse within 1 year after allo-HSCT and late relapse was defined as relapse beyond 1 year after allo-HSCT. CR or CR with incomplete hematologic recovery (CRi) are defined as per 2017 ELN AML recommendations.
The diagnosis of acute GVHD (aGVHD) and chronic GVHD (cGVHD) is based on the 2016 Mount Sinai Acute GVHD International Consortium guidelines and the 2014 National Institutes of Health (NIH) consensus [7, 8]. prDFS was defined as the time from the first CR/CRi after relapse to relapse or death. prOS was defined as the time from relapse to death from any cause. prNRM was defined as relapsed patients whose primary disease was in CR/CRi and who died from complications such as lung infection, cerebral bleeding during the low-cell phase after therapy, or GVHD. Post-relapse aGVHD (praGVHD) and cGVHD (prcGVHD) were defined as aGVHD or cGVHD onset post-relapse.
Statistical analysis
Categorical variables were reported as counts and percentages, and continuous variables as medians and ranges. Group comparisons of CR/CRi were conducted using chi-square and Fisher’s exact tests, while Poisson regression was employed for multivariate analysis. Competing risk analysis was used to calculate the cumulative incidence of prNRM, and Gray’s test was used to compare the differences between the two groups. The prOS and prDFS were estimated using the Kaplan-Meier method, and the log-rank test. Variables with P < 0.05 in univariate analysis and variables that might be clinically meaningful were entered into Cox proportional hazards regression model for multivariate analyses. All statistical analyses were performed using SPSS 26.0 and R 4.1.2 (http://www.r-project.org).
Results
Patient characteristics
One hundred and seventy-eight patients had relapse after transplantation. The median (range) duration from transplantation to relapse was 259 days (18-2130). In all relapsed individuals, 81 patients (45.5%) were acute myeloid leukemia (AML), 95 patients (53.4%) were acute lymphoblastic leukemia (ALL), and 2 patients (1.1%) were acute mixed-lineage leukemia (AMLL). The median (range) days to relapse was 312 (18-2130) in AML, 229 (45-1729) in ALL, and 154 (119-189) in AMLL. The parameters of patients were summarized in Table 1.
Clinical response to salvage therapy
After receiving salvage therapies, 69 out of 178 relapsed patients (38.3%) attained CR/CRi. The median duration of remission was 154 days (range: 22–2564).The cumulative incidence of CR/CRi at 30 days and 1 year was 12.9% (95%CI, 7.9–17.7%) and 38.3% (95%CI, 30.6–44.9%), respectively.
In AML patients, 26 out of 81 (32.1%) achieved CR/CRi, while in ALL patients, 43 out of 95 (45.3%) achieved CR/CRi; however, none of the patients with AMLL achieved CR/CRi after salvage therapy. The median remission durations were 238 days (range: 34–1511) and 153 days (range: 22–2564) for AML and ALL, respectively. Patients achieved CR/CRi after relapse by receiving chemotherapy combined with DLI (n = 42), chemotherapy alone (n = 12), chimeric antigen receptor T-cell therapy (CART) (n = 6), and secondary transplantation (n = 4).
Univariate analysis in the relapsed AML group revealed that patients with BM blasts ≤20% at relapse had a higher CR/CRi rate (P = 0.022). Moreover, receiving DLI after relapse was significantly associated with a higher CR/CRi (P = 0.013), as shown in Supplementary Table 1. BM blasts and DLI treatment were included in murltivariate analysis. Results of multivariate analysis showed that DLI treatment after relapse was related to better treatment response in AML patients (RR = 2.216, 95% CI, 1.054–4.657, P = 0.036).
Univariate analysis from ALL patients showed that CR/CRi rate was higher for patients with cGVHD history (P < 0.001), late relapse after allo-HSCT (P = 0.005), and BM blast cells ≤20% at relapse (P = 0.026). Also, CR/CRi rates for patients with BM recurrence, solitary extramedullary disease (EMD) recurrence, and both BM and EMD involvement were 35.8%, 80.0%, and 36.4%, respectively (P = 0.002). (Supplementary Table 1). Factors above with P < 0.05 were included in murltivariate analysis. The results indicated a significant association between the post-transplantation cGVHD and higher CR/CRi rate in patients relapsed from ALL (RR = 1.660, 95% CI, 1.050–2.624, P = 0.030).
