Abstract
The association of graft-versus-host disease (GVHD) and graft-versus-leukemia effect after stem-cell transplantation (SCT) is well established but with limited data in the setting of haploidentical SCT (haploSCT) with post-transplant cyclophosphamide (PTCy). We used a series of landmark analyses to investigate this association in 805 AML patients following haploSCT. On day +100, 707 patients were alive and leukemia-free, 500 had no prior acute GVHD, 137 had acute GVHD grade II and 70 had grade III–IV. Subsequent relapse rates were 20.3%, 23.2% and 15.0%, respectively (P = 0.52). Subsequent non-relapse mortality (NRM) was 8.6%, 17.8% and 38.6%, respectively (P < 0.0001). Leukemia-free survival (LFS) was 71.0%, 59.0% and 46.3%, respectively (P < 0.0001). Multivariate analysis showed that acute GVHD grade II and grade III–IV were not associated with relapse (HR 1.21, P = 0.37 and HR 1.03, P = 0.94), but were associated with increased NRM (HR 2.09, P = 0.005 and HR 6.41, P < 0.0001) and lower LFS (HR 1.47, P = 0.02 and HR 2.59, P = < 0.0001). Chronic GVHD was not associated with subsequent relapse. Extensive chronic GVHD was associated with higher NRM (HR 6.72, P < 0.0001) and inferior LFS (HR 3.29, P = < 0.0001). GVHD of any type or grade is not associated with lower relapse after haploSCT with PTCy. Severe forms are associated with higher NRM and lower survival.
Similar content being viewed by others
Introduction
Allogeneic stem cell transplantation (SCT) is a curative therapy for acute myeloid leukemia (AML). It provides both dose-intensive chemo-radiotherapy and enhancement of a graft-versus leukemia effect (GvL). The major causes of treatment failure are recurrent disease and non-relapse causes such as graft-versus-host disease (GVHD) and infections. The association between GVHD and GvL is well established [1,2,3]. Horowitz et al. have shown that both acute and chronic GVHD are associated with a lower risk for relapse after SCT [3]. However, only the mild forms were associated with better survival as the more severe GVHD forms also resulted in increased non-relapse mortality (NRM). Chronic GVHD was more important in controlling relapse in patients with AML.
Marked changes have been introduced in modern SCT over the last two decades. These include SCT in older patients, the use of reduced-intensity conditioning (RIC), the use of older sibling donors, more unrelated donors, as well as alternative donors such as haploidentical and umbilical cord blood donors, and a change to the more common use of peripheral blood stem cells (PBSC) as the stem-cell source. These changes as well as the marked improvement in supportive care improved NRM, but did not markedly change the rate of disease relapse [4]. All of these changes may have an impact on the association of GVHD and GVL in these new SCT settings. A more recent analysis of the correlation between relapse and GVHD in a mega-file of >48,000 transplants reported to the European Society for Blood and Marrow Transplantation (EBMT) confirmed the well-known association of GVHD and GVL [5] However, the strength of the association was different between diseases. A strong association was seen in chronic myeloid leukemia and acute lymphoblastic leukemia. However, the correlation was relatively weak in patients with AML suggesting that GvL effects may be operating in the absence of GVHD in this disease [3, 5].
The use of haploSCT has markedly increased over the last decade [6]. Results have constantly improved with time, more than most types of transplants [7]. This is mostly related to the shift towards non-T cell-depleted transplants, such as with the use of post-transplant cyclophosphamide (PTCy) [8]. The outcome of patients with AML after haploSCT is now similar to that after HLA- matched related or unrelated donors [9, 10]. Haploidentical transplant with extensive T-cell depletion is associated with low rates of GVHD and alloreactivity is mostly related to natural killer (NK) cell activity [11]. The role of NK alloreactivity in non-T cell-depleted haploidentical transplants is much more controversial [12]. PTCy limits the rate of severe acute GVHD and of chronic GVHD. However, there is limited data regarding the association of GVHD and GVL in this setting [13, 14].
In this study, we explored the association of GVHD and GvL in a relatively large cohort of patients with AML given haploSCT with PTCy.
Patients and methods
Study design and data collection
This is a retrospective multicenter analysis. Patient data were obtained from the EBMT registry. The EBMT is an international research collaborative group comprising over 650 transplant centers required to report on an annual basis on all transplants performed. Quality control measures of this multicenter registry include confirmation of the validity of the entered data by the reporting team, cross-checking with the national registries, and regular in-house and external data audits. The study was approved by the acute leukemia working party (ALWP) and was performed in compliance with the Helsinki declaration and under guidance of the EBMT. All patients provided written informed consent authorizing the use of information for research purposes.
