Abstract
This is an update on acute and chronic graft-versus-host disease (GvHD) in 425 patients with hematologic malignancies, undergoing an unmanipulated haploidentical (HAPLO) graft from related donors, with a modified post-transplant cyclophosphamide (PT-CY) regimen. All patients received a myeloablative conditioning regimen, either based on thiotepa busulfan fludarabine (TBF), or on full-dose total body irradiation (TBI). The cumulative incidence of acute GvHD-grade II–IV was 29%, and the CI of GvHD-grade III–IV was 4%. We found older donors and older patients to have higher rates of grade II–IV acute GvHD; female donors, diagnosis, disease phase, year of transplant, and the conditioning regimen had no predictive effect on acute GvHD. There was no impact of grade II GvHD, but a significant impact of grade III–IV acute GvHD, on overall survival. The CI of moderate–severe chronic GvHD was 18%: the major predictor was a previous acute GvHD, followed by combined donor and recipients age. In conclusion, PT-CY given on days+3 + 5 results in a relatively low, but not insignificant risk of acute and chronic GvHD, in patients grafted from the related HAPLO donors. The use of young donors appears to reduce this risk.
Similar content being viewed by others
Introduction
Graft-versus-host disease (GvHD) remains one of the major problems in patients undergoing an allogeneic hemopoietic stem cell transplant (HSCT), in its acute, and also in its chronic form (cGvHD), and has significant impact on survival and quality of life [1, 2]. Post-transplant cyclophosphamide (PT-CY) has been reported to be very effective in controlling severe GvHD, following unmanipulated haploidentical transplants (HAPLO), when given on days +3 + 4, followed on day + 5, by a calcineurin inhibitor (CNI) and mycophenolate mofetil (MMF). The original paper was published by the Baltimore group and recently updated [3, 4], and very encouraging results have been reproduced worldwide [5,6,7,8,9,10]. We will refer to this regimen as PT-CY +3 + 4. We have reported the use of a modified PT-CY regimen, with CNI given on day 0, MMF day +1, and PT-CY on days +3 and +5 [11], together with a myeloablative regimen: we had designed PT-CY +3 + 5, because of the fear of increased toxicity due to the introduction of a myeloablative conditioning; the patient population was also at high risk, and more graft versus leukemia was welcome [11]. We will refer to this regimen as PT-CY +3 + 5. Because the CNI is given before PT-CY, one may have expected more acute and/or chronic GvHD [12]. We have now used this modified PT-CY regimen in 425 patients, prepared with a myeloablative regimen between 2011 and 2017, in two transplant units (Genova and Rome Gemelli). The aim of this study is to update the incidence, risk factors, and outcome of GvHD in this patient population.
Patients and methods
Patients were selected for HAPLO grafts in the absence of a suitable HLA-matched related or unrelated donor. The median age of the patients was 52 years (14–74), with 101 patients aged over 60. Remission status was as follows: CR1 (n = 171), CR2 (n = 112), and advanced disease (n = 161). The median donor age was 34 years (18–67). The diagnosis was AML (n = 154), ALL (n = 87), MDS (n = 83), myelofibrosis (n = 47), non-Hodgkin lymphoma (n = 31), and other (n = 44).
Conditioning regimens
We used two myeloablative conditioning regimens, one chemotherapy based (n = 350), including thiotepa, busulfan, and fludarabine (TBF) as described (BBMT 2013), and one radiation based (n = 99) with full-dose radiation (999–1200 rads) (TBI) and fludarabine (BBMT 2013) (n = 75). The TBF regimen was used with full-dose busulfan 3.2 mg/kg × 3, (patients ≤ 60 years), or 3.2 mg/kg × 3 (patients over 60 years) and BU 3.2 mg/kg × 1 (patients over 70 years). The median age for the TBF regimen was 55 years (18–74), whereas for the TBI, it was 35 years (14–64).
GvHD prophylaxis for all patients was cyclosporin (CsA) 2 mg/kg i.v. starting day 0, mycophenolate 2 g/day p.o, starting day +1 to day +30, and PT-CY 50 mg/kg day +3 and day +5. When possible, CsA was tapered starting day +100 and discontinued at day +180. All patients received an unmanipulated marrow as a stem cell source.
Age score
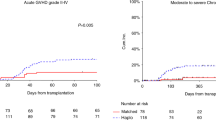
We scored donors age as 0–2 with a cutoff at 30 and 40 years, and we scored recipients age as 0–2 with a cutoff at 40 and 60 years. When added together, patients could be scored 0–4 as shown in Fig. 1a, b.