Post-relapse NRM
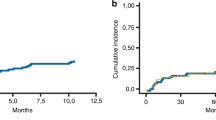
One hundred and thirty-nine of the 178 patients who experienced relapse eventually died. Among them, 3.9% (7/178) died while in CR/CRi. The cumulative prNRM at 1-year, 3-year and 5-year was 2.5%, 3.8, and 5.1%, respectively (Fig. 2). The cumulative relapse-related mortality at 1-year, 3-year and 5-year was 65.0%, 78.9% and 84.7%, respectively (Fig. 2). The causes of death were infectious diseases (n = 5), severe pulmonary rejection (n = 1), and another tumor (tongue cancer) (n = 1). Univariate analysis indicated that praGVHD (P = 0.032) and prcGVHD (P = 0.003) were associated with increased prNRM.
Post-relapse DFS
After salvage therapy, 69 out of 178 relapsed patients (38.3%) achieved CR/CRi, with a median prDFS of 197 days (95%CI, 101.7–292.3). The prDFS rates at 1-, 2-, and 3-years were 37.7% (95%CI, 27.0–52.5%), 28.0% (95%CI, 18.3–42.5%), and 25.6% (95%CI, 16.2–40.5%), respectively. Patients with cGVHD after transplantation had a significantly longer median prDFS of 440 days (95%CI, 0.0–1653.5), compared to patients without cGVHD, whose median prDFS was 155 days (95%CI, 138.5–171.5) (P = 0.020). On the contrary, patients with praGVHD had a shorter median prDFS of 153 days (95%CI, 98.4–207.6) compared to those without praGVHD, whose median prDFS was 322 days (95%CI, 63.5–580.5) (P = 0.038). In addition, status at HSCT, presence or absence of preemptive DLI, relapse site, and whether or not receiving DLI after relapse also affected prDFS (Table 2). Factors mentioned above were included in Cox analysis. The Cox multivariate analysis demonstrated a significant relationship between cGVHD following transplantation and improved prDFS (HR = 0.483, 95%CI, 0.243–0.962, P = 0.038) (Fig. 3a).
Among patients with AML (n = 26, Table 2), those who received a transplant from an unrelated donor (URD) had median prDFS of 77.0 days (95%CI, 69.2–84.8), worse than those from related donors (P = 0.024). Patients with solitary EMD had better prDFS (P = 0.030). Furthermore, patients who received preemptive DLI had significantly lower median prDFS compared to those who did not (110.0 days, 95%CI, 33.3–186.7 vs 308.0 days, 95%CI, 0.0–623.6, P = 0.004). There were no independent factors affecting prDFS in the results of multivariate analysis.
Upon analyzing relapsed ALL patients (n = 43, Table 2), we observed that pre-transplant CRn (n > 1) patients (P = 0.005) and patients in the high and very high-risk group had a worse prDFS (P = 0.048). In contrast, patients with cGVHD after transplantation (P = 0.024), solitary EMD (P = 0.018) and BM blast cells ≤20% at relapse (P = 0.039) had a better prDFS. Furthermore, patients who experienced grade III-IV aGVHD after transplantation had a significantly lower prDFS (30.0 days, 95%CI, 17.2–42.8) compared to those with grade 0-II aGVHD (197.0 days, 95%CI, 0.0–412.2) (P < 0.001). Multivariate analysis including factors above showed that the occurrence of grade III-IV aGVHD after transplantation was an independent factor for prDFS (HR = 67.949, 95%CI, 6.568–702.918, P < 0.001).
Post-relapse OS
After relapse, the median survival time of 178 patients was 204 days (95%CI, 160.7–247.3), with follow-up data available up to June 30, 2021. Of these patients, 39 (21.9%) survived at the last follow-up with a median (range) follow-up of 185 days (3–2633) following recurrence. The prOS at 1 year was 31.9% (95%CI, 25.5–40.0%), decreasing to 17.8% (95%CI, 12.5–25.3%) at 3 years and 10.5% (95%CI, 5.7–19.4%) at 5 years.