Patients were eligible for the study if they had de-novo or secondary AML and had received a haploSCT, in first complete remission (CR1) or second CR (CR2), between the years 2009–2017. Only patients engrafting after SCT were included in the analysis. Haploidentical donors were defined as two or more mismatches from a family related donor. Data collected included recipient and donor characteristics, disease features, transplant-related factors including drugs and total doses used in the conditioning regimen, and outcome variables including the occurrence and timing of acute and chronic GVHD, relapse, and survival data.
Conditioning regimens
The conditioning regimen was selected at the participating center’s discretion. Dose intensity was defined according to standard criteria based on the reversibility and expected duration of cytopenia after SCT [15]. All transplants were non T-cell-depleted and were based on PTCy. Additional GVHD prophylaxis was selected according to the participating center policy and consisted of a calcineurin inhibitor (cyclosporine A or tacrolimus) with mycophenolate mofetil in most cases. No ex-vivo manipulation was allowed. Patients given anti-thymocyte globulin were excluded from the analysis. Both bone marrow (BM) and PBSC were eligible stem cell source.
Evaluation of outcomes
Overall survival (OS) was calculated from the day of SCT until the death of any cause or the date of the last follow-up. Leukemia-free survival as survival with no relapse. Disease relapse was defined according to standard hematological criteria. NRM was defined as death without prior disease recurrence. Acute GVHD was graded and staged according to the consensus criteria [16]. Chronic GVHD was graded according to the Seattle criteria [17].
Statistical analysis
The primary endpoint of the study was relapse for assessing the impact of acute and chronic GVHD on post transplant outcome. Secondary endpoints were acute and chronic GVHD rates, NRM, LFS, and OS The probabilities of OS and LFS were calculated using the Kaplan–Meier method [18]. Relapse, NRM, and GVHD rates were estimated using cumulative incidence analysis, considering competing risks. In the estimation of acute and chronic GVHD we considered relapse and death to be competing events. Univariate analyses were performed using log rank test for OS and LFS and Gray’s test for cumulative incidence functions [19]. For all univariate analyses, continuous variables were categorized and the median was used as a cut-off point. To study the effect of GVHD on SCT outcomes, we used a series of landmark analyses [20, 21]. From the original data set, we constructed data sets for four landmark time points at days +30, +100, +180, and +360, selecting patients alive in remission at these time points. At each landmark point, we fitted a simple Cox model. Variables were included if considered relevant based on the univariate analysis (P value < 0.2), or known to be so from the literature. In order to take into account the “overlap” between landmark data sets, and since the data of the same patient are used repeatedly in the different landmark strata, we used a stacked data set containing all the landmark data sets, and the final model was stratified by the landmark and standard errors obtained by taking into account the “clustering” of the data using the sandwich estimators of Lin and Wei (1989) [22]. We also used a Cox proportional hazards model including GVHD as a time-dependent variable for relpase, NRM, and LFS. Hazard ratios (HR) and 95% confidence intervals (95% CI) are reported. In the univariate analyses, the P values gave the global comparison of the 3 groups (No GVHD, grade II, grade III–IV). However, the interpretation of the results is based on the results of multivariate analyses where P values are given versus a reference group which is the absence of GVHD. Statistical analyses were performed with R 3.4.0 (R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.), packages ‘survival’, ‘cmprsk’ and ‘dynpred’.
Results
Patient characteristics
The study included 805 patients with AML in CR1 or CR2 given a first T-cell replete haploSCT with PTCy, during the years 2009–2017. Patient characteristics are outlined in Table 1. The median patient age was 53 years (range, 18–76). The median donor age was 37 years (range, 13–72). Fifteen percent had secondary AML and 16% had poor cytogenetics. The conditioning regimen was myeloablative in 52% of patients and RIC in 48%. GVHD prophylaxis included a calcineurin inhibitor and mycophenolate mofetil in addition to PTCy in most patients. The stem cell source was BM in 47% of patients and PBSC in 53%.