Cumulative incidence (CI) of acute GvHD grade II–IV in patients stratified according to donor and recipients age (see text). Score 0–1 represents young patients and young donors, and score 4 represents older patients (>60 years) grafted from older donors (>40 years); score 2–3 represent donor and recipients of intermediate age
Statistical analysis
The NCSS 11 Data for Windows (Kaysville, UT, USA) was used for contingency tables, rank-sum test, cumulative incidence (CI) rates, and actuarial survival. When calculating the CI of GvHD, the competing risk was death without GvHD. The log-rank test was used for differences between survival curves; the Grays’ test was used to assess differences between cumulative incidence curves. Multivariate Cox analyses on GvHD was run, testing the effect of donor and recipients age, gender, diagnosis, disease phase, and the conditioning regimen.
Results
Acute GvHD
The CI of acute GvHD II–IV was 28% and 3% for aGvHD grade III–IV. In univariate analysis, risk factors for acute GvHD grade II–IV, were patients over the age of over 60 (p = 0.01; as a continuous variable p = 0.06), donor age over 33 (p = 0.02), and also as a continuous variable (p = 0.03). A female donor in a male recipient, disease phase (remission vs. advanced), diagnosis (acute vs. chronic disorders), conditioning regimen (TBF vs. TBI), year of transplant (</>2014), and ABO compatibility were not predictive factors. There was a trend for patients receiving a larger dose of total nucleated cells (×108/kg) to have more GvHD grade II–IV: 14% for cell dose <2 × 108/kg, 19% for cell dose 2.1–4 × 108/kg, and 22% for a cell dose >4 × 108/kg (p = 0.3). The CI of aGvHD grade II–IV in patients under 60 was 16% vs. 28% for patients over 60 years; the CI of aGvHD grade II–IV in patients grafted from donors under 34 years (median age) was 15% vs. 23% for older donors (p = 0.02). We then classified donor and recipient age as follows: donors age < 30 (0), 1–40 (1), and >40 (2); recipients age <40 (0), 41–60 (1), and >60 (2). When donor and recipient scores were added together, there were 176 patients with score 0–1, 221 patients with score 2–3, and 28 patients with score 4. The CI of aGvHD grade II–IV for patients with score 0–1, 2–3, and 4 was, respectively, 13%, 21%, and 41% (p = 0.003) (Fig. 1a).
In a multivariate analysis, combined donor and recipient age was the only predictor, with a risk of 2.3 of developing grade II–IV acute GvHD.
The effect was not significant, but there was a clear trend for combined donor/recipient age to predict severe grade III–IV acute GvHD (3% vs. 4% vs. 8%) for score 0–1 and 2–3 and (p = 0.4).
Chronic GvHD
The overall CI of chronic GvHD was 61%: the proportion of patients surviving 100 days, with no, minimal, moderate, or severe cGvHD was 39%, 38%, 17%, and 6%, respectively. The CI of moderate–severe chronic GvHD was 18%. It was strongly predicted by donor age: it was 11% vs. 24% vs. 26% for donors <30 years of age, aged 31–40 or over 40 years (p = 0.005). Recipients age was not predictive of moderate–severe cGvHD (p = 0.5). However, the same scoring system adopted for acute GvHD was predictive of moderate–severe cGvHD (11% vs. 25% vs. 29%) (Fig. 1b).
Variables with no predictive effect on cGvHD were disease phase, diagnosis (acute vs. chronic disorders), ABO match vs. mismatch, and conditioning regimen (radiation based vs. TBF). In multivariate analysis, the strongest predictive factor for moderate–severe cGvHD was acute GvHD grade II–IV (RR 4.2, p < 0.00001), followed by patients and recipient age scores: score 2–3 had a risk of 2.3 (p = 0.01) as compared with score 0–1, and score 4 had a risk of 4.1 (p = 0.1).
GvHD and survival, TRM and relapse
The effect of acute GvHD on overall survival (OS) is shown in Fig. 2: the 5-year OS was comparable for grades 0–I (52%) and II acute GvHD (52%), whereas there was a significant detrimental effect for a small number of patients (n = 15), with grade III–IV acute GvHD (5-year OS 16%). As to chronic GvHD, there was a positive effect of minimal cGvHD in patients with early disease (CR1 + CR2) (5-year OS of 81%), whereas patients with severe cGvHD (n = 13) had a very poor outcome (3-year OS 0%) (Fig. 3a). There was no significant effect of cGvHD in patients with advanced disease (Fig. 3b). The CI of relapse for early disease was 24%, 21%, 17%, and 7%, respectively, for patients with no, minimal, moderate, or severe cGvHD (p = 0.4); the CI of NRM was 5%, 4%, 15%, and 55% again for different severity of cGvHD (p < 0.00001).