Univariate analysis (Table 2) revealed that the grade of aGVHD after HSCT was strongly associated with prOS (P = 0.004), with median survival time of 92 days (95%CI, 29.3-154.7) and 217days (95%CI, 182.2–251.8) for grade III-IV aGVHD and none-II aGVHD, respectively. Conversely, patients who developed cGVHD after HSCT had better prOS (median: 506 days, 95%CI, 0.0-1364.7) compared to cGVHD-free individuals (median: 169 days, 95%CI, 118.7–220, P < 0.001). Patients who had early relapse had lower prOS (median: 164 days, 95%CI, 111.4–216.6) compared to those who had late relapse (median: 302 days, 95%CI, 177.7–426.3, P = 0.028). The median prOS for patients with BM blast cells ≤20% and >20% at relapse was 358 days (95%CI, 160.0–556.0) and 132 days (95%CI, 92.5–171.5), respectively (P = 0.001). Patients with solitary EMD relapse had better prOS (not reached) than those with BM relapse (median: 139 days, 95%CI, 105.6–172.4) or both BM and EMD involvement (median: 204 days, 95%CI, 160.7–247.3) (P < 0.001). Of note, 63.2% of patients with solitary EMD relapse were still alive. Patients with CRn (n > 1) at HSCT (P = 0.033), with the high and very high risk group (P = 0.010) and with preemptive DLI had a worse prOS (P = 0.045).
Factors with P < 0.05 in univariate analysis were subjected to multivariate analysis. The results showed that grade III-IV aGVHD (HR = 2.091, 95%CI, 1.180–3.705, P = 0.012) and BM blasts at relapse >20% (HR = 2.509, 95%CI, 1.707–3.688, P < 0.001) were independently associated with inferior prOS. Conversely, cGVHD (HR = 0.412, 95% CI, 0.262–0.647, P < 0.001), late relapse (HR = 0.682, 95%CI, 0.467–0.998, P = 0.049), and relapse not involving the BM (HR = 0.792, 95%CI, 0.639–0.981, P = 0.033) were associated with superior prOS (Fig. 3b).
The median prOS of patients who underwent different treatment modalities following relapse was compared. The median prOS for patients who received palliative care (n = 8), chemotherapy (n = 40), chemotherapy combined with DLI (n = 84), secondary transplantation (n = 7), and CART (n = 13) was 22 days, 184 days, 213 days, 393 days, and 345 days, respectively (P = 0.024, Fig. 4).
Prognostic factors exhibit variation across different disease types (Table 2). In patients with AML, the median prOS for those with solitary EMD was not reached. In contrast, the median prOS for isolated BM and both BM and EMD recurrence was 135.0 days (95%CI, 64.9–205.1 days) and 321.0 days (95%CI, 158.2–483.8 days), respectively (P = 0.002). Patients with BM blasts ≤20% and >20% at relapse had median prOS of 310.0 days (95%CI, 143.3–476.7) and 132.0 days (95%CI, 83.6–180.4), respectively (P = 0.001). Multivariate analysis revealed that the relapse site after transplantation (HR = 0.617, 95%CI, 0.439–0.866, P = 0.005) and the proportion of blasts at BM (HR = 2.731, 95%CI, 1.570–4.750, P < 0.001) were independent factors significantly affecting prOS.
Among patients with ALL, the median prOS was significantly shorter for patients who developed transplant-related III-IV aGVHD (93.0 days, 95%CI, 58.9–127.1) than those who did not (231.0 days, 95%CI, 185.0–277.0, P = 0.003). Patients who developed cGVHD after transplantation had a significantly longer median prOS (1305.0 days, 95% CI, 397.8–2212.2) than those who did not (150.0 days, 95% CI, 82.7–217.3, P < 0.001). Furthermore, the median prOS for patients with BM blasts ≤ 20% and > 20% at relapse were 476.0 days (95%CI, 0.0–1038.4) vs 145.0 days (95%CI, 95.2–194.8), respectively (P < 0.001). In addition, we observed that pre-transplant CRn (n > 1) patients (P = 0.046) and patients in the high and very high risk group (P = 0.031) had a worse prOS. On the contrary, patients with solitary EMD had a better prOS (P < 0.001) (Table 2). Multivariate analysis revealed that grade III-IV aGVHD after transplantation (HR = 2.816, 95%CI, 1.304–6.081, P = 0.008) and the BM blasts >20% at recurrence (HR = 2.818, 95%CI, 1.637–4.851, P < 0.001) were independent prognostic factors for inferior prOS; while cGVHD after transplantation (HR = 0.284, 95%CI, 0.140–0.574, P < 0.001) was an independent factor associated with improved prOS.