Acute GVHD and SCT outcomes
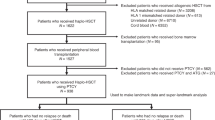
Transplantation outcomes are presented in Fig. 1 and Supplementary Table 1. The overall rate of acute GVHD grade II–IV and III–IV was 30.3% (95% CI, 27.2–33.6) and 11.6% (95% CI, 9.5–13.9), respectively. Seven hundred and seven patients were alive and leukemia-free 100 days after transplant; 500 had no prior acute GVHD at this landmark, 137 had acute GVHD grade II and 70 had grade III–IV. The overall rates of relapse subsequent to the day +100 landmark was 20.3%, 23.2% and 15.0%, respectively (P = 0.52). The overall rates of subsequent NRM were 8.6%, 17.8% and 38.6%, respectively (P < 0.0001). The rates of LFS were 71.0%, 59.0% and 46.3%, respectively (P < 0.0001). Multivariate analysis showed that acute GVHD grade II before day +100 was not associated with subsequent relapse (HR) 1.21, P = 0.37), but it was associated with increased NRM (HR 2.09, P = 0.005) and lower LFS (HR 1.47, P = 0.02). Similarly, acute GVHD grade III–IV before day +100 was not associated with subsequent relapse (HR 1.03, P = 0.94) but was associated with higher NRM (HR 6.41, P < 0.0001) and inferior LFS (HR 2.59, P = < 0.0001). Acute GVHD grade III–IV was associated with subsequent chronic GVHD (HR 1.75, P = 0.01). Similar findings were observed at landmark day +30 (Supplementary Table 2).
Chronic GVHD and SCT outcomes
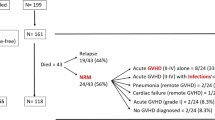
Transplantation outcomes in relation to chronic GVHD are presented in Fig. 2 and Supplementary Table 3. The overall rates of chronic GVHD and extensive chronic GVHD were 28.9% (95% CI: 25.6–32.3) and 11.2% (95% CI: 9.0–13.6), respectively. Four hundred and ninety-three patients were alive and leukemia-free 360 days after transplant; 338 had no prior chronic GVHD at this landmark, 100 had limited grade chronic GVHD and 55 had extensive chronic GVHD. The overall rates of relapse subsequent to the day +360 landmark were 7.9%, 5.2% and 10.9%, respectively (P = 0.52). The overall rates of subsequent NRM were 2.5%, 3.4% and 23.5%, respectively (P < 0.0001). The rates of LFS were 89.5%, 91.4% and 65.6%, respectively (P = 0.0001). Multivariate analysis showed that limited chronic GVHD before day +360 was not associated with subsequent relapse (HR 0.63, P = 0.27), NRM (HR 1.61, P = 0.35) or LFS (HR 0.87, P = 0.67). Extensive chronic GVHD before day +360 was also not associated with subsequent relapse (HR 1.74, P = 0.22), but it was associated with higher NRM (HR 6.72, P < 0.0001) and inferior LFS (HR 3.29, P = < 0.0001). Similar findings were observed at landmark day +180 (supplementary Table 4).
Multivariable models
In a final model that was stratified by four landmark points (days +30. +100, +180, +360) the only factor that predicted relapse was the use of RIC (HR 1.67, P = 0.007, Table 2). Acute and chronic GVHD of any grade did not protect from relapse. The factors predicting an increased NRM were acute GVHD of both grade II (HR 1.86, P = 0.01) and grade III–IV (HR 4.61, P < 0.0001). Extensive chronic GVHD (HR 2.81, P = 0.002) and advanced age (HR 1.3, P = 0.008) also predicted higher NRM (Table 3). The factors predicting reduced LFS were acute GVHD of both grade II (HR 1.42, P = 0.03) and grade III–IV (HR 2.05, P = 0.0004), extensive chronic GVHD (HR 1.96, P = 0.008) and RIC (HR 1.52, P = 0.003).