At 1 year post transplant, 88% of patients were off cyclosporin A and 83% were off steroids; the average Karnofsky score was 97%. Chronic GvHD was scored as absent (68.7%) minimal (24.8%), moderate (4.8%), and severe (1.4%).
Discussion
In this study, we describe the incidence of GvHD in HAPLO transplants grafted with an unmanipulated marrow, with PT-CY +3 + 5, and a CsA on day 0, following a myeloablative conditioning regimen. The cumulative incidence of GvHD grade II–IV and III–IV was 28% and 3%; the cumulative incidence of moderate–severe chronic GvHD was 18%. The strong and only predictive factor for acute GvHD grade II–IV was the combination of donor and recipient age: young patients grafted from young donors had a 13% cumulative incidence of acute GvHD grade II–IV, whereas old patients (>60) grafted from old donors (>40) had a 41% incidence of grade II–IV acute GvHD. If we look only at patients over 60 years, the risk of GvHD II–IV is 17%, 26%, and 40%, when grafted from donors aged ≤30, 31–40, and >40 years. The combined age score was not significantly predictive of acute GvHD grade III–IV, but there was a definite trend (3%, 4%, and 8%) for older patients grafted from older donors to have at least twice the risk of severe acute GvHD, as compared with young patients grafted from young donors. As for chronic GvHD, the strongest predictor was acute GvHD grade II–IV: the risk of moderate–severe cGvHD was 16% vs. 49% for patients with grade 0–I or II–IV acute GvHD (p < 0.0001). The combination of donor and recipients age remained a strong predictor of chronic GvHD. In a multivariate analysis including diagnosis, disease phase, conditioning regimen, and donor–recipient gender mismatch, the strongest predictor was a previous acute GvHD II–IV, followed by donor–recipient age score. Chronic GvHD seen in these HAPLO transplants appears to be treatable, since at 1 year post transplant, 83% were off cyclosporine and 88% were off steroids.
How do these results compare with other reports of HAPLO transplants, using PT-CY on days +3 + 4 with a CNI starting the day after CY (day +5)? In a relatively recent study, the Center for International Blood and Marrow Transplantation Research (CIBMTR) reported 104 AML patients receiving a HAPLO graft, with PT-CY given on days +3 + 4 [13]. We compared these 104 AML patients with 150 AML patients reported in a multicenter trial, all receiving PT-CY +3 + 5 [14]. The two groups were comparable for disease phase (CR1 46% vs. 45%; CR2 20% vs. 21%) (p = 0.8) and percentage of patients over 50 years of age (42% vs. 51%, p = 0.2), respectively, for PT-CY +3 + 4 and PT-CY +3 + 5. Acute GvHD grade II–IV was reported in 16% of patients receiving PT-CY +3 + 4, vs. 22% for PT-CY +3 + 5 (p = 0.08). The overall risk of chronic GvHD was, respectively, 30% for the original regimen vs. 56% for the modified regimen (p = 0.001). There is thus a trend for PT-CY +3 + 5, with CsA starting before PT-CY, to give more acute and more chronic GvHD. A very recent editorial has suggested that increased GvHD may be due to a lower number of regulatory T cells, as shown in vitro when cells are exposed to CsA before mafosfamide, and compared with mafosfamide alone or mafosfamide followed by CsA [14]. The results in our clinical study with PT-CY +3 + 5, were obtained with a marrow as the only stem cell source, and it may be that the use of unmanipulated PB would further increase the risk of severe GvHD, when a CNI is given before PT-CY. If PT-CY +3 + 5 produces more GvHD, one may expect less leukemia relapse: in the two studies just outlined [13, 14], the percentage of patients dying with relapse was 44% for PT-CY +3 + 4 vs. 23% for PT-CY +3 + 5 (p = 0.0006) [13, 14].
Finally, we looked at the impact of GvHD on survival: only the small number of patients with acute GvHD grade III–IV (n = 14, 3%) or with severe chronic GvHD (n = 20, 6%) had a reduced probability of survival at 5 years, and this was mostly evident in patients with early disease (CR1 + CR2). Grade II acute GvHD has no impact on OS. Patients with minimal chronic GvHD had the best outcome, due to reduced relapse and low transplant-related mortality.
Conclusion
We confirm a relatively low, but not insignificant incidence of GvHD in patients receiving an unmanipulated HAPLO bone marrow transplant, with PT-CY given on days +3 + 5, and CsA given before cyclophosphamide, which can be reduced when using young donors. The risk factors are mainly donors age, although the combination of donor and recipient age is able to predict both acute and chronic GvHD. Whether this modified regimen is appropriate for patients at high risk of relapse, and less so for patients with indolent disorders, remains to be ascertained. Finally, these data are obtained with an unmanipulated marrow as a stem cell source: the risk of severe acute GvHD could be significantly increased with the use of unmanipulated peripheral blood and PT-CY on days +3 + 5 [12].