Prognostic score for survival after post-transplant relapse
Based on the presence of risk factors such as grade III-IV aGVHD after transplantation, no cGVHD after transplantation, early relapse, involvement of BM at relapse, and BM blasts >20% at relapse, three groups were formed for patients who relapsed after transplantation between 2013 to 2018: 0-2 risk factors (n = 51), 3 risk factors (n = 62), and 4–5 risk factors (n = 62). The 1-year prOS of the three groups were 61.7%, 29.4%, and 11.8%, respectively. The median survival time was 871 days (95%CI, 0.0–1997.7), 230 days (95%CI, 199.8–260.2), and 97 days (95%CI, 75.6–118.4) for the three groups, respectively (P < 0.001) (Table 3, Fig. 5a).
A total of 333 AL patients had allo-HSCT in our center from 2019 to 2020. The follow-up was conducted until April 1, 2022, and 54 patients experienced recurrence. Based on the number of risk factors, the relapsed patients were categorized into three groups: 0–2 risk factors (n = 15), 3 risk factors (n = 19), and 4–5 risk factors (n = 20). The 1-year prOS for these groups were 70.1%, 43.0% and 6.7%, respectively.
The median survival for each of the three groups was 479 days (95%CI, 316.3–641.7), 322 days (95%CI, 0.0–757.1), and 115 days (95%CI, 88.7–141.3), respectively, with statistically significant difference (P < 0.001) (Table 3, Fig. 5b).
Discussion
Allo-HSCT provides the possibility of curing malignant hematological diseases, which relies on high-dose conditioning and graft-versus-leukemia (GVL) effect to kill leukemia cells. The GVL is originated by donor immune cells to mount a furious allogeneic response and wipe out patient-derived leukemia cells [9]. Still, the most common cause of death following transplantation is relapse due to immunological evasion and clonal development of leukemia cells [10, 11].
According to the Seattle group’s research on 307 adult patients who relapsed after transplantation, 2-year survival rates ranged from 3 to 19% [12]. The European Society of Blood and Marrow Transplantation reported a 5-year prOS of 1% for 465 adults with ALL [13]. A retrospective registry analysis of 698 relapsed patients treated with different strategies showed a median survival of 4.7 months and a 2-year prOS of 17.7% [14]. Reports from the European Blood and Marrow Transplantation Acute Leukemia Working Group on 263 AML patients who relapsed after reduced-dose transplantation showed a 2-year prOS of 14%, comparable to standard conditioning [15]. In our center, the survival of patients who relapsed after transplantation is likewise subpar. Currently, haplo-HSCT with MAC is the predominant approach utilized in our center. However, in cases where relapse occurs, it is an indicator of a highly malignant disease with a poor prognosis. Our retrospective analysis examined the factors influencing the prognosis of patients with AL who experienced relapse after allo-HSCT. Our findings indicate that early vs late recurrence, recurrence site, tumor burden at recurrence, and occurrence of GVHD are significant predictors of prognosis. This study provides valuable insights for clinicians to quickly identify relapsed patients at high risk and implement prompt interventions and more efficacious treatment strategies.
According to our findings, the BM blast count during relapse is a crucial prognostic indicator. Patients with lower BM blast count at relapse had better survival. It is challenging to treat leukemia because the high BM blast cells at recurrence show that the illness is extremely malignant and the leukemia cells are in a highly proliferating state. In a study of 89 patients with AL who relapsed after transplantation, the 2-year progression-free survival rate of patients with lower (5-19%) and higher (≥20%) blasts before treatment was 28.0% vs 16.1% (P = 0.045), respectively; and the 2-year OS was 43.4% vs 25.1% (P = 0.041), respectively. Moreover, multivariate analysis showed that the BM blasts at initial diagnosis was an independent factor for progression-free survival [16]. The study by Christoph Schmid et al found that patients with AML who relapsed following RIC conditioning had different 2-year prognoses depending on whether they had lower (<27%) or higher (>27%) blasts at relapse, which was further confirmed by multivariate analysis [15]. Therefore, the prognosis of patients could be improved by preemptively intervening when the tumor burden is low, such as an upwards MRD.