We also constructed a Cox proportional hazards model including GVHD as a time-dependent variable (Tables 2–4). The same predicting factors were identified. RIC was the only factor predicting relapse (HR 1.77, P = 0.001). Acute GVHD of both grade II, (HR 1.62, P = 0.04) and grade III–IV (HR 6.51, P < 0.0001), extensive chronic GVHD (HR 2.65, P = 0.0004) and advanced age (HR 1.34, P = 0.0002) predicted NRM. Acute GVHD of both grade II, (HR 1.34 P = 0.04) and grade III–IV (HR 2.74, P < 0.0001), extensive chronic GVHD (HR 1.61, P = 0.02) and RIC (HR 1.53, P = 0.001) predicted a reduced LFS. We also performed a Cox analysis limited to 387 patients given RIC. Similar observations were seen in the entire group. Acute GVHD grade II–IV was not associated with relapse (HR 1.04, P = 0.86) but was associated with higher NRM (HR 3.08, P < 0.0001) and lower LFS (HR 1.71, P = 0.0008). Chronic GVHD was also not associated with relapse rate (HR 0.92, P = 0.76) but was associated with higher NRM (HR 1.92, P = 0.03)
Discussion
In this registry-based study, we show in a relatively large cohort of patients with AML given haploSCT with PTCy that acute and chronic GVHD of any grade are not associated with reduction of relapse rate after SCT. The more severe forms are associated with increased NRM and reduced survival. This suggests that PTCy allows the separation of GvL from GVHD in this transplant setting. This observation contrasts with the known association of GVHD and GvL in the HLA-matched setting [1,2,3].
These observations may be explained in part by the mechanism of action of PTCy. This mechanism of action has not been completely defined. Most of the data come from comes from experiments with MHC- matched murine models of skin allograft rejection [8]. These experiments suggested that alloreactive T-cell elimination and thymic clonal deletion are the major mechanisms for GVHD prevention. Theoretically, rapidly proliferating T-cells directed against HLA antigens will easily be eliminated following PTCy in the haploidentical setting, while T-cells that target underlying leukemia and infectious agents without causing GVHD will be spared. Therefore, there will not be any additional advantage in disease control for those having GVHD. Recent data suggest a major role of regulatory T-cells (Tregs) in promoting long-term tolerance after SCT with PTCy [23]. Tregs, similarly to hematopoietic stem cells express high levels of aldehyde dehydrogenase which confers resistance to Cy [24]. Acute GVHD grade II is common after PTCy but progression to more severe forms and to chronic GVHD is less common, suggesting that alloreactive T-cells are not eliminated but are controlled and that alloreactivity is reduced with time [8]. PTCy modulates alloreactivity and abrogates the impact of most of the traditional risk factors for GVHD, such as the number of mismatches and donor gender. The potential to use Tregs for control of GVHD while enhancing GvL has been reported by the Perujia group with the infusion of Tregs with conventional T-cells after T- cell-depleted haploSCT that enhanced GvL with no concomitant GVHD [25].
The degree of correlation between GVHD and GVL in AML is somewhat controversial [5]. After allogeneic SCT patients with no GVHD have a lower relapse rate than the rate observed after identical twin or autologous transplant, supporting this relation [3, 26]. However, other studies showed only a relatively weak correlation. There is data to support GVL in the absence of GVHD in this disease [26]. Results of SCT with T-cell depletion have not shown excess post-transplant relapse rates in early-stage AML [27]. The threshold level of T-cells necessary to trigger GvL is lower compared to GVHD and a lower level of GVHD may be sufficient to reduce the risk of relapse [28]. Mechanisms other than general T-cell alloreactivity may be effective in AML. These mechanisms may include NK cell alloreactivity or targeting of leukemia-specific antigens.
There is limited data on the association between GvL and GVHD in the haploidentical setting. The Baltimore group explored this association in 340 patients with various hematological malignancies following haploSCT with nonmyeloablative conditioning and PTCy [13]. They used a similar statistical methodology as in the current study. They showed that acute GVHD grade II was associated with reduced relapse rates, similar NRM, and improved OS compared to no GVHD. Acute GVHD grade III–IV did not reduce relapse, probably due the high immune suppression burden, markedly increased NRM, and reduced OS. Chronic GVHD showed a trend towards reduction of relapse but no effect on survival. Similar observations were seen with the use of PTCy after HLA-matched transplants with PTCy [29]. Mo et al., investigated the same question in a group of 324 patients with AML/ MDS following haploSCT with the Chinese platform using ATG in the conditioning. Chronic GVHD reduced the risk of relapse and improved survival, particularly in the mild-moderate forms [14]. The differences between these studies and the current study may be related to the use of different platforms, different groups of patients, conditioning regimens, and stem cell source. The Baltimore group used nonmyeloablative conditioning and BM as the stem cell source. The Chinese platform uses a different concept with no use of PTCy. In the current study, PTCy was used, but the conditioning regimen was myeloablative and PBSC was given in a significant fraction of patients. The association of GVHD and GvL has been shown to be more predominant in the reduced- intensity than in the myeloablative setting [5, 30, 31]. We observed the same finding when limiting the analysis to RIC recipients. Still, the nonmyeloablative Baltimore platform with minimal intensity may show different relations of GVHD and GVL. Similarly, PBSC may show stronger GVL that may not be dependent of GVHD.