References
Gratwohl A, Brand R, Apperley J, et al. Graft vs host diseaseand outcome in HLA identical sibling transplantation, for chronic myeloid leukemia. Blood. 2002;100:3877–86.
Palmer J, Chai X, Martin PJ, Weisdorf D, Inamoto Y, Pidala J, et al. Failure-free survival in a prospective cohort of patients with chronic graft-versus-host disease. Haematologica 2015;100:690–5.
Luznik L, Jalla S, Engstrom LW, et al. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98:3456–64.
McCurdy SR, Kasamon YL. Kanakry comparable composite endpoints after HLA-matched and HLA-haploidentical transplantation with post-transplantation cyclophosphamide. Haematologica. 2017;102:391–400.
Sugita J, Kagaya Y, Miyamoto T. Japan Study Group for Cell Therapy and Transplantation (JSCT). Myeloablative and reduced-intensity conditioning in HLA-haploidentical peripheral blood stem cell transplantation using post-transplant cyclophosphamide. Bone Marrow Transplant 2019;54:432–41.
Ruggeri A, Sun Y, Labopin M, et al. Post-transplant cyclophosphamide versus anti-thymocyte globulin as graft- versus-host disease prophylaxis in haploidentical transplant. Haematologica 2017;102:401–10.
Bashey A, Zhang MJ, McCurdy SR, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell–replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35:3002–9.
Ciurea SO, Saliba R, Rondon G, et al. Reduced-intensity conditioning using fludarabine, melphalan and thiotepa for adult patients undergoing haploidentical SCT. Bone Marrow Transplant 2010;45:429–36.
Katsanis E, Sapp LN, Varner N, et al. Haploidentical bone marrow transplantation with post-transplant cyclophosphamide/bendamustine in pediatric and young adult patients with hematologic malignancies. Biol Blood Marrow Transplant 2018;24:2034–9.
Devillier R, Bramanti S, Furst S, et al. T-replete haploidentical allogeneic transplantation using post-transplantation cyclophosphamide in advanced AML and myelodysplastic syndromes. Bone Marrow Transplant. 2016;51:194–8.
Raiola AM, Dominietto A, Ghiso A, et al. Unmanipulated haploidentical bone marrow transplant and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant 2013;19:117–22.
Kanakry CG, Luznik L. Teaching a young dog new tricks: modifications to the post-transplantation cyclophosphamide haploidentical transplantation platform. Biol Blood Marow Transplant. 2018;24:1108–10.
Ciurea SO, Zhang MJ, Bacigalupo A. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126:1033–40.
Chiusolo P, Bug G, Olivieri A, et al. A modified post-transplant cyclophosphamide regimen, for unmanipulated haploidentical marrow transplantation, in acute myeloid leukemia: a multicenter study. Biol Blood Marrow Transplant 2018;24:1243–9.
Funding
Publication of this supplement was sponsored by Gilead Sciences Europe Ltd., Cell Source, Inc., The Chorafas Institute for Scientific Exchange of the Weizmann Institute of Science, Kiadis Pharma, Miltenyi Biotec, Celgene, Centro Servizi Congressuali, and Almog Diagnostic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bacigalupo, A., Maria Raiola, A., Dominietto, A. et al. Graft versus host disease in unmanipulated haploidentical marrow transplantation with a modified post-transplant cyclophosphamide (PT-CY) regimen: an update on 425 patients. Bone Marrow Transplant 54 (Suppl 2), 708–712 (2019). https://doi.org/10.1038/s41409-019-0594-1
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0594-1
- Springer Nature Limited
This article is cited by
-
Possible prognostic impact of WT1 mRNA expression at day + 30 after haploidentical peripheral blood stem cell transplantation with posttransplant cyclophosphamide for patients with myeloid neoplasm: a multicenter study from the Okayama Hematological Study Group
International Journal of Hematology (2022)
-
Cord blood resilience in a patient with relapsing Ph + B lymphoblastic acute leukemia after hematopoietic stem cell transplantation
Annals of Hematology (2022)
-
Post-transplant cyclophosphamide alters immune signatures and leads to impaired T cell reconstitution in allogeneic hematopoietic stem cell transplant
Journal of Hematology & Oncology (2022)
-
Single-cell transcriptomic analysis reveals disparate effector differentiation pathways in human Treg compartment
Nature Communications (2021)
-
Early administration of cyclosporine may reduce the incidence of cytokine release syndrome after HLA-haploidentical hematopoietic stem-cell transplantation with post-transplant cyclophosphamide
Annals of Hematology (2021)