Another important prognostic factor is the occurrence and extent of GVHD after transplantation. Wang et al found that the presence of cGVHD at relapse could prolong survival time after relapse, which may be related to a stronger GVL effect and a delay in time to relapse [17]. Thanarajasingam et al reported that GVHD was a drawback of prOS, irrespective of the GVHD type. They posited that the occurrence of relapse following GVHD signified a disease state that was unresponsive to the GVL effects [18]. The observed discrepancy could potentially be attributed to the heterogeneity of the primary diseases encompassed in their study. T.H. Terwey et al investigated the relationship between the GVHD occurrence and the prognosis of ALL patients. Their findings indicated that grade III-IV aGVHD harmed OS due to increased NRM. On the other hand, any degree of cGVHD was associated with higher OS [19]. Our research indicates that patients who develop grade III-IV aGVHD following transplantation experience poorer pOS, possibly due to increased NRM and impaired treatment tolerance resulting from compromised organ function. cGVHD is associated with improved prOS, likely due to delayed relapse and sustained GVL effects. Since patients with ALL have a substantial GVL effect, GVHD has a more significant impact on the prognosis of ALL patients with relapse after transplantation [20].
The correlation between the timing of HSCT recurrence and survival outcomes has been consistently demonstrated across multiple studies. In a retrospective study analyzing the survival of 102 patients with AL who experienced relapse after transplantation, those who relapsed within 180 days had a significantly lower 2-year probability of overall survival (4%) compared to those who relapsed after 180 days (22%, P < 0.001) [21]. Raynier Devillier et al. conducted a retrospective analysis to evaluate the prognosis of 54 patients with AML who relapsed after allo-HSCT. The study found that patients who relapsed within 6 months of transplantation had a significantly poorer 1-year OS rate of 9% compared to those who relapsed after 6 months (32%, P = 0.012). Furthermore, multivariate analysis revealed that time to relapse (HR = 3.7) and performance status at relapse (HR = 2.2) were the only independent predictors of OS [2]. The occurrence of early relapse suggests that leukemic cells may be less sensitive to conditioning regimens and/or the GVL effect, resulting in a higher relapse rate and decreased likelihood of achieving remission. Both our primary study cohort and subsequent validation cohort demonstrated that patients who experienced early relapse had a significantly worse prognosis for prOS.
This study has limitations. First, it is a retrospective study conducted at a single center, which resulted in limited data collection from patients. Second, due to the unavailability of genetic data, we could not evaluate whether specific molecular alterations had a greater impact on predicting prognosis. Third, the analysis may be limited by selection bias as the treatment of post-transplant relapse was not standardized and was based on individual patients. Additionally, the high heterogeneity of conditioning regimens may have influenced the outcomes of the patients.
Conclusion
The incidence of post-transplant GVHD, location and timing of relapse, and tumor burden are significant predictors of prognosis in patients with acute leukemia following allo-HSCT. Identification of unfavorable prognostic factors and tailoring treatment are essential to improve survival outcomes for patients.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Craddock C, Hoelzer D, Komanduri KV. Current status and future clinical directions in the prevention and treatment of relapse following hematopoietic transplantation for acute myeloid and lymphoblastic leukemia. Bone Marrow Transpl. 2019;54:6–16.
Devillier R, Crocchiolo R, Etienne A, Prebet T, Charbonnier A, Furst S, et al. Outcome of relapse after allogeneic stem cell transplant in patients with acute myeloid leukemia. Leuk Lymphoma. 2013;54:1228–34.
D’Souza A, Fretham C, Lee SJ, Arora M, Brunner J, Chhabra S, et al. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transpl. 2020;26:e177–e82.
Wu X, Liu Q. Prophylaxis and treatment of relapse after haploidentical stem cell transplantation: what is known vs unknown? Semin Hematol. 2019;56:209–14.