GVHD and GvL in the HLA- matched setting are directed against disparities in minor histocompatibility antigens between the recipient and donor. In the HLA- mismatched setting major HLA antigens may become targets for alloreactive T- cells [32]. The frequency of T-cells with direct alloreactivity against HLA- antigens is 0.1–1%, which is higher than against any other antigen [33]. This suggests theoretically, that the GvL effect will be stronger following HLA- mismatched unrelated or haploSCT. There is evidence for reduced relapse risk with an increased number of mismatches in umbilical cord blood transplantation [34]. However, in clinical practice, there is no evidence for a lower relapse rate after haploidentical transplants [35]. The exploitation of HLA- mismatch as a target for GvL is hampered by the need for intensive interventions to prevent GVHD. There is also a strong possibility for immune escape through the loss of the mismatched haplotype leading to the loss of the targets for GvL and relapse occurring in up to one-third of relapses [36]. However, there are some locus-specific exceptions such as permissible or low-expression mismatches in HLA-C or HLA-DPB1 that are sufficient to provoke GvL but with no excess GVHD [32].
In conclusion, GVHD of any type or grade is not associated with improved relapse rate after T-cell replete haplo SCT with PTCy and offers no survival advantage. Severe forms are associated with higher NRM and lower survival. Future novel strategies for the prevention of significant GVHD are warranted.
References
Sullivan KM, Weiden PL, Storb R, Witherspoon RP, Fefer A, Fisher L, et al. Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood 1989;73:1720–8.
Weiden PL, Flournoy N, Sanders JE, Sullivan KM, Thomas ED. Antileukemic effect of graft-versus-host disease contributes to improved survival after allogeneic marrow transplantation. Transpl Proc. 1981;13:(1 Pt 1) 248–51.
Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood 1990;75:555–62.
Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–101.
Stern M, de Wreede LC, Brand R, van Biezen A, Dreger P, Mohty M, et al. Sensitivity of hematological malignancies to graft-versus-host effects: an EBMT megafile analysis. Leukemia 2014;28:2235–40.
Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transpl. 2017;52:191–6.
Shouval R, Fein JA, Labopin M, Kröger N, Duarte RF, Bader P, et al. Outcomes of allogeneic haematopoietic stem cell transplantation from HLA-matched and alternative donors: a European Society for Blood and Marrow Transplantation registry retrospective analysis. Lancet Haematol. 2019;6:e573–e584.
Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol. 2016;13:132.
Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood 2015;126:1033–40.
Versluis J, Labopin M, Ruggeri A, Socie G, Wu D, Volin L, et al. Alternative donors for allogeneic hematopoietic stem cell transplantation in poor-risk AML in CR1. Blood Adv. 2017;1:477–85.
Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood 2007;110:433–40.
Shimoni A, Labopin M, Lorentino F, Van Lint MT, Koc Y, Gülbas Z, et al. Killer cell immunoglobulin-like receptor ligand mismatching and outcome after haploidentical transplantation with post-transplant cyclophosphamide. Leukemia 2019;33:230–9.
McCurdy SR, Kanakry CG, Tsai HL, Kasamon YL, Showel MM, Bolaños-Meade J, et al. Grade II Acute Graft-versus-Host Disease and Higher Nucleated Cell Graft Dose Improve Progression-Free Survival after HLA-Haploidentical Transplant with Post-Transplant Cyclophosphamide. Biol Blood Marrow Transpl. 2018;24:343–52.
Mo XD, Xu LP, Zhang XH, Liu DH, Wang Y, Chen H, et al. Chronic GVHD induced GVL effect after unmanipulated haploidentical hematopoietic SCT for AML and myelodysplastic syndrome. Bone Marrow Transpl. 2015;50:127–33.
Spyridonidis A, Labopin M, Savani BN, Niittyvuopio R, Blaise D, Craddock C, et al. Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transpl. 2020;55:1114–25.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transpl. 1995;15:825–8.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–17.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assos. 1958;53:457–81.
Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706.
Van Houwelingen HC. Dynamic prediction by landmarking in event history analysis. Scand J Stat. 2007;34:70–85.
Van Houwelingen HC, Putter H. Dynamic predicting by landmarking as an alternative for multi-state modeling: an application to acute lymphoid leukemia data. Lifetime Data Anal. 2008;14:447–63.
Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–8.
Wachsmuth LP, Patterson MT, Eckhaus MA, Venzon DJ, Gress RE, Kanakry CG. Post-transplantation cyclophosphamide prevents graft-versus-host disease by inducing alloreactive T cell dysfunction and suppression. J Clin Invest. 2019;129:2357–73.
Kanakry CG, Ganguly S, Zahurak M, Bolaños-Meade J, Thoburn C, Perkins B, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med. 2013;5:211ra157.
Pierini A, Ruggeri L, Mancusi A, Carotti A, Falzetti F, Terenzi A, et al. T cell depletion and no post transplant immune suppression allow separation of graft versus leukemia from graft versus host disease. Bone Marrow Transpl. 2019;54:775–9. Suppl 2
Ringdén O, Labopin M, Gorin NC, Schmitz N, Schaefer UW, Prentice HG, et al. Is there a graft-versus-leukaemia effect in the absence of graft-versus-host disease in patients undergoing bone marrow transplantation for acute leukaemia? Br J Haematol. 2000;111:1130–7.
Devine SM, Carter S, Soiffer RJ, Pasquini MC, Hari PN, Stein A, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transpl. 2011;17:1343–51.
Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–50.
McCurdy SR, Kanakry CG, Tsai HL, e Gojo I, Smith BD, Gladstone DE, et al. Development of grade II acute graft-versus-host disease is associated with improved survival after myeloablative hla-matched bone marrow transplantation using single-agent post-transplant cyclophosphamide. Biol Blood Marrow Transpl. 2019;25:1128–35.
Weisdorf D, Zhang MJ, Arora M, Horowitz MM, Rizzo JD, Eapen M. Graft-versus-host disease induced graft-versus-leukemia effect: greater impact on relapse and disease-free survival after reduced intensity conditioning. Biol Blood Marrow Transpl. 2012;18:1727–33.
Baron F, Labopin M, Niederwieser D, Vigouroux S, Cornelissen JJ, Malm C, et al. Impact of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation for acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European group for blood and marrow transplantation. Leukemia 2012;26:2462–8.
Fleischhauer K, Beelen DW. HLA mismatching as a strategy to reduce relapse after alternative donor transplantation. Semin Hematol. 2016;53:57–64.
Archbold JK, Macdonald WA, Burrows SR, Rossjohn J, McCluskey J. T-cell allorecognition: a case of mistaken identity or déjà vu? Trends Immunol. 2008;29:220–6.
Brunstein CG, Petersdorf EW, DeFor TE, Noreen H, Maurer D, MacMillan M, et al. Impact of allele-level HLA mismatch on outcomes in recipients of double umbilical cord blood transplantation. Biol Blood Marrow Transpl. 2016;22:487–92.
Ringdén O, Labopin M, Ciceri F, Velardi A, Bacigalupo A, Arcese W, et al. Is there a stronger graft-versus-leukemia effect using HLA-haploidentical donors compared with HLA-identical siblings? Leukemia 2016;30:447–55.
Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MT, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361:478–88.
Acknowledgements
The authors acknowledge the contribution of all the EBMT centers contributing patients to this study.
Author information
Authors and Affiliations
Contributions
AS, ML, MM and AN, designed the research, analyzed and interpreted data, and wrote the manuscript; AS, ML, EA, DB, FC, YK, ZG, DMJL, BB, LC, MM, MR, MM and AN provided patients, collected and analyzed data, and critically reviewed the manuscript before submission. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Shimoni, A., Labopin, M., Angelucci, E. et al. The association of graft-versus-leukemia effect and graft-versus host disease in haploidentical transplantation with post-transplant cyclophosphamide for AML. Bone Marrow Transplant 57, 384–390 (2022). https://doi.org/10.1038/s41409-021-01493-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-021-01493-6
- Springer Nature Limited
This article is cited by
-
Effect of graft-versus-host disease on outcomes of HLA-haploidentical peripheral blood transplantation using post-transplant cyclophophamide
Bone Marrow Transplantation (2024)
-
Allogeneic hematopoietic cell transplantation from alternative donors in acute myeloid leukemia
Annals of Hematology (2024)
-
Trends in allogeneic transplantation for favorable risk acute myeloid leukemia in first remission: a longitudinal study of >15 years from the ALWP of the EBMT
Bone Marrow Transplantation (2024)
-
GVHD occurrence does not reduce AML relapse following PTCy-based haploidentical transplantation: a study from the ALWP of the EBMT
Journal of Hematology & Oncology (2023)