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71.
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the mount sinai acute GVHD international consortium. Biol Blood Marrow Transpl. 2016;22:4–10.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transpl. 2015;21:389–401 e1.
Orti G, Barba P, Fox L, Salamero O, Bosch F, Valcarcel D. Donor lymphocyte infusions in AML and MDS: enhancing the graft-versus-leukemia effect. Exp Hematol. 2017;48:1–11.
Bremnes RM, Al-Shibli K, Donnem T, Sirera R, Al-Saad S, Andersen S, et al. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: emphasis on non-small cell lung cancer. J Thorac Oncol. 2011;6:824–33.
Christopher MJ, Petti AA, Rettig MP, Miller CA, Chendamarai E, Duncavage EJ, et al. Immune escape of relapsed AMl cells after allogeneic transplantation. N Engl J Med. 2018;379:2330–41.
Mielcarek M, Storer BE, Flowers ME, Storb R, Sandmaier BM, Martin PJ. Outcomes among patients with recurrent high-risk hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2007;13:1160–8.
Spyridonidis A, Labopin M, Schmid C, Volin L, Yakoub-Agha I, Stadler M, et al. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia. 2012;26:1211–7.
Schmid C, de Wreede LC, van Biezen A, Finke J, Ehninger G, Ganser A, et al. Outcome after relapse of myelodysplastic syndrome and secondary acute myeloid leukemia following allogeneic stem cell transplantation: a retrospective registry analysis on 698 patients by the Chronic Malignancies Working Party of the European Society of Blood and Marrow Transplantation. Haematologica. 2018;103:237–45.
Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119:1599–606.
Sun W, Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, et al. Chemotherapy plus DLI for relapse after haploidentical HSCT: the biological characteristics of relapse influences clinical outcomes of acute leukemia patients. Bone Marrow Transpl. 2019;54:1198–207.
Wang Z, Yin C, Zhang W, Tang W, Song X, Hu X, et al. The benefit of chronic graft-versus-host disease in patients with acute myeloid leukemia relapsed after allogeneic stem cell transplantation. Ann Hematol. 2019;98:1765–73.
Thanarajasingam G, Kim HT, Cutler C, Ho VT, Koreth J, Alyea EP, et al. Outcome and prognostic factors for patients who relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2013;19:1713–8.
Terwey TH, Le Duc TM, Hemmati PG, le Coutre P, Nagy M, Martus P, et al. NIH-defined graft-versus-host disease and evidence for a potent graft-versus-leukemia effect in patients with acute lymphoblastic leukemia. Ann Oncol. 2013;24:1363–70.
Porter DL, Alyea EP, Antin JH, DeLima M, Estey E, Falkenburg JH, et al. NCI first international workshop on the biology, prevention, and treatment of relapse after allogeneic hematopoietic stem cell transplantation: report from the committee on treatment of relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2010;16:1467–503.
Matsumoto K, Yamamoto W, Ogusa E, Ishigatsubo Y, Kanamori H. Prognostic index for relapsed acute leukemia after allogeneic stem cell transplant. Leuk Lymphoma. 2014;55:2808–12.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant nos. 82170210).
Author information
Authors and Affiliations
Contributions
YG and HW contributed to collecting and analyzing the data and writing the manuscript; ZS and FG reviewed the literature and approved the final draft; JS, YL, JY, XL, HF and LL supported the treatment course of these patients and approved the final draft; HH and YZ are corresponding authors and they critically reviewed the patients and the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, Y., Wu, H., Shi, Z. et al. Prognostic factors and clinical outcomes in patients with relapsed acute leukemia after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 58, 863–873 (2023). https://doi.org/10.1038/s41409-023-01989-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-01989-3
- Springer Nature Limited
This article is cited by
-
Risk factors and long-term outcomes for human herpesvirus 6 encephalitis in the early period after allogeneic stem cell transplantation
Bone Marrow Transplantation (2024)
-
A case of posttransplant isolated extramedullary relapse of acute lymphoblastic leukemia achieving durable treatment-free remission with blinatumomab and donor lymphocyte infusion
International Journal of Hematology (2